Effects of Lobeglitazone, a Novel Thiazolidinedione, on Bone Mineral Density in Patients with Type 2 Diabetes Mellitus over 52 Weeks
Article information
Abstract
Background
The aim of this multicenter, randomized, double-blind study was to examine the effect of lobeglitazone, a novel thiazolidinedione, on the changes in bone mineral density (BMD) in patients with type 2 diabetes mellitus.
Methods
A 24-week, double-blinded phase was followed by a 28-week, open-label phase, in which the placebo group also started to receive lobeglitazone. A total of 170 patients aged 34 to 76 years were randomly assigned in a 2:1 ratio to receive lobeglitazone 0.5 mg or a matching placebo orally, once daily. BMD was assessed using dual-energy X-ray absorptiometry at week 24 and at the end of the study (week 52).
Results
During the double-blinded phase, the femur neck BMD showed decreasing patterns in both groups, without statistical significance (−0.85%±0.36% and −0.78%±0.46% in the lobeglitazone and placebo groups, respectively). The treatment difference between the groups was 0.07%, which was also not statistically significant. Further, minimal, nonsignificant decreases were observed in both groups in the total hip BMD compared to values at baseline, and these differences also did not significantly differ between the groups. During the open-label phase, the BMD was further decreased, but not significantly, by −0.32% at the femur neck and by −0.60% at the total hip in the lobeglitazone group, and these changes did not significantly differ compared with the original placebo group switched to lobeglitazone.
Conclusion
Our results indicate that treatment with lobeglitazone 0.5 mg over 52 weeks showed no detrimental effect on the BMD compared to the placebo.
INTRODUCTION
The bone mineral density (BMD) tends to be increased in patients with type 2 diabetes mellitus (T2DM) because they are commonly overweight or obese [1]. However, the risk of osteoporotic fracture is significantly increased in patients with T2DM, with approximately a two-fold relative risk compared to those without diabetes [23]. The impaired bone quality and increased risk of falls might attenuate or offset the positive effect of the relatively large body weight on bone strength in subjects with T2DM [45]. Moreover, the mortality related to osteoporotic fracture is much higher in diabetic patients compared to their nondiabetic counterparts [2]. Given the deterioration of bone strength and higher mortality and morbidity in diabetic patients with fracture, the skeletal effects of antidiabetic drugs represent a major issue [6].
Thiazolidinediones (TZDs) are a class of antidiabetic drugs that work by increasing insulin sensitivity by activating peroxisome proliferator-activated receptor γ (PPARγ) [78]. TZDs are known to inhibit differentiation of osteoblasts by overweighing adipogenesis from mesenchymal stem cells, which are the common progenitors of both adipocytes and osteoblasts [9]. Furthermore, some data suggest that TZDs may also partially promote osteoclastogenesis [10]. In many clinical trials, the BMD was found to decrease and the risk of fractures was found to increase in diabetic patients treated with rosiglitazone [1112]. Accordingly, there are many concerns about whether long-term administration of TZDs is associated with decreased BMD and increased osteoporotic fractures [1213]. These negative effects of TZDs on bone strength have become a major limitation in prescribing TZDs to diabetic patients, particularly those at high risk of fractures.
Several TZDs have demonstrated similar glucose-lowering efficacies and are currently being used as antidiabetic drugs. However, these drugs have shown distinguishable properties in terms of their effects on diverse organs, systems, and processes, including energy metabolism, skeletal biology, the vasculature, and lipid profiles [1415]. Especially pioglitazone has been reported to show fewer negative effects on bone metabolism compared to rosiglitazone [1617]. Based on these findings, it is conceivable that the effects on bone metabolism might differ between different TZDs, independently of their glucose-lowering potency.
Lobeglitazone is a novel PPARγ agonist with proven glucose-lowering potency in previous clinical trials and has been used to treat diabetic patients in several Asian countries [1819]. In the present study, we evaluated the effects of 52 weeks of treatment with lobeglitazone on BMD as a means to determine whether lobeglitazone treatment shows negative effects on BMD compared to a placebo.
METHODS
Study design and patients
This was a 52-week, multicenter, randomized, controlled trial to investigate the effect of lobeglitazone on BMD. This study consisted of two phases: a 24-week, double-blinded study followed by a 28-week open-label extension study in which the placebo group also started to receive lobeglitazone. This study was conducted at nine centers in South Korea between 2009 and 2011. In the first double-blinded phase, the patients were randomized into two groups in a 2:1 ratio: one group treated with lobeglitazone 0.5 mg and one group administered a placebo drug for 24 weeks. After completing the first phase of the study, a 28-week, open-label extension phase was conducted, with the patients receiving lobeglitazone continuing on the same drug, whereas the patients in the placebo group were switched to lobeglitazone and continued on this drug for 28 weeks. In our previous studies using the same cohort, the glucose-lowering efficacy and safety of lobeglitazone were demonstrated [1819]. In the present study, the main inclusion criteria were as follows: age 18 to 80 years; T2DM diagnosed at least 3 months prior to the study enrollment; glycosylated hemoglobin (HbA1c) 6.5% to 9.0% at the screening test if medication with oral hypoglycemic agents had been stopped less than 3 months ago, or HbA1c 7.0% to 10.0% at the screening test if patients were drug-naïve or had ceased medication with antidiabetic drugs more than 3 months previously; body mass index between 21 and 40 kg/m2; fasting serum C-peptide level >1.0 ng/mL. The exclusion criteria were as follows: fasting plasma glucose (FPG) level >250 mg/dL; triglyceride level >500 mg/dL; treatment with insulin or TZDs within 60 days; uncontrolled hypertension; history of cardiovascular diseases, including myocardial infarction, uncompensated heart failure, cerebral infarction, cerebral hemorrhage, or unstable angina within 6 months; severe hepatic or renal dysfunction; anemia for any reason; uncontrolled other disease or diabetic complications; and a history of cancer within 5 years. Subjects who used drugs affecting bone metabolism, including bisphosphonates, raloxifene, sex hormone-replacement therapy, or teriparatide, were also excluded. Subjects who were using lipid-lowering agents were allowed to maintain those drugs. This study was approved by each study center's Institutional Review Board (B-0704-044-009) and was carried out in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki). All patients provided informed consent to participate.
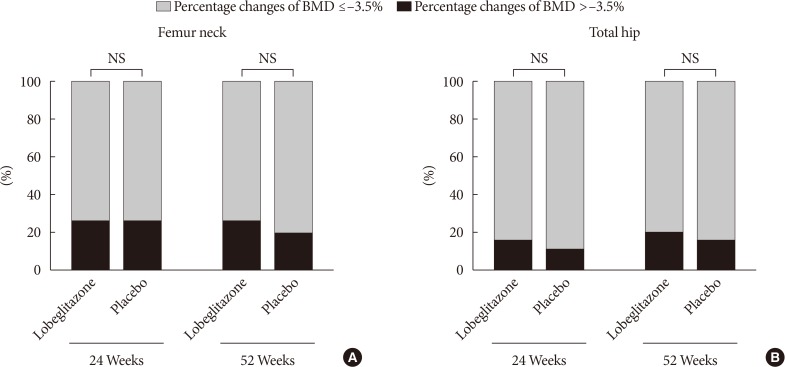
The primary outcome was the changes in the femur neck BMD from baseline to the end of treatment. The secondary endpoints were the changes in total hip BMD during the study period and the percent of patients who showed changes of BMD more than −3.5% at the femur neck or total hip in each study phase.
Dual-energy X-ray absorptiometry
The BMDs at the femur neck and total hip area were measured by dual-energy X-ray absorptiometry (DXA) (Hologic Inc., Bedford, MA, USA; or GE Healthcare Lunar, Madison, WI, USA) in each center. DXA scans were performed at baseline and at week 24 and at the end of the study. BMD values from both systems were converted to standardized BMD values using a conversion formula, which is previously defined [20]. Changes in the BMD were calculated as the percentage of changes of the absolute BMD during the study period. The coefficient variations of BMD measurements in each center ranged from 0.8% to 1.8% for both the femur neck and total hip.
Biochemical parameters
After overnight fasting for at least 12 hours, serum was collected. The serum concentrations of total cholesterol, triglycerides, high density lipoprotein cholesterol, low density lipoprotein cholesterol, blood urea nitrogen, creatinine, HbA1c, and FPG were measured by standard biochemical methods.
Statistical analysis
Data are expressed as mean±standard deviation for continuous variables or as the number and the percentage of patients for categorical variables. The mean percent changes of BMD were calculated using the BMD values at baseline and at weeks 24 and 52. The comparisons of the changes from before and after the treatment within the groups were analyzed by the paired t-test, and differences between the study groups were assessed by repeated measures analysis of variance at the end of the study. Significant BMD loss was defined as a reduction of >3.5% in each study phase than 3.5% loss in each study phase, which was assumed as the least significant change calculated with precision multiplied by 2.77. All statistical analyses were performed using SPSS version 18 (SPSS Inc., Chicago, IL, USA). Values of P<0.05 were considered significant.
RESULTS
Demographics and baseline characteristics
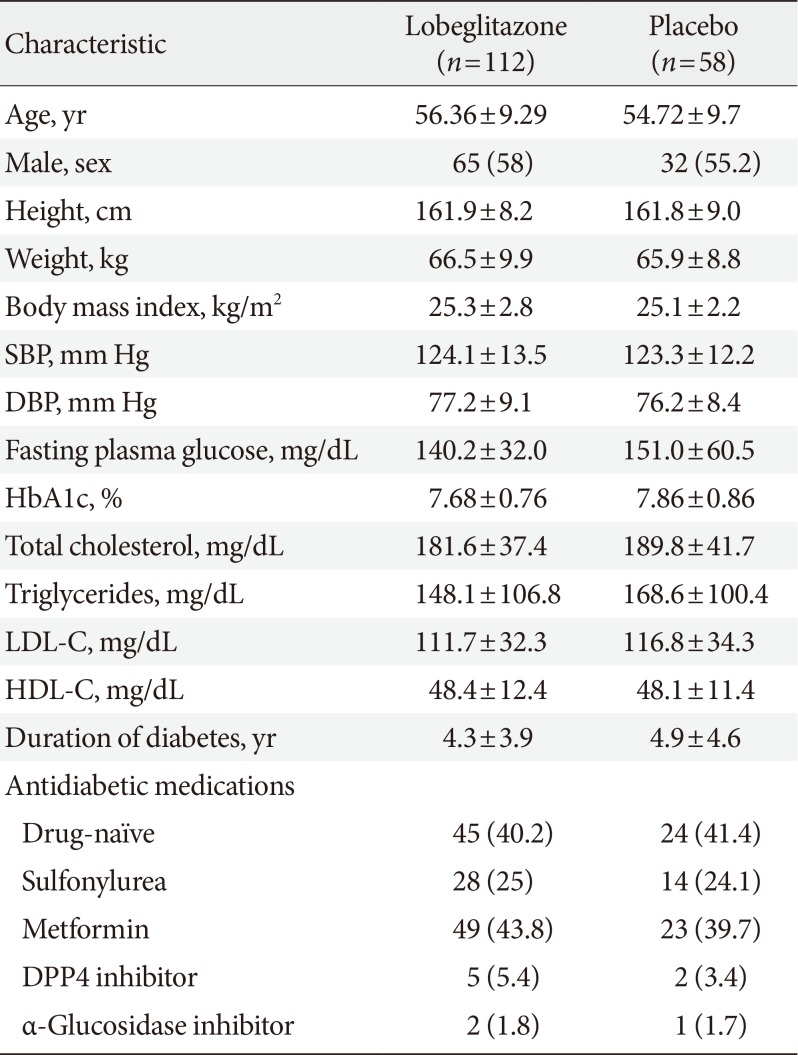
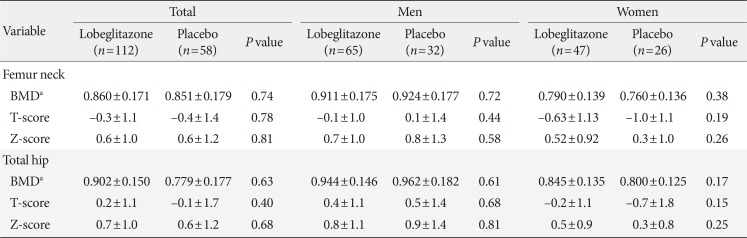
The detailed information about the subject enrollment and randomization has been previously described [1819]. A total of 173 patients were randomly assigned to the lobeglitazone or control group in a 2:1 ratio. The BMD values in three patients in the lobeglitazone group from the original study were not provided, and those patients were thus excluded from the present analysis. The baseline characteristics of the 170 patients who were finally included in the analyses are provided in Table 1. There were no differences in any of the demographic, anthropometric, or clinical characteristics at baseline between the two groups. Further, the BMD values were comparable between the groups for all patients and for men and women when analyzed separately (Table 2).
Changes in bone mineral density
Changes in BMD at the femur neck and total hip in the total subjects and in men and women are presented in Figs. 1 and 2. During the placebo, controlled, blinded phase, the femur neck BMD at 24 weeks had decreased by −0.85%±0.36% in the lobeglitazone group and by −0.78%±0.46% in the placebo group compared to values at baseline; however, these changes were not statistically significant. The difference between the groups was 0.07%, and this was also statistically insignificant (Figs. 1A and 2A). Minimal nonsignificant decreases were observed in both groups in total hip BMD, with no treatment difference noted between the groups (−0.17%±0.30% in the lobeglitazone group vs. −0.35%±0.34% in the placebo group; treatment difference, 0.17%; P>0.05 for all) (Figs. 1B and 2B). During the open-label phase, in which the placebo was switched to lobeglitazone, the BMD was further decreased by −0.32% in the femur neck and by −0.60% in the total hip, without statistical significances, in the lobeglitazone group (Figs. 1A, B and 2). In the group that switched to lobeglitazone during the open-label phase, there was a slight increase in the femur neck BMD and a decrease in the total hip BMD; however, these changes similarly did not reach statistical significance, and there were no significant differences compared to the lobeglitazone-maintained group (Figs. 1A, B and 2). Similar trends were observed in both men and women when analyzed separately (Fig. 1C and F).
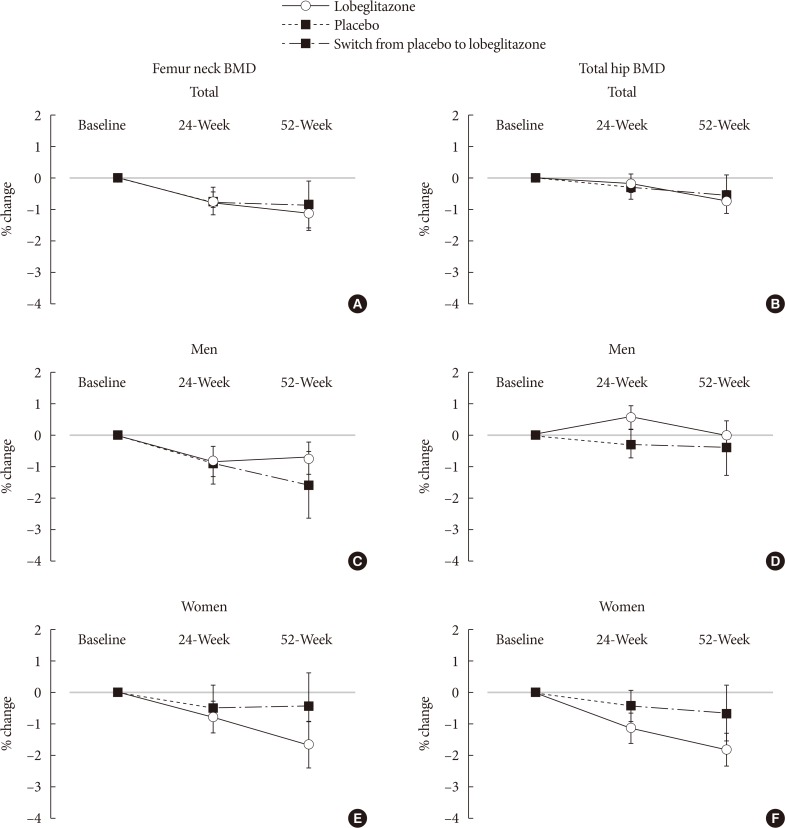
Mean percentage of changes in bone mineral density (BMD) during the study period for the femur neck and total hip in (A, B) all subjects, (C, D) men, and (E, F) women.

Mean percentage changes in bone mineral density (BMD) for the (A) femur neck and (B) total hip during the double-blinded and open-labeled phases. NS, not significant.
We also compared the BMD changes in women and men older than 50 years or younger. Consequently, the BMD changes were not different between the treatment groups regardless of their ages, indicating that lobeglitazone administration did not show any significant effects on BMD changes in subgroups that have higher risk of rapid bone loss (data not shown).
The ratios of patients showing significant bone loss during the double-blinded or open-label phase did not differ between the groups at either the femur neck (25.5% in the lobeglitazone group vs. 25.2% in the placebo group during the double-blinded phase; 26.2% in the lobeglitazone group vs. 18.5% in the group switched from placebo to lobeglitazone during the open-label phase) or total hip (15.0% vs. 10.9% and 20.0% vs. 14.8%, respectively; P>0.05 for all) (Fig. 3).
DISCUSSION
In this study, we investigated the effects of a novel TZD, lobeglitazone, on BMD in subjects with T2DM after 52 weeks of treatment. The results from the present study revealed neutral effects of lobeglitazone on BMD changes in this study population. No statistically significant differences were observed between the lobeglitazone and placebo groups in the changes in BMD at the femur neck and total hip compared to baseline during both the double-blinded and open-labeled periods.
TZDs are insulin-sensitizing agents [2122] and are considered one of the major therapeutic drug classes for the management of diabetes [7]. TZDs increase insulin sensitivity by activating PPARγ, mainly in the adipose tissue and muscle, and show beneficial effects in preventing vascular complications in patients with insulin resistance [2324]. However, despite these benefits, severe clinical issues are related to TZDs, especially the predisposition to fractures [12]. Both adipocytes and osteoblasts differentiate from the same mesenchymal stem cells [2526], and TZDs are known to induce adipocyte differentiation [8] and suppress osteoblastogenesis [27]. Therefore, TZDs might have potentially negative effects on bone metabolism. Accordingly, rapid bone loss and increased risk of fractures have been reported in several clinical studies on TZDs [1128]. Thus, given the increased fragility of diabetes patients, the negative influence on bone has become a significant limitation in TZD prescription.
Interestingly, rosiglitazone and pioglitazone show somewhat different profiles in their effects on diverse organs. Rosiglitazone, which was once withdrawn from the market because of an increased risk of cardiovascular events [29], has been reported to decrease BMD and increase fracture risks [11]. On the other hand, basic and clinical studies with pioglitazone revealed cardiovascular benefits [3031], although its effect on the skeletal system remains inconclusive, with several studies finding no meaningful influences on bone [1632]. In addition, these two drugs also exert distinct effects on lipid profiles and skeletal muscle biology [1415].
Lobeglitazone, a novel PPARγ agonist, has proven glucose-lowering efficacy [181933] and a higher affinity with PPARγ compared to rosiglitazone or pioglitazone [3334]. This profile enables lower dosage as an antidiabetic therapeutic drug; thereby, decreasing the risks of dose-related side-effects. Moreover, the detrimental effects of rosiglitazone on bone are known to result partially from stimulated osteoclastogenesis [35]. However, this phenomenon has not been demonstrated with other TZDs [36]. In the present study, treatment with lobeglitazone 0.5 mg did not affect bone metabolism. The lower dosage of lobeglitazone and its differential effects on bone cells might contribute to the current study findings. However, mechanistic studies to elucidate its effects of bone biology are required to confirm this speculation.
There are several strengths to this study. First, this was a multicenter, placebo-controlled, randomized clinical study. Second, BMD was measured in 98% of the participants. Third, diabetic patients taking other medications such as glucagon-like peptide-1 agonists or sodium-glucose cotransporter inhibitors were excluded because these agents might affect bone metabolism.
However, several limitations should be noted. First, the primary outcome of the original study was changes in the HbA1c level. Therefore, the sample size was calculated based on the determination of the glucose-lowering efficacy, and not powered to investigate BMD changes. Second, approximately half of the study subjects were treatment-naïve patients whose bone quality is thus not expected to be greatly affected by long-term hyperglycemia. Therefore, we cannot extend the current study findings to diabetic populations with a long duration of disease. Third, bone formation and resorption markers were not assessed in the present study, which made it impossible to assess the effect of lobeglitazone on the bone turnover rate per se. Fourth, BMDs were measured only at the femur neck and total hip areas, not at the lumbar spine. Fifth, the study sample size was too small to evaluate the lobeglitazone effects on fracture risks, even though any new fractures had not happened during the study periods in both groups. Last, the study duration was only 52 weeks, which is not enough time to conclude the long-term cumulative effects of lobeglitazone on bone health.
The prevalence of diabetes is increasing substantially worldwide, mainly due to increasing rates of obesity and insulin resistance [37383940], as well as in the increasing number of elderly people, in whom fracture is a critical factor affecting quality of life and mortality [2]. Under these circumstances, TZDs will continue to play a crucial role in diabetes management, and awareness of both the beneficial and side effects of these drugs is important in daily clinical practice [41]. In this study, treatment of lobeglitazone 0.5 mg once a day for 52 weeks showed no detrimental effect on BMD at the femur neck and total hip area compared to placebo. Longer-term and mechanistic studies are needed to confirm this finding.
ACKNOWLEDGMENTS
The original investigators were responsible for the study design and protocol. The current investigators were responsible for analysis plans, statistical analysis, and reporting of the results. The decision to submit the manuscript for publication was made jointly by all authors.
Notes
CONFLICTS OF INTEREST: The original study was funded by CKD Pharmaceutical Corporation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.