Feasibility of a Patient-Centered, Smartphone-Based, Diabetes Care System: A Pilot Study
Article information
Abstract
Background
We developed a patient-centered, smartphone-based, diabetes care system (PSDCS). This study aims to test the feasibility of glycosylated hemoglobin (HbA1c) reduction with the PSDCS.
Methods
This study was a single-arm pilot study. The participants with type 2 diabetes mellitus were instructed to use the PSDCS, which integrates a Bluetooth-connected glucometer, digital food diary, and wearable physical activity monitoring device. The primary end point was the change in HbA1c from baseline after a 12-week intervention.
Results
Twenty-nine patients aged 53.9±9.1 years completed the study. HbA1c and fasting plasma glucose levels decreased significantly from baseline (7.7%±0.7% to 7.1%±0.6%, P<0.0001; 140.9±39.1 to 120.1±31.0 mg/dL, P=0.0088, respectively). The frequency of glucose monitoring correlated with the magnitude of HbA1c reduction (r=–0.57, P=0.0013). The components of the diabetes self-care activities, including diet, exercise, and glucose monitoring, were significantly improved, particularly in the upper tertile of HbA1c reduction. There were no severe adverse events during the intervention.
Conclusion
A 12-week application of the PSDCS to patients with inadequately controlled type 2 diabetes resulted in a significant HbA1c reduction with tolerable safety profiles; these findings require confirmation in a future randomized controlled trial.
INTRODUCTION
Diabetes mellitus is a threat to public health and the economy. As of 2013, the number of people with diabetes was estimated to be 382 million worldwide, and this number is expected to increase to 471 million by 2035 [1]. The diabetes-related health expenditure worldwide in 2013 was estimated to be US dollar 548 billion [1]. Because diabetes is a manageable chronic disease rather than a curable disease, more emphasis should focus on life-long lifestyle modifications and medications. Therefore, diabetes self-management education and adherence to the treatment plans are considered the key components for the clinical management of diabetes [23].
A variety of information technology (IT)-based interventions have been developed to support diabetes self-management [456]. However, mixed results have been reported with regard to glycosylated hemoglobin (HbA1c) reduction [78910111213141516171819202122232425262728293031]. In a systematic review that compared the computerized decision support systems (CDSS) with conventional care [4], CDSS with feedback on the patient's performance and case management by health care providers reduced HbA1c [789101112], whereas CDSS without feedback or case management demonstrated no effect [131415161718192021]. The HbA1c-lowering effect of the various computer-based interventions, including clinic-, Internet-, and mobile phone-based systems, was as small as –0.2% (95% confidence interval [CI], –0.4 to –0.1) compared with control interventions [5]. Among the interventions, three mobile phone-based interventions decreased HbA1c by –0.5% (95% CI, –0.7 to –0.3) compared with the control interventions [5]. In a meta-analysis including 10 studies, educational intervention via text messaging showed an overall –0.6% reduction of HbA1c compared with the control intervention [6]. These results suggest that an IT-based diabetes care system with feedback and case management modules can be an effective tool for maintaining glycemic control and that the benefits might be larger by using mobile phone-based interventions.
Recently, the revolutionary generation of a mobile phone, the smartphone, became the mainstream of IT-based communications, with 1.4 billion users worldwide as of 2013 [32]. Similarly, mobile healthcare systems are rapidly evolving. Numerous applications have been developed for patients with chronic diseases requiring daily monitoring and management. However, a systematic review, including 71 smartphone applications for diabetes self-management available from the Apple App Store (as of 2012), revealed that the majority of those applications were not sufficient or comprehensive for daily diabetes self-management [33]. They primarily focused on monitoring blood glucose levels, medications, and diet. However, the proportion of applications supporting physical activity, diabetes education, and decision support was relatively low [33]. In addition, the majority of the applications were not equipped with other convenient modules, such as automated data entry, social networking, and data integration with a patient health record system [33]. Therefore, more comprehensive smartphone-based interventions, including various components of diabetes self-management, are necessary.
In this study, we developed a new patient-centered, smartphone-based, diabetes care system (PSDCS) featuring individualized diabetes management algorithm, automatic input of daily glucose levels and physical activity, guidance for basal insulin dosage, and various interactive components, including social network system (SNS). The PSDCS particularly supports patient's decision making to cope with high or low glucose and suggests insulin dosage, if the patient is on insulin treatment. The aim of this study was to test the feasibility of HbA1c reduction using PSDCS in patients with type 2 diabetes.
METHODS
Study participants
Patients with type 2 diabetes who were between 20 and 80 years and whose HbA1c levels were greater than or equal to 6.5% were recruited from the outpatient clinic of the Seoul National University Hospital during December 2013 and January 2014. The HbA1c cut-off value of 6.5% was according to clinical practice guidelines by International Diabetes Federation [34] and Korean Diabetes Association [35]. Patients were excluded from the study for the following: severe diabetic complications (severe non-proliferative retinopathy or proliferative retinopathy, stage 4 or 5 of chronic kidney disease, severe diabetic neuropathy, history of diabetic foot, and history of angina pectoris, myocardial infarction, cerebrovascular disease, and peripheral arterial disease), type 1 diabetes, insulin therapy other than insulin glargine, insulin pump, history of drug addiction, psychotic disorder, and pregnancy. Participants were divided into four groups based on the type of treatment. The patients without antidiabetic medication were assigned to group A, and those who were taking oral antidiabetic medication(s) with minimal risk of hypoglycemia (e.g., metformin, α-glucosidase inhibitors, thiazolidinediones, and dipeptidyl peptidase-4 inhibitors) were assigned to group B. The patients taking oral antidiabetic medication(s) with increased risk of hypoglycemia (e.g., sulfonylureas and meglitinides) were assigned to group C, and insulin glargine users were assigned to group D. According to the four different treatment groups, the patients received different feedback when their glucose levels were entered into the system. This study was conducted according to the guidelines of the Declaration of Helsinki. The study protocol was approved by the Institutional Review Board of the Seoul National University Hospital (IRB No. H-1301-075-459) and the Ministry of Food and Drug Safety of the Republic of Korea (Approval No. 426). Written informed consent was obtained from all participants before any study-related procedures were commenced.
Study design
In this 12-week, single-arm, pilot study, the participants used PSDCS for diabetes self-management, and the feasibility of PSDCS was evaluated. It was installed manually by our study team to enrolled participants. Before initiating the smartphone application, the participants were engaged in a face-to-face instruction session to understand the detailed use of the PSDCS. The smartphone application displayed individualized daily recommendations for calorie intake and physical activity for each individual, based on their baseline calorie intake, physical activity, and body weight. The participants were recommended to input their dietary intake in the smartphone application, which was used for self-monitoring. The blood glucose level was monitored using a Bluetooth glucometer (MyGlucoHealth; Entra Health Systems, Seoul, Korea), and the participants were recommended to measure their glucose level at least once a day, preferably before breakfast. In addition, the participants monitored their physical activity status using a Bluetooth activity tracker (LG LifeGram LA11M-BS; LG Electronics, Seoul, Korea).
Using the PSDCS, we investigated efficacy and safety measures at the baseline and after 12 weeks of intervention. We measured HbA1c, fasting plasma glucose (FPG), body weight, blood pressure, total cholesterol, triglyceride, high density lipoprotein cholesterol (HDL-C), low density lipoprotein cholesterol (LDL-C), aspartate transaminase, and alanine transaminase. Summary of diabetes self-care activities (SDSCA) was used to evaluate the overall self-management activities for diabetes [36]. SDSCA contains a total of 11 questions in six categories (general diet, specific diet, exercise, blood glucose testing, foot care, and medication); we evaluated the number of days during the previous week in which the specific activity occurred. The primary end point was the change in HbA1c at 12 weeks of intervention compared with baseline. The secondary end points were the changes in FPG, lipids, body weight, and the SDSCA scores. Changing the prescription of medication, except the insulin dose, was not allowed during the study period. The insulin dose was recommended according to a specific algorithm in PSDCS.
Patient-centered smartphone-based diabetes care system
The PSDCS had an android-based application, which was composed of four modules: a glucose module, a diet module, a physical activity module, and an SNS module. The glucose module consisted of a Bluetooth glucometer, feedback messages according to the glucose levels, recommendations for basal insulin dosage, and algorithms for detecting and coping with hypoglycemia and serious hyperglycemia. The diet module consisted of recording daily dietary intake and calculating total calories and nutrients based on a predefined food database. The physical activity module consisted of an activity tracker, a semi-automatic energy expenditure calculator, and video clips that guided resistance excise. The individualized target goal for diet and physical activity was set at baseline. The SNS module could motivate the participants by sharing their thoughts, opinions, and tips for diabetes self-care.
As soon as the blood glucose was measured and transmitted to the smartphone application, a feedback message immediately popped up, according to the glycemic control algorithm. A glucose level between 70 and 130 mg/dL was set as the target. A glucose level between 130 and 180 mg/dL was considered to be fair. When the glucose level was between 180 and 250 mg/dL, the patient received an alarm message. If the glucose level was between 250 and 320 mg/dL for 3 consecutive days or higher than 320 mg/dL once, an alarm message was sent to a pre-registered family member of the patient, and an automatic phone call was made from the patient's smartphone to the research clinic to assess the patient's condition. When hypoglycemia (glucose <70 mg/dL) occurred, an acute management pathway that has been described elsewhere [37] was automatically activated. If the patient did not execute the acute management plan within 1 minute, an alarm message was sent to the designated family member and an automatic phone call to the research clinic was made
For participants on basal insulin glargine therapy, an insulin dose was recommended, according to the following algorithm. When the median fasting glucose level from the 3 previous days was in the range of 130 to 159, 160 to 189, 190 to 219, 220 to 249, 250 to 279, and 280 to 319 mg/dL, an increase in the insulin dosage by 2, 3, 4, 5, 6, and 7 units, respectively, was recommended. If the patient did not follow the recommendation, the actual insulin dosage was manually entered into the system. For safety reasons, the total increase in the insulin dosage from baseline via the PSDCS during the 12-week study period was limited to 10 units. When the glucose level was still inadequately controlled using the maximal recommended insulin dosage, the patient was instructed to make an unscheduled visit to the research clinic. If the patient experienced hypoglycemia with a blood glucose level below 70 mg/dL on any of the previous 3 days, the insulin dose was not allowed to be increased. After acute management of the hypoglycemic event, the insulin dose was recommended to be reduced according to the glucose level (–2 units when the glucose levels were 55 to 70 mg/dL and –4 units when the glucose levels were <55 mg/dL).
Each participant was instructed to record the daily dietary intake using the smartphone application. The PSDCS counted and displayed the daily total calorie intake. In addition, it analyzed the participant's dietary habit and food preferences. The amount of exercise and energy expenditure was estimated by the activity tracker and could be manually adjusted by the participant, according to the specific types and duration of exercise (e.g., swimming). It also included 238 video clips, mostly guided resistance exercise and stretching. For overweight patients, the PSDCS recommended scheduled exercise programs every week. The PSDCS also contained diabetes self-management educational material that provided detailed information concerning how to manage various diabetes-related conditions and specific situations, including sick-day rules. The SNS was designed for sharing information about diabetes management and posting anything that the participant wanted to share. The SNS displayed the scores and ranks according to the number of access to the PSDCS.
The website for physicians was developed to show the baseline characteristics, including anthropometric data, prescribed medications, and laboratory data, and to monitor readouts of the glucometer, amounts of daily calorie intake, and daily physical activity. All the participants were informed that their physician could observe their condition, progress, and adherence to the PSDCS through the website. The data in this website were used for discussion with the participants in the clinic at the end of the study.
Statistical analysis
The results are presented as the mean±standard deviation values. After confirmation of normal distribution of parameters using the Kolmogorov-Smirnov test, the comparison of each parameter taken at baseline and at the end of the study was performed using the paired t-test. To compare the mean values of multiple groups, one-way analysis of variance was used. The correlations between continuous variables were assessed using Pearson correlation analysis. A Wilcoxon matched pairs test was used to analyze the changes in several parameters that did not exhibit a normal distribution and subgroup analysis. Statistical significance was assumed when P<0.05. Statistical analyses were performed using SPSS 21.0 version (IBM Co., Armonk, NY, USA) or Prism 5.0 (GraphPad, San Diego, CA, USA).
RESULTS
Thirty-one patients were screened, and one patient was excluded because of low HbA1c. One patient withdrew from the study. A total of 29 patients completed the 12-week intervention. The mean age of the participants was 53.9±9.1 years. Baseline HbA1c was 7.7%±0.7%; baseline FPG was 140.9±39.1 mg/dL; baseline body mass index was 25.0±2.8 kg/m2. The baseline characteristics of the study participants are shown in Table 1.
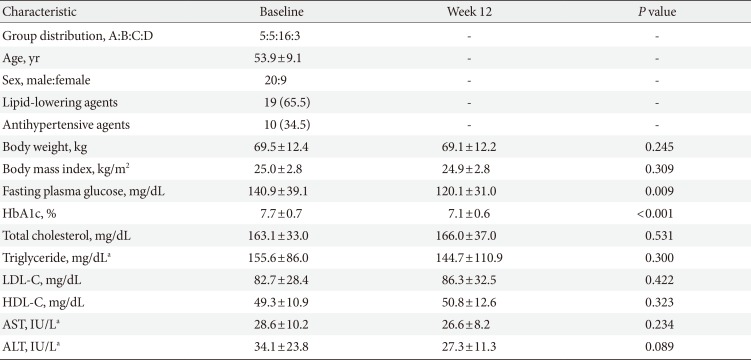
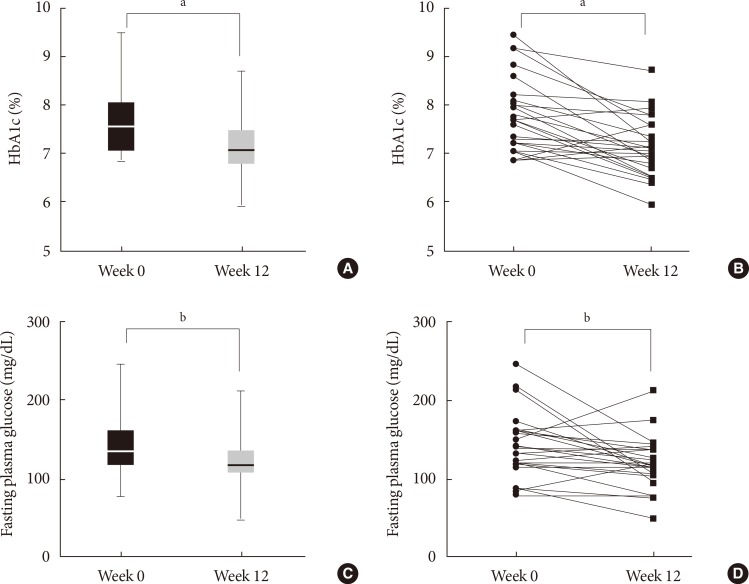
After the 12-week intervention, HbA1c significantly decreased by 0.6%±0.7% from baseline (P<0.0001) (Fig. 1A and B), and FPG decreased by 20.8±39.9 mg/dL from baseline (P=0.0088) (Fig. 1C and D). There were no differences in the change in HbA1c and FPG among the four groups (data not shown), although the numbers of participants were not evenly distributed and the number of participants in each group was too small to initiate comparisons. There was a linear correlation between baseline HbA1c and the amount of change in HbA1c (r=–0.68, P<0.0001) (Fig. 2A). The HbA1c reduction was correlated with the average number of daily glucometer input (r=–0.57, P=0.0013) (Fig. 2B). The number of glucometer inputs tended to be higher in older patients (0.7±0.4/day in patients ≤44 years, 1.0±0.6/day in patients 45 to 54 years, 1.3±0.9/day in patients 55 to 64 years, and 1.6±0.4 /day in patients ≥65 years; P=0.10), although it was not statistically significant. A subgroup analysis was performed with the patients whose glucometer input frequency was at least once a day (n=18) and the patients whose glucometer input frequency was less than once a day (n=11). In the former group, HbA1c changed from 7.9%±0.7% to 7.1%±0.5% (P=0.0005) (Fig. 2C). However, in the latter group, HbA1c did not decrease significantly (7.4%±0.7% to 7.2%±0.7%, P=0.24) (Fig. 2D).
Change in glycosylated hemoglobin (HbA1c) and fasting plasma glucose after 12 weeks of intervention. (A, C) Mean values of HbA1c and fasting plasma glucose levels, respectively. (B, D) Individual data of HbA1c and fasting plasma glucose levels, respectively. aP<0.001, bP<0.01.

Factors correlated with glycosylated hemoglobin (HbA1c) reduction. Baseline HbA1c and the average number of daily glucometer input showed linear correlation to HbA1c reduction (A, B). HbA1c significantly decreased in the patients whose glucometer input frequency was minimal once a day (C) but did not decrease in the patients whose glucometer input frequency was less than once a day (D). aP<0.001.
After 12 weeks, body weight and the levels of total cholesterol, triglyceride, HDL-C, and LDL-C did not change (Table 1). In terms of the SDSCA, the scores of "general diet," "exercise," and "blood glucose testing" increased significantly after 12 weeks (Fig. 3A). When the patients were divided into three groups according to the average number of the glucometer inputs, the upper tertile presented more improved SDSCA scores than the lower tertile (Fig. 3B and C). When the patients were divided into three groups according to the magnitude of HbA1c reduction, the SDSCA scores increased more in the upper tertile of HbA1c reduction (Fig. 3D and E).
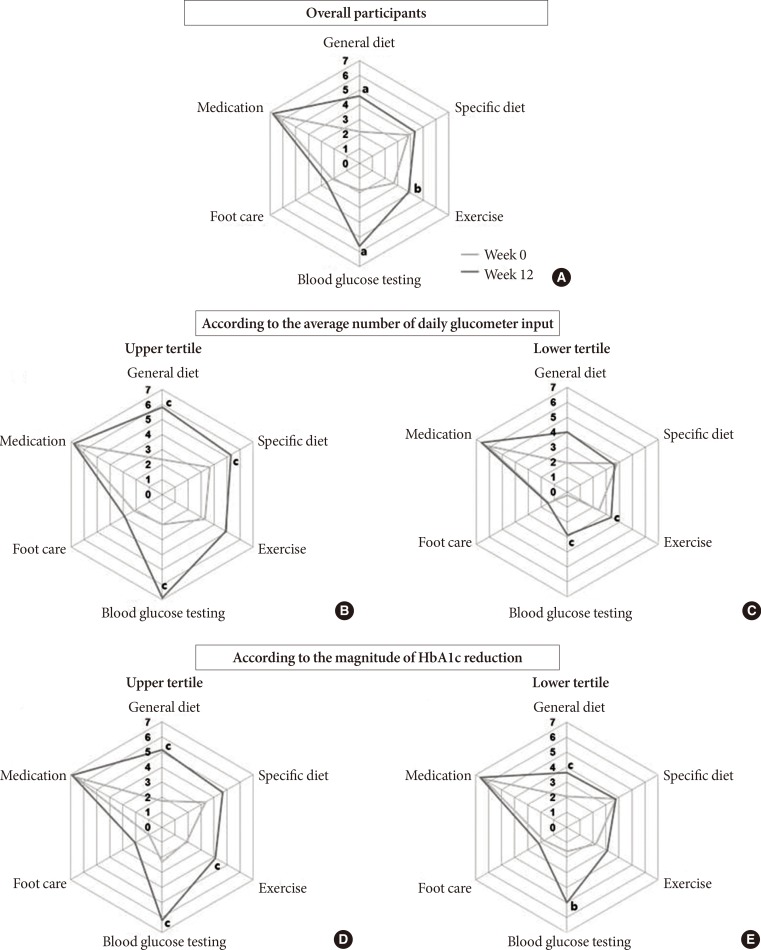
Changes in the summary of diabetes self-care activities (SDSCA) score after 12 weeks of intervention. (A) Overall change and (B, C) change in the SDSCA scores according to the average number of daily glucometer inputs (upper tertile vs. lower tertile, respectively). (D, E) Change in the SDSCA scores according to the magnitude of glycosylated hemoglobin reduction (upper tertile vs. lower tertile, respectively). aP<0.001, bP<0.01, cP<0.05.
Twelve serious hyperglycemia events, which were predefined to be 320 mg/dL or higher by the glucometer readouts, were reported in seven patients. All of the hyperglycemia events spontaneously resolved, except in one patient, who showed aggravation of glucose control associated with upper respiratory tract infection and who was requested to make an unscheduled visit to the research clinic to adjust the insulin dose. Hypoglycemia (<70 mg/dL) occurred in one patient using glimepiride (group C) before eating breakfast, which was immediately resolved by eating breakfast. Severe adverse events did not occur during the study period.
DISCUSSION
In this study, HbA1c and FPG decreased significantly after 12 weeks of the PSDCS intervention. HbA1c changed by –0.6% from baseline, which was consistent with previous reports using mobile phone interventions [56]. The PSDCS significantly improved diabetes self-management in terms of diet, exercise, and blood glucose monitoring. Overall, we found that PSDCS is a promising tool for aiding self-management and effectively lowering blood glucose levels without significant increase in adverse events.
Because our PSDCS was based on a smartphone application, one may be concerned that elderly patients would have a decreased usability presumably because of a decreased interest in the program and because of the complexity of the program. However, the number of daily glucometer inputs tended to be higher with increasing age, although this difference was not statistically significant (P=0.10). In a study of a web-based intervention in patients with diabetes aged 60 years or older, all 31 participants in the intervention group used the system well, resulting in a significant HbA1c reduction [9]. Another study with a ubiquitous healthcare system using a telephone network-connected glucometer and short message service on mobile phones in elderly patients showed a completion rate of more than 90% with beneficial effects on glycemic control [26]. Considering that the number of elderly diabetes patients is increasing [38], the PSDCS would be a good tool for improving their diabetes care.
An important outcome of the PSDCS should be the change in the patient's behavior concerning the self-management of diabetes. In this regard, there was a significant correlation between average daily glucometer input numbers and HbA1c reduction. It was reported that more frequent measurements of blood glucose levels improved glycemic control even without IT applications [3940]. In terms of the SDSCA, a 12-week intervention with the PSDCS improved the patients' behaviors, including general diet, exercise, and blood glucose testing. Those patients who showed a greater HbA1c reduction exhibited an improved SDSCA score. Collectively, it is plausible that our current PSDCS improved glycemic control by increasing the frequency of blood glucose monitoring and by improving diabetes self-care behaviors.
No severe adverse events occurred during the study period. However, one episode of mild hypoglycemia and one episode of persistent serious hyperglycemia occurred during the study period. In previous randomized controlled studies of mobile diabetes care systems, the frequency of hypoglycemia was rare [28] or even absent [27]. Although a study performed in elderly patients with type 2 diabetes reported that minor hypoglycemia was numerically higher in the ubiquitous healthcare service group (32.2%) than in the control group (21.8%), the incidence of severe hypoglycemia was low in both groups [26].
There are limitations in this study because of its nature as a pilot study. There was no control group with which to compare the efficacy and safety. The number of participants was small, and the study period was short. In addition, the number of patients in each subgroup was too small and unevenly distributed. In particular, the number of patients using insulin (group D) was too small to test the efficacy and safety of the insulin-dosing algorithm.
In conclusion, a 12-week study of the new smartphone-based PSDCS for patients with inadequately controlled type 2 diabetes resulted in a significant reduction of HbA1c with tolerable safety profiles. A greater HbA1c reduction was associated with better compliance and better diabetes self-care activities. The PSDCS may be used as an efficacious tool for managing patients with type 2 diabetes. Based on the results of this pilot study, a randomized controlled trial is in preparation, with an upgraded version of the PSDCS, to confirm the therapeutic efficacy and safety.
ACKNOWLEDGMENTS
We thank Dr. Jung Hun Ohn and Yoon Ji Kim for their support in the development of the study protocol.
Notes
CONFLICTS OF INTEREST: This study was sponsored by Health Connect Co. Ltd. S.B. and S.L.L. are employees of Health Connect Co. Ltd.