Metabolic Surgery for Type 2 Diabetes Mellitus: Experience from Asia
Article information
Abstract
Type 2 diabetes mellitus (T2DM) is a current global health priority and Asia is the epicenter of this epidemic disease. Unlike in the west, where older population is most affected, the burden of diabetes in Asian countries is disproportionately high in young to middle-age adults. The incidence of diabetic nephropathy is alarmingly high in patients with early onset T2DM, especially in those with poor glycemic control. How to control this chronic and debilitating disease is currently a very important health issue in Asia. Bariatric surgery has proven successful in treating not just obesity but also T2DM in morbid obese patients (body mass index [BMI] >35 kg/m2). Gastrointestinal metabolic surgery recently has been proposed as a new treatment modality for obesity related T2DM for patients with BMI <35 kg/m2. Many studies from Asia reported promising results of metabolic surgery to treat obese patients with T2DM which is not well controlled. It has been demonstrated that changes in gastrointestinal hormone secretion after gastrointestinal surgery would favor an early improvement of T2DM in Asians. New procedures have also been designed and proposed specifically for the treatment of diabetes in Asia. This article examines clinical trial data and accepted algorithms with a view toward elucidating the application of metabolic surgery for the treatment of T2DM in the Asia. We propose a systematic approach to surgical treatment, addressing current evidences, patient selection, procedure of choice, and timing and guideline for new procedures.
INTRODUCTION
Type 2 diabetes mellitus (T2DM) has reached to a pandemic level and is currently a significant challenge to health care system worldwide. More than 60% of the world's population with diabetes comes from Asia and the incidence of T2DM in Asia is increasing more rapidly than the rest of the world [1]. Unlike in the West, where older population is most affected, the burden of diabetes in Asian countries is disproportionately high in young to middle-age adults. The incidence of diabetic nephropathy is alarmingly high in patients with early onset T2DM, especially in those with poor glycemic control [2]. In Asia, most of newly diagnosed end-stage renal disease patients are due to T2DM. How to control this chronic and debilitating disease is currently a very important health issue in Asia. Unfortunately, current medical treatment has been relatively unsatisfactory as more than half of the patients cannot achieve the therapeutic goal [34]. Those with poorly controlled T2DM are exposed to high risk of developing microvascular and macrovascular complications such as blindness, limb amputation, end-stage renal disease, and cardiovascular accidents. It also carries a heavy burden of psychosocial and health-economic consequences. Recently, a potential cure for diabetes, bariatric surgery, has arisen in an unexpected way. Bariatric surgery, a weight reduction surgery, has been shown not only an effective treatment for severe obesity (body mass index [BMI] >35 kg/m2) but also result in marked improvement of T2DM control [5]. In addition to weight loss, many mechanisms, including calorie restriction, improved β-cell function, improved insulin sensitivity, alterations in gut physiology, bile acid metabolism, and gut microbiota may contribute in T2DM remission after metabolic surgery [6]. Therefore, gastrointestinal metabolic surgery recently has been proposed as a new treatment modality for obesity related T2DM in patients with BMI <35 kg/m2 [7]. Although bariatric surgery was introduced in Asia later than the Western countries and relatively less well established, the use of bariatric surgery to treat T2DM is pioneered from this region as Asia is in the epicenter of T2DM epidemic. This review summarized the development of metabolic surgery and the current evidences on efficacy of metabolic surgery to treat T2DM in Asia over the past decade. This review will build a foundation for metabolic surgery and the development of further clinical trials to provide more evidence in metabolic surgery for the treatment of T2DM.
HISTORY
The initiation of metabolic surgery started from the report by Pories et al. [8] in 1995. In this landmark paper, the authors reported that gastric bypass is the most effective therapy for T2DM in morbidly obese patients. Rubino and Marescaux [9], then, rejuvenated the metabolic surgery by publishing the provoke concept of duodenum exclusion by an elaborate animal experiment in 2004. In Asia, Lee et al. [10] published the first paper in the world to report the effectiveness of bariatric surgery on the treatment of metabolic syndrome in 2004. Lee et al. [11] then published the first two reports about the efficacy of bariatric surgery on T2DM treatment in Asian in 2008 and 2009. However, Asian Pacific Metabolic and Bariatric Surgery Society was the first society to propose using bariatric/metabolic surgery for T2DM treatment and recommended to set the indication for metabolic surgery at lower BMI <32 kg/m2 in 2005 [7]. The first randomized controlled trial (RCT) in the world specific to metabolic surgery is from Asia [12]. This study compared laparoscopic sleeve gastrectomy (LSG) to laparoscopic gastric bypass (LGB) for the treatment of T2DM in Asian patients with BMI <35 kg/m2 and was the first study to prove that LGB (a duodenum exclusion procedure) had better efficacy on T2DM remission than LSG (non-duodenum exclusion procedure). Following this landmark study, many reports, clinical trials, and researches had come out from this region and contributed to the development of metabolic surgery. After many Asian societies advocated new consensus of using metabolic surgery to treat T2DM for Asians, the international society incorporated the new consensus for Asian [13]. At this time, metabolic surgery has been formally put into the algorithm of T2DM treatment.
EFFICACY OF DIFFERENT METABOLIC PROCEDURES
Bariatric surgery has been evolving over the past 50 years. There have been numerous surgical approaches to weight loss surgery. The application of laparoscopic techniques to bariatric surgery in the past decade has reduced perioperative morbidity and has contributed to a remarkable increase in popularity of bariatric surgery and metabolic surgery for the treatment of T2DM. Among all the procedures, LSG, LGB, and adjustable gastric banding were the three most commonly performed bariatric/metabolic procedures in Asia (Fig. 1) [14].
Laparoscopic sleeve gastrectomy
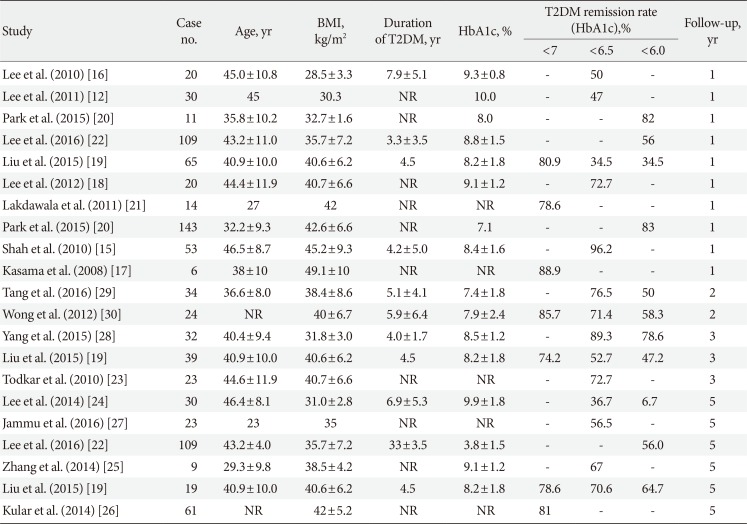
LSG removes approximately 75% of the stomach from greater curvature side and leave a long narrow gastric tube and antrum. This procedure not only restricts the food intake but also increases both gastric emptying and intestinal transit time. Because of the relative simplicity, good weight result and less long-term nutritional problems, LSG becomes the most commonly performed bariatric/metabolic surgery worldwide nowaday [14]. The first report of using LSG to treat T2DM was from India [15]. The remission rate of T2DM (glycosylated hemoglobin <6.5% without antidiabetic medications) 1 year after LSG was 96.2%. However, the remission rate decreased to 50% in patients with BMI <30 kg/m2 [16]. Many reports have been published from different areas of Asia [1718192021222324252627282930]. Table 1 summarizes the reported T2DM remission rate according to different criteria and follow-up years. The remission rate was higher in high BMI group but a trend of decreasing glycemic control with increasing follow-up duration was observed. The advantages of LSG were its efficacy, relative simplicity, less long-term malnutrition, less complication and avoidance of remnant gastric cancer. It does not require insertion of foreign body and avoids band related complications. However, two randomized trials reported that the overall glycemic control was better in LGB over LSG for T2DM treatment in low BMI patients, possibly due to the duodenum exclusion effect [412]. A meta-analysis also supported the superiority of LGB over LSG for T2DM treatment [31]. Therefore, all the T2DM patients should receive detailed evaluation and counselling before receiving metabolic surgery.
Laparoscopic gastric bypass
Five-decades old gastric bypass surgery has become a time honored procedure and is currently regarded as a standard bariatric/metabolic procedure. Following the introduction of laparoscopic era, LGB has accelerated the development both of the bariatric and metabolic surgery [32]. LGB isolates a small gastric pouch from the rest of the stomach. The ingested food in the small gastric pouch was then channeled into the distal part of small bowel via an anastomosis. Thus, the gastric bypass procedure (1) restricts the food and calorie intake, (2) bypasses the gut hormone rich duodenum and proximal jejunum, and (3) expedites the undigested food to the distal intestine, stimulating glucagon-like peptide-1 (GLP-1) and peptide-YY (PYY) secretion, as well altering the gut microbiota environment and bile acid metabolism [6]. The drainage procedure is by either Roux-en-Y gastric bypass (RYGB) or a simplified loop bypass, single anastomosis (mini-) gastric bypass (SAGB) [33]. The first report of gastric bypass for the treatment of T2DM in Asian was from Lee et al. [11] in 2008. The authors reported a very high T2DM remission rate of more than 80% after SAGB in those with BMI >35, but remission rate decreased to 75% in those with BMI <35. Since then, there have been reports about LGB for T2DM treatment from many areas of Asia [343536,37383940414243]. Table 2 summarized the reported T2DM remission after gastric bypass according to different criteria and follow-up years. The remission rate was higher in high BMI group. A decreasing trend of glycemic control along with the increasing follow-up years was observed again. However, in general, the remission rate after LGB was higher than that of LSG.
Laparoscopic adjustable gastric banding
Laparoscopic adjustable gastric banding (LAGB) is the safest bariatric surgical procedure but the efficacy is less favored than other bariatric procedures. Although some randomized trial had shown the better efficacy of LAGB for the treatment of T2DM compared to medical treatment, LAGB is now rarely performed in Asia because of its unpredictability in outcome [171830]. The reported T2DM remission rate after LAGB was only 26.3%, less significant than the 58.3% in LSG [30], and around 80% in gastric bypass [1718].
NEW BARIATRIC/METABOLIC PROCEDURES
Laparoscopic duodenal-jejunal bypass surgery was inspired from Rubino's animal experiment [21]. However, simple exclusion of duodenum was found to be less effective than conventional bariatric/metabolic surgery [44]. Therefore, duodenojejunal bypass with sleeve gastrectomy (DJB-SG) has recently been introduced from this region as a novel metabolic surgery by adding a duodenal switch procedure to SG, which combines the principles and advantages of SG and duodenal switch [45]. The efficacy of DJB-SG was similar to convention gastric bypass [46]. Although gastric bypass is the standard metabolic surgery, gastric bypass had a major problem of precluding the screening of excluded stomach. This may raise a great concern in Asian countries with high incidence of gastric cancer. Therefore, the major advantage of DJB-SG compared to RYGB is avoidance of the risk of gastric cancer arising from remnant stomach by leaving no excluded stomach. Other theoretical advantage of DJB-SG is related to the preservation of the pylorus, which include the prevention of dumping syndrome and facilitating the iron, calcium, vitamin B12, and protein absorption by preserving the acid and intrinsic factor. A recent study showed that by adding a duodenal exclusion to SG, the DJB-SG can increase 10% more weight loss and improve the glycemic control as well as reduce the uric acid level [47]. This finding further supported the important role of duodenum exclusion in the treatment of T2DM. A 5-year report was published recently from Japan to support the efficacy of this procedure [48].
Other novel procedure, such as ileal transportation [49] or proximal jejunal bypass were either too complicated or without evidence to support in clinical usage.
EFFICACY OF METABOLIC SURGERY IN ASIANS IN CONTRAST TO NON-ASIAN POPULATION
Several studies in Asian and Western population have supported unequivocal advantage of metabolic surgery over life style modification and intensive medical treatment alone in terms of diabetes remission (Table 3) [3,4,40,50,51,52,53,54]. The outcomes of metabolic surgery on T2DM remission was comparable between Asians and Western populations. T2DM remission was reported around 40% to 75% following metabolic surgery in contrast to poor remission rate in non-surgical group which was reported around 0% to 10% in both Asian and non-Asian populations. Even though the follow-up period in most of the published studies were short; only 1 to 2 years, T2DM resolution following metabolic surgery is very promising. More reports on longer term metabolic outcomes are expected to come out in near future to provide stronger evidences on durability of metabolic effects of bariatric surgery.
RANDOMIZED CONTROL TRIALS
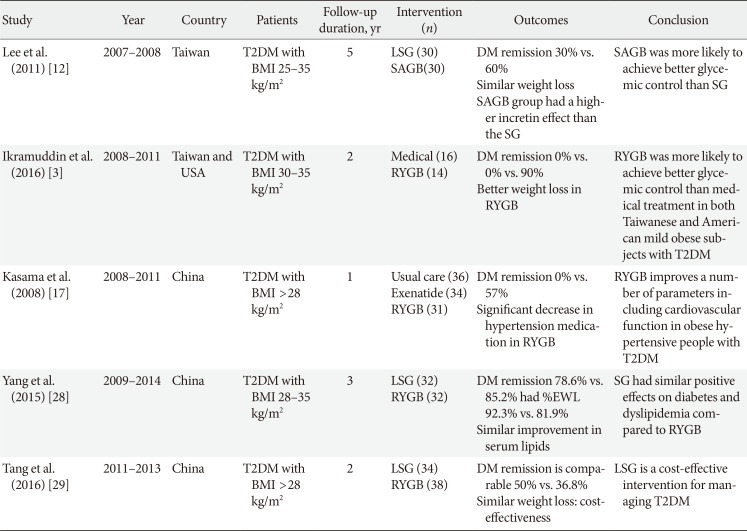
Six RCTs about metabolic surgery had been published from Asia [31217252829]. Table 4 listed the detail of each studies. Two focused on the comparison of bariatric surgery and best medical treatment for the treatment of T2DM [317]. One study recruited 120 patients with BMI between 30 to 40 from Taiwan and USA [3]. Among those patients, 71 patients were mild obesity (BMI 30 to 35 kg/m2), 35 participants (19 USA and 16 Taiwanese) enrolled in medical treatment group, where 36 participants (22 USA and 12 Taiwanese) enrolled RYGB. The other study recruited 120 patients with BMI >28 kg/m2 T2DM patients with hypertension into three groups [17]. Both groups achieved significantly better glycemic control and reduction of hypertension medication in surgical treatment comparing to medical treatment.
The other three studies focused on the comparison of LSG and LGB for the treatment of T2DM [122528]. One studies found that SAGB is better than LSG in control low BMI patients [12]. The other two studies found similar efficacy between RYGB and LSG in the treatment of T2DM [1728]. One RCT compared cost effectiveness between LSG and RYGB with 2-year follow-up [29]. The author concluded that LSG is as effective as RYGB and is slightly more cost-effective than RYGB. Another two on-going studies are awaiting to be published.
MECHANISM OF EFFECT
Overwhelming evidence have supported that effective diabetes resolution was achieved in obese T2DM patients after undergoing metabolic surgery. The underlying mechanism for diabetes remission after metabolic surgery is intriguing. Initially, four possible mechanisms had been proposed, including the starvation followed by weight-loss hypothesis, the ghrelin hypothesis, the lower intestinal (hind-gut) hypothesis, and the upper intestinal (fore-gut) hypothesis. More theories were proposed recently [13]. None of these theories necessarily precludes the others. Therefore, any combination of these mechanisms may contribute to some degree in T2DM remission and it is very difficult to design a study to elucidate the exact mechanism.
Although Asian tends to accumulate fat in abdomen and develop T2DM relatively at a younger age, lower BMI and much higher incidence [12], metabolic surgery still works very well in Asian. A rapid reduction of insulin resistance and recovery of early insulin secretion have been documented after both LSG and LGB [16]. However, the recovery of incretin effect was found to be higher in LGB than LSG [24]. This discrepancy was attributed to different level of gut hormone and cytokine secretion [55]. It has been suggested that the dramatic reduction in ghrelin after SG may explain the weight loss mechanism. In addition to the dramatic reduction of ghrelin effect, SG was also reported to have hind-gut effect with increasing of GLP-1 and PYY due to increasing transit time after SG [55]. It was also reported that weight loss to be similar between GB and SG but duodenum exclusion do play a role in T2DM remission [1247].
OPERATIVE RISKS AND LONGTERM NUTRITIONAL EFFECTS
Although the safety of bariatric/metabolic surgery had been improved significantly recently, metabolic surgery still carried a major complication rate of 2% with a mortality rate 0.2% [31]. Once major complication occurred, the severity was usually higher in those with T2DM because of the associated medical conditions. Therefore, detailed preoperative cardiovascular, renal, and ophthalmologic evaluation is mandatory for T2DM patients before receiving metabolic surgery. Metabolic surgery should also be performed by experienced surgeons and in high volume center to reduce the surgical risk and maximize the outcome.
Along with weight reduction and T2DM remission, metabolic surgery may also bring some side-effects and long-term nutritional problems [356]. A certain degree of malnutrition commonly accompanies the food restriction and duodenum exclusion, including the iron, calcium, vitamin B12, and protein deficiency. Therefore, T2DM patients with metabolic surgery should be regularly followed up, monitored and treated by a multidisciplinary team. Routine vitamin and minor element supplements are also indicated after metabolic surgery.
PREDICTORS OF DIABETES REMISSION AND PATIENT SELECTION
Optimal outcomes for diabetes remission after metabolic surgery can be achieved if patients best suited to the surgery are selected and those who will predictably have a poor result are excluded. To be able to make such decisions, we need some predictors and guideline from Asia for the Asian. This information is helpful for applying metabolic surgery for T2DM treatment in clinical practice.
Current indications for bariatric surgery were based on BMI and metabolic surgery was recommended for T2DM Asian with BMI >27.5 kg/m2 [11]. Although T2DM remission is closely associated with BMI, many other factors, such as abdominal obesity, β-cell function, duration of disease, and age are all important predictor of T2DM remission after metabolic surgery in Asian.
Since T2DM is a progressive disease with continuously depleting β-cell function, the longer the duration of T2DM, the poorer the response to T2DM is expected. Patients with long history of diabetes usually have poor β-cell function and not suitable for metabolic surgery as they may be taking unnecessary surgical risks without achieving desirable remission. Therefore, current international guideline recommends metabolic surgery should be performed as earlier as possible. Metabolic surgery is now recommended as a priority treatment of choice for severely obese (BMI >32.5 for Asian) T2DM patients.
Metabolic surgery is indicated in those with acceptable β-cell function and C-peptide is recommended for a useful tool to evaluate T2DM patient for metabolic surgery [57]. C-peptide is a connecting peptide to insulin. Pro-insulin is formed by one C-peptide molecule attached to each insulin molecule. Pro-insulin splits into insulin and C-peptide after it is release from the pancreas into the blood in response to a rise in blood glucose. C-peptide is a valuable test in the classifying different types of diabetes. Therefore, C-peptide levels in T2DM patients may reflect the status of pancreas islet cell preservation and predict the success of surgical treatment of T2DM.
Age is also an important consideration for metabolic surgery. The incidence of young-onset T2DM is high in Asians. As these patients tend to have severe disease and more complications, metabolic surgery is especially worth in this group of patients. A recent study showed that young onset (<40 years age) T2DM Asian had more severe disease than late onset T2DM patients but had a better response to metabolic surgery [58]. This study highlighted the importance of metabolic surgery to be considered as priority for those Asian with young onset and poorly controlled T2DM.
To combine those important predictors, a scoring system has recently been developed [59]. This ABCD Diabetes Surgery Score system consisted of four variables; age, BMI, C-peptide level, and duration of diabetes. A 4-point score ranging from 0 (lowest value) to 3 (maximal value) was used for BMI, C-peptide level, and duration of diabetes. For age, only a one-point score was used. The summated ABCD score ranges from 0 to 10 points. The higher the score, the higher the chance of T2DM remission after metabolic surgery can be expected. Young patients with high BMI, short duration of disease, and good β-cell function are associated with high ABCD score. ABCD score is especially designed for predicting the success of metabolic surgery and has been validated in many studies [60]. In clinical practice, this score system can help the endocrinologist to set the priority for referring patients for metabolic surgery and the surgeon to counsel the patients for metabolic surgery and choice of surgical procedure.
CONCLUSIONS
The success of bariatric surgery in obese diabetic individuals (BMI >35 kg/m2) has led to the paradigm shift of metabolic surgery for the treatment of T2DM, including patients with a BMI <35 kg/m2. Data from Asian studies supported that metabolic surgery is the promising therapy for glycemic control in poorly controlled Asian T2DM patients with BMI >27.5 kg/m2. The mechanisms of metabolic gastrointestinal surgery are thought to be dependent on the dramatic enterohormonal changes after physioanatomical re-arrangement of the gastrointestinal tract. Right selection of patients and metabolic surgery procedure is paramount importance to achieve high T2DM remission and successful outcomes after surgery. Further researches are required to establish new guidelines and indications for metabolic surgery in less obese or lean patients. How to provide a safe bariatric surgery, train qualified bariatric surgeon and continue to develop better techniques will be the important issues in surgical treatment of obesity in future.
Notes
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.