Glucagon-Like Peptide-1 Receptor Agonists for the Treatment of Type 2 Diabetes Mellitus: A Position Statement of the Korean Diabetes Association
Article information
Abstract
The glucagon-like peptide-1 receptor agonists (GLP-1RAs) were recommended as a monotherapy or combination therapy with oral hypoglycemic agents or basal insulin in the position statement of the Korean Diabetes Association 2017 for pharmacological therapy. Many randomized clinical trials and systematic reviews report that GLP-1RAs have considerable glucose-lowering effect and lead to weight reduction and low risk of hypoglycemia when used as a monotherapy or combination therapy. The cardiovascular safety of GLP-1RAs has been assessed in several randomized clinical trials and systematic reviews. The results of cardiovascular outcome trials of long-acting GLP-1RAs (liraglutide, semaglutide) demonstrated cardiovascular benefits in subjects with type 2 diabetes mellitus and a high risk of cardiovascular disease. The GLP-1RA may be a choice of therapy when weight control and avoidance of hypoglycemia are important, and patients with high risk of cardiovascular disease might also favor choosing GLP-1RA.
INTRODUCTION
Incretin hormone includes glucagon-like peptide-1 (GLP-1) from L-cells and glucose-dependent insulinotrophic polypeptide from K-cells of the small intestine. GLP-1 stimulates glucose-dependent insulin release, inhibits glucagon release, delays gastric emptying, and suppresses the appetite through GLP-1 receptor binding. However, GLP-1 has a short half-life of 1 to 2 minutes due to degradation by didpeptidyl peptidase-4 (DPP-4), which has been a limitation for its use as an anti-hyperglycemic agent. GLP-1 receptor agonists (GLP-1RAs) are synthetic analogues that are resistant to DPP-4 degradation and have more stable pharmacodynamic profiles [12]. The GLP-1RAs are effective in improving glycemic control with reduction in body weight and a low risk of hypoglycemia. Recently, there have been many clinical trials and systematic reviews to determine the efficacy, safety, and cardiovascular effects of GLP-1RAs [345678910].
In the position statement of the Korean Diabetes Association (KDA) 2017 for pharmacological therapy in non-pregnant adult patients with type 2 diabetes mellitus (T2DM), the Committee of Clinical Practice Guidelines of KDA updated the previous recommendations published in 2015 after extensive review of the scientific evidence. In particular, GLP-1RAs were recommended as a monotherapy or combination therapy with oral hypoglycemic agents or basal insulin, which was unlike the previous guideline that recommended them only as a combination therapy.
In this article, we will review the glycemic and metabolic effects and cardiovascular outcome trials and provide the rationale for the recommendation of GLP-1RAs in the position statement of the KDA 2017.
GLYCEMIC AND METABOLIC EFFECTS
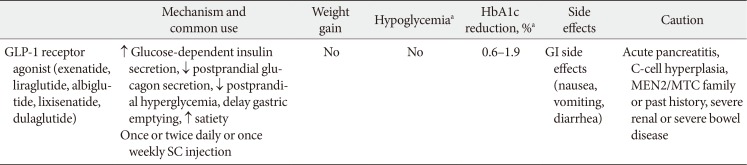
GLP-1RAs reduce fasting glucose concentrations, by up to 50 mg/dL, and glycosylated hemoglobin (HbA1c) by 0.5% to 1.3% with a lower risk of hypoglycemia due to a glucose-dependent mechanism of modulating insulin and glucagon secretion (Table 1) [345611]. The short-acting GLP-1RAs have more pronounced effects on gastric emptying time and a greater effect on postprandial glucose, whereas the longer-acting agents result in greater effect on fasting glucose and HbA1c concentrations [3456]. The risk of hypoglycemia with GLP-1RAs is low due to the glucose-dependent mechanism. In addition to their effects on HbA1c, the GLP-1RAs are associated with the reduction of appetite and food intake resulting in weight loss of between 2 and 4 kg on average [34561213]. In clinical trials, increased concentration of high-density lipoprotein cholesterol by 0.01 to 0.02 mmol/L and decreased concentration of triglyceride by 0.15 to 0.7 mmol/L were shown after treatment with GLP-1RAs [5]. The other characteristics, side effects and caution, were introduced in Table 1.
CARDIOVASCULAR OUTCOME TRIALS
The cardiovascular safety of GLP-1RAs has been assessed in several randomized clinical trials (RCTs) and systematic reviews [45678910]. In the ELIXA (Evaluation of Lixisenatide in Acute Coronary Syndrome) trial, 6,068 patients with T2DM who had experienced a myocardial infarction or who had been hospitalized for unstable angina in the past 180 days were randomized and assigned to a lixisenatide group or placebo group. There was no reduction in overall cardiovascular risk and no difference in heart failure or serious adverse events between the two groups [7]. In contrast, the long-acting liraglutide and semaglutide reduced the cardiovascular events. In the LEADER (Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results) trial, 9,340 patients with T2DM and high cardiovascular risk were randomized and assigned to either a liraglutide or placebo group, which was added to standard care. Liraglutide reduced the risk for the combined primary outcome of cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke by 13%, versus 14.9% in the placebo group (hazard ratio [HR], 0.87; 95% confidence interval [CI], 0.78 to 0.97). The rates of nonfatal myocardial infarction, nonfatal stroke, and hospitalization for heart failure were not significantly lower in the liraglutide group than in the placebo group [8]. The Trial to Evaluate Cardiovascular and Other Long-term Outcomes with Semaglutide in patients with T2DM (SUSTAIN-6) (semaglutide is not currently marketed in Korea) was designed as a noninferiority safety study and randomized 3,297 patients with T2DM who were at high risk of cardiovascular disease. The primary composite outcome (the first occurrence of cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke) occurred in fewer patients in the semaglutide group, 6.6% versus 8.9% in the placebo group (HR, 0.74; 95% CI, 0.58 to 0.95) [9]. The difference of results in cardiovascular outcome trials may be related to the pharmacokinetics and potency of the glucose-lowering effect of the respective agents. Differences in study design including duration, patient risk profile, and study size may also influence the results of trials. However, the cardiovascular safety trials have been performed in very high risk populations aiming at captureing cardiovascular safety issue, and the study duration is relatively short. There are few data on cardiovascular disease safety or benefits in lower-risk patients. Several systematic reviews and meta-analyses summarizing the effect of GLP-1RAs did not show an increased risk for hospitalization for heart failure. This was unlike administration of DPP-4 inhibitors, the other incretin-based therapy [4510].
Microvascular outcomes
There is no trials assessing the effect of GLP-1RA on microvascular complication as the primary outcome. In trials designed to assess cardiovascular outcomes, LEADER, SUSTAIN-6, liraglutide and semagluitde reduced the risk of nephropathy [89]. In SUSTAIN-6 study, rates of diabetic retinopathy (vitreous hemorrhage, blindness, or conditions requiring treatmemt with an intravitreal agent or photocoagulation) were higher significantly in semaglutide group compared to placebo group (HR, 1.76; 95% CI, 1.11 to 2.78) [9]. However, we cannot conclude yet because these trials were not designed and were of short duration to assess microvascular outcomes and there was no evaluation for retinopathy at baseline of study. Further studies about the effects of GLP-1RA on microvascular complications in lower-risk patients are needed.
RECOMMENDATION
GLP-1RAs
A GLP-1RA can be used as a monotherapy or combination therapy with oral antihyperglycemic agents or basal insulin [A].
COMMENTS ON RECOMMENDATIONS
GLP-1RAs as monotherapy
In accordance with other guidelines, metformin monotherapy is recommended as an initial therapy for patients with T2DM in the position statement of the KDA 2017 for pharmacological therapy [1415161718]. However, if metformin is not tolerated or is contraindicated, then another class of antihyperglycemic agent including GLP-1RA can be chosen according to the patient's circumstances, with the goal of controlling blood glucose levels with fewer side effects. There are many studies demonstrating that GLP-1RAs have a considerable glucose-lowering effect leading to weight reduction and a low risk of hypoglycemia when used as a monotherapy [192021]. Weight control and avoidance of weight gain are important factors in T2DM management. Therefore, GLP-1RA may be appropriate when weight loss and avoidance of hypoglycemia is a primary consideration. In addition, patients with a high risk of cardiovascular disease might also favor choosing GLP-1RA based on the results of cardiovascular outcome studies.
GLP-1RAs as combination therapy with basal insulin
When basal insulin has been titrated to an acceptable fasting glucose level but satisfactory HbA1c levels have not been achieved, GLP-1RA can be added to basal insulin therapy. Many RCTs and systematic reviews have demonstrated that a combination of GLP-1RA with basal insulin showed equal or slightly superior efficacy of HbA1c reduction and less hypoglycemia and better weight control compared to combination therapy of prandial insulin and basal insulin [2223242526]. In another study, the Basal Insulin Glargine plus Exenatide twice daily versus Basal Insulin Glargine plus Bolus Insulin Lispro (4B) study, exenatide twice daily or mealtime insulin lispro was given to patients with T2DM and insufficient glycemic control receiving titrated insulin glargine and resulted in comparable reductions in HbA1c. Exenatide was associated with weight loss and less hypoglycemia, but more gastrointestinal adverse effects, including nausea, vomiting, and diarrhea [23]. Subgroup analysis for Korean patients in the 4B study reported that exenatide twice daily and prandial insulin lispro both reduced HbA1c effectively [24]. In a 26-week trial, compared the addition of liraglutide versus a single daily dose of insulin aspart to insulin degludec in patients who were already treated with metformin (BEGIN: VICTOZA ADD-ON), liraglutide reduced HbA1c levels significantly more than insulin aspart, and it was also associated with more weight loss and less hypoglycemia [25]. In the AWARD (Aseessment of Weekly AdministRation of Dulaglutide)-9 trial, weekly dulaglutide added to basal insulin glargine was efficient for glucose control and well-tolerated by patients [26].
GLP-1RAs as combination therapy with oral hypoglycemic agents
GLP-1RAs have been studied in combination with one or two oral hypoglycemic agents. Liraglutide was effective when used in combination with one (metformin, sulfonylurea) or two (sulfonylurea plus metformin, thiazolidinedione plus metformin) oral hypoglycemic agents [27282930]. In a 26-week randomized study, exenatide weekly therapy resulted in a greater reduction of HbA1c and more frequent weight loss compared with insulin glargine therapy [31]. In HARMONY 4: an RCT comparing once-weekly albiglutide and insulin glargine, albiglutide was noninferior to insulin glargine at reducing HbA1c at week 52, and produced modest weight loss and less hypoglycemia [32]. Dulaglutide has been evaluated in combination with one or two oral hypoglycemic agents (metformin, sulfonylurea, pioglitazone) and showed good glucose-lowering effect and weight loss but with gastrointestinal adverse events [3334]. Combined use of the most recently approved antihyperglycemic agent classes, GLP-1RA (exenatide weekly) and sodium-glucose co-transporter-2 inhibitors (dapagliflozin), improved glycemic control and were well-tolerated in patients with T2DM which was inadequately controlled by metformin monotherapy [35]. Recently a meta-analysis comparing the clinical effects of short- or long-acting GLP-1RAs versus insulin treatment in head-to-head studies reported that GLP-1RAs achieved better glycemic control with benefits regarding hypoglycemia, body weight, blood pressure, and lipoprotein when added on oral hypoglycemic agents [36].
CONCLUSIONS
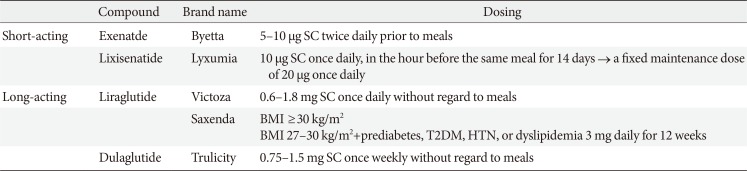
GLP-1RAs have benefits as antihyperglycemic agents which can achieve reduction of glucose levels and body weight with a low risk of hypoglycemia. In the position statement of the KDA 2017, GLP-1RAs were recommended as a monotherapy or combination therapy with oral hypoglycemic agents or basal insulin based on sufficient clinical evidence. Table 2 shows compounds and dosing of currently available GLP-1 RAs in Korea [37]. We can choose a GLP-1 RA according to the patient's status, for example, level and pattern of hyperglycemia and life style.
Cardiovascular disease is the major cause of mortality and morbidity in patients with T2DM [3839] and reduction of cardiovascular risk is one of the treatment goals in management of T2DM. Each therapeutic agent's capacity to modify cardiovascular risk has been investigated and is one of the important considerations for choice of an antihyperglycemic agent. Therefore, recent reports of cardiovascular outcome trials of GLP-1RAs yield informative data. We should consider the limitation; however, that these trials were performed in very high risk populations. Hypoglycemia is a major barrier to achieving good glycemic control in patients with T2DM, and the risk of hypoglycemia is an important factor for the choice of antihyperglycemic agents [40]. The percentage of those who are obese has increased among Korean patients with diabetes, and nearly half of all patients with diabetes are obese according to a Diabetes Fact Sheet in Korea [4142]. Weight control is one of the important factors in obese diabetes. Therefore, GLP-1RA may be a choice of therapy when weight control and avoidance of hypoglycemia are important and in patients with high risk of cardiovascular disease. In addition, further studies about cardiovascular disease safety or benefits in lower-risk patients and concerning efficacy and safety in Korean patients are needed.
ACKNOWLEDGMENTS
This position statement on antihyperglycemic-agent therapy was written by the Korean Diabetes Association Committee of Clinical Practice Guidelines. We gratefully acknowledge the following experts who provided a critical review and discussion of this update.
Notes
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.