Serum Total Bilirubin Levels Provide Additive Risk Information over the Framingham Risk Score for Identifying Asymptomatic Diabetic Patients at Higher Risk for Coronary Artery Stenosis
Article information
Abstract
Background
The diagnosis of coronary artery disease (CAD) is often delayed in patients with type 2 diabetes. Serum total bilirubin levels are inversely associated with CAD. However, no studies have examined whether this can be used as a biochemical marker for identifying asymptomatic diabetic patients at higher risk for having obstructive CAD.
Methods
We performed a cross-sectional study of 460 consecutive asymptomatic patients with type 2 diabetes. All patients underwent coronary computed tomographic angiography, and their serum total bilirubin levels were measured. Obstructive CAD was defined as ≥50% diameter stenosis in at least one coronary artery.
Results
Serum total bilirubin tertiles showed an inverse association with the prevalence of obstructive CAD. In multivariate logistic regression analysis, the odds ratio for the highest versus the lowest tertile of total bilirubin was 0.227 (95% confidence interval [CI], 0.130 to 0.398), and an increment of 1 µmol/L in serum total bilirubin level was associated with a 14.6% decrease in obstructive CAD after adjustment for confounding variables. Receiver operating characteristic curve analysis showed that the area under the curve for the Framingham Risk Score (FRS) plus serum total bilirubin level was 0.712 (95% CI, 0.668 to 0.753), which is significantly greater than that of the FRS alone (P=0.0028).
Conclusion
Serum total bilirubin level is inversely associated with obstructive CAD and provides additive risk information over the FRS. Serum total bilirubin may be helpful for identifying asymptomatic patients with type 2 diabetes who are at higher risk for obstructive CAD.
INTRODUCTION
Coronary artery disease (CAD) is the major cause of morbidity and mortality in patients with type 2 diabetes. The diagnosis of CAD is often delayed in patients with type 2 diabetes due to a lack of symptoms [1]. Thus, diabetic patients have more extensive CAD at the time of diagnosis and worse outcomes than nondiabetic subjects [23]. Indeed, the CAD mortality rate after the first myocardial infarction is two to four times higher in patients with diabetes than in those without [3]. Therefore, development of screening approaches for the early detection of CAD is warranted in patients with type 2 diabetes.
Coronary computed tomographic angiography (CCTA) is a rapidly evolving noninvasive method for evaluating CAD and is currently considered to be a reliable alternative to conventional coronary angiography [45]. However, radiation exposure, the use of iodinated contrast agents, and the high cost all limit its use as a screening tool for CAD in asymptomatic patients. Additionally, CCTA is currently not recommended for the routine diagnosis of CAD in asymptomatic patients with diabetes [6]. Thus, identifying the subgroup of patients who might benefit from CCTA could be important in clinical practice.
Bilirubin, the end product of heme catabolism, is derived primarily from circulating hemoglobin [7]. Although bilirubin has long been considered a waste product, it is currently recognized as a potent endogenous antioxidant. A growing number of epidemiological studies report a negative association between serum bilirubin levels and the prevalence of CAD [8910]. Recently, a large prospective study of primary care patients with no previous history of CAD demonstrated that the serum bilirubin level is an independent risk factor for CAD events and all-cause death [11]. Therefore, much attention has been focused on the potential role of serum bilirubin as a predictive or prognostic marker for CAD in clinical practice. However, the potential role of the serum bilirubin level in identifying patients with obstructive CAD has not been investigated in asymptomatic patients with type 2 diabetes.
Here, we used CCTA to examine whether serum total bilirubin levels are independently associated with coronary artery stenosis in asymptomatic patients with type 2 diabetes and asked whether this biochemical marker may help to identify a subgroup of patients at increased risk for obstructive CAD.
METHODS
Study population
Four hundred seventy-eight consecutive asymptomatic patients with type 2 diabetes who visited the diabetes clinic at the Asan Medical Center between October 2009 and December 2010 were enrolled in the study. All patients underwent 64-slice dual-source multi-detector computed tomography (MDCT) for CCTA. The exclusion criteria were as follows: chest pain or angina-equivalent symptoms, as determined using the Rose angina questionnaire; abnormal findings on a resting electrocardiogram, including pathological Q waves, ischemic (≥1 mm depression) ST segments, deep negative T waves, or complete left bundle branch block; a previous history of myocardial infarction/angina or percutaneous coronary intervention/coronary artery bypass grafting; ventricular or supraventricular arrhythmia; an estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2; and a previous history of allergies to iodinated contrast agents. This study was originally designed to identify potential risk factors for obstructive CAD in asymptomatic patients with type 2 diabetes. An initial evaluation of this population is published elsewhere [12]. For the present analysis, 18 patients with chronic liver disease were also excluded: 16 with positive hepatitis B surface antigen and 2 with positive hepatitis C antibodies. Therefore, a total of 460 patients were included in the final analysis. All patients provided written informed consent. The study protocol was approved by the Institutional Review Board of the Asan Medical Center.
Clinical and biochemical assessment
Basic demographic data were obtained through personal interviews. All patients were asked about their history of angina, myocardial infarction, revascularization, concomitant noncardiac comorbidities, age at diagnosis of diabetes, smoking and drinking habits, and current medication profiles. The data were confirmed by a review of their medical records. Type 2 diabetes was diagnosed according to the Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus [13]. Hypertension was defined as a blood pressure ≥140/90 mm Hg or the use of antihypertensive medications. Smoking habits were defined as smoking currently or having stopped smoking within 1 year of the interview.
The body mass index (BMI) was calculated as weight in kilograms divided by the square root of the height in meters. Blood pressure was measured on the right arm after a ≥5-minute rest using an automatic manometer fitted with an appropriate size cuff. Blood samples were obtained after a fast of at least 12 hours. Serum total bilirubin levels were measured by the vanadate oxidation method using the Toshiba 200FR (Toshiba, Tokyo, Japan). The levels of total cholesterol, low density lipoprotein cholesterol (LDL-C), high density lipoprotein cholesterol (HDL-C), aspartate aminotransferase (AST), alanine aminotransferase (ALT), and triglycerides were determined by an enzymatic colorimetric method using the Toshiba 200FR. The serum creatinine levels were measured by the quantitative capillary photometry method using the TEST 1 automated analyzer (Alifax SpA, Polverara, Italy). The glycosylated hemoglobin (HbA1c) levels were determined by ion-exchange high-performance liquid chromatography using the Variant II automated analyzer (Bio-Rad Laboratories, Hercules, CA, USA). The eGFR was calculated using the Modification of Diet in Renal Disease study equation [14]. A standard 12-lead electrocardiogram was recorded on the same day that the 64-slice dual-source MDCT was performed. The presence of diabetic retinopathy was assessed using retinal photography performed with a wide-angle camera and scored centrally by an experienced ophthalmologist. The extent of albuminuria was determined from the albumin-creatinine ratio in a random spot urine collection. The presence of diabetic nephropathy was defined as microalbuminuria (30 to 300 µg albumin/mg creatinine) or overt albuminuria (>300 µg albumin/mg creatinine) in the absence of other conditions capable of causing proteinuria. The Framingham Risk Score (FRS) for estimating the 10-year risk for coronary heart disease was calculated based on age, sex, total cholesterol and HDL-C levels, blood pressure, smoking status, and history of diabetes [15].
Evaluation of coronary stenosis and plaque morphology
For all eligible patients, coronary artery calcium (CAC) measurements were followed immediately by CCTA using a 64-slice dual-source MDCT scanner (Somatom Definition, Siemens Medical Solutions, Erlangen, Germany). Patients with baseline heart rates >85 beats per minute were given oral β-blockers before CCTA imaging. Each patient was also given 0.6 mg of nitroglycerin sublingually 1 minute before image acquisition. A standard scanning protocol was applied with 2×32×0.6 mm collimation, 64-slice acquisition per rotation, 330 ms rotation time, 100 to 120 kVp tube voltage, and 250 to 380 mAs/rot tube current according to the patient's body habitus. All scans were performed using electrocardiogram-gated dose modulation. A bolus of 60 to 80 mL of iomeprol (Iomeron 400; Bracco, Milan, Italy) was intravenously injected (4 to 5 mL/sec), followed by 50 mL of saline chaser. A region of interest was located in the ascending aorta, and image acquisition was automatically initiated once a selected threshold (120 Hounsfield units [HU]) had been reached with bolus tracking. Each patient's electrocardiogram was simultaneously recorded to allow for retrospective segmental data reconstruction. The images were initially reconstructed at the mid-diastolic phase (75% of the R-R interval) of the cardiac cycle.
All scans were analyzed on a 3-dimensional workstation (Syngo Workstation; Siemens Medical Solutions). An experienced radiologist who was blinded to the patients' clinical information calculated the CAC scores according to the Agatston method [16]. All coronary computed tomography angiograms were interpreted by two experienced radiologists who were blinded to the patients' clinical information and CAC scores and who reached a decision by consensus. Each lesion was identified using a multiplanar reconstruction technique and the maximum intensity projection of short-axis, two-chamber, and four-chamber views. Coronary artery stenosis and plaque characteristics were analyzed on a per-segment basis according to the classification of the American Heart Association criteria [17]. The contrast-enhanced portion of the coronary lumen was semi-automatically traced at the maximal stenotic site and compared with the mean values for the proximal and distal reference sites. Obstructive CAD was defined as ≥50% diameter stenosis in at least one coronary artery. Plaques were defined as structures >1 mm2 within and/or adjacent to the vessel lumen. Plaques consisting of calcified tissue occupying more than 50% of the plaque area (density >130 HU in native scans) were classified as calcified plaques, plaques with <50% calcium were classified as mixed plaques, and plaques without any calcium were classified as noncalcified plaques.
Statistical analysis
Continuous variables showing a normal distribution are expressed as the mean±standard deviation, continuous variables showing a skewed distribution are expressed as the median (and interquartile range), and categorical variables are expressed as percentages (%). The study population was divided into the following tertiles according to serum total bilirubin levels: T1 (<12 µmol/L), T2 (12 to 17 µmol/L), and T3 (>17 µmol/L). The clinical and biochemical characteristics according to the tertiles of serum total bilirubin levels were compared using analysis of variance for continuous variables and the chi-square trend test for categorical variables. The presence of any plaque subtype (calcified, mixed, or noncalcified) was analyzed on a per-patient basis. Multivariate logistic regression analyses were used to calculate the odds ratios of serum total bilirubin level as a group or continuous variable to predict the presence of obstructive CAD, a CAC score ≥400, or different plaque subtypes. To compare the abilities of the FRS plus serum total bilirubin level and FRS alone to predict obstructive CAD, receiver operating characteristic (ROC) curves were plotted and the area under the curve (AUC) calculated. MedCalc version 11.6.1.0 (MedCalc Software, Mariakerke, Belgium) was used to compare AUC values. All other statistical analyses were performed using SPSS version 14.0 (SPSS Inc., Chicago, IL, USA). A P<0.05 was considered statistically significant.
RESULTS
Clinical and biochemical characteristics of the study population according to serum total bilirubin tertiles
The study population was composed of 460 asymptomatic patients with type 2 diabetes and no history of CAD (age, 63.0±8.3 years; duration of diabetes, 13.3±7.9 years; 62% male). A longer duration of diabetes (P<0.001), higher systolic blood pressure (P=0.040), current smoking (P=0.012), diabetic retinopathy (P=0.001), diabetic nephropathy (P<0.001), lower level of HDL-C (P=0.017), higher level of triglycerides (P=0.045), lower eGFR (P=0.015), and higher FRS (P=0.002) were each significantly associated with lower total bilirubin tertiles (Table 1). The prevalence of hypertension tended to decrease across the total bilirubin tertiles; however, the statistical significance was marginal (P=0.068). There were no significant differences in age, male gender, BMI, diastolic blood pressure, levels of HbA1c, total cholesterol, LDL-C, AST, and ALT, current use of insulin and statins, or alcohol consumption among the groups.
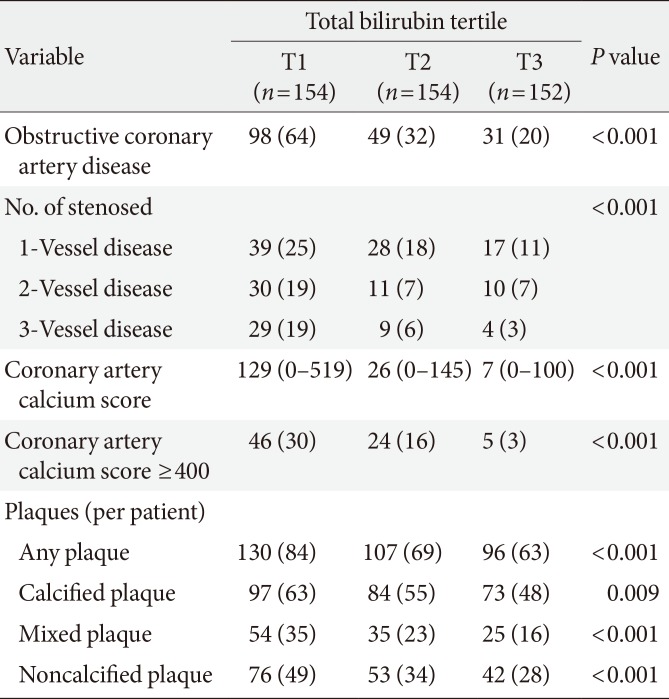
Prevalence of obstructive CAD and coronary plaque subtypes according to serum total bilirubin tertiles
Among the 460 patients, 333 had some form of coronary atherosclerotic plaque and 178 had obstructive (>50% luminal narrowing) CAD (Table 2). The prevalence of patients with obstructive CAD significantly decreased across the total bilirubin tertiles (T1, 64%; T2, 32%; T3, 20%; P<0.001). There was also a significant decrease in the extent of CAD, defined by the number of stenosed vessels, across the tertiles (P<0.001). A lower total bilirubin tertile was significantly associated with a higher CAC score (P<0.001). The percentage of patients with a CAC score ≥400 showed a negative relationship with the tertile (T1, 30%; T2, 16%; T3, 3%; P<0.001). In addition, a higher total bilirubin tertile was significantly associated with a lower prevalence of any (T1, 84%; T2, 69%; T3, 63%; P<0.001), calcified (T1, 63%; T2, 55%; T3, 48%; P=0.009), mixed (T1, 35%; T2, 23%; T3, 16%; P<0.001), or noncalcified (T1, 49%; T2, 34%; T3, 28%; P<0.001) plaques.
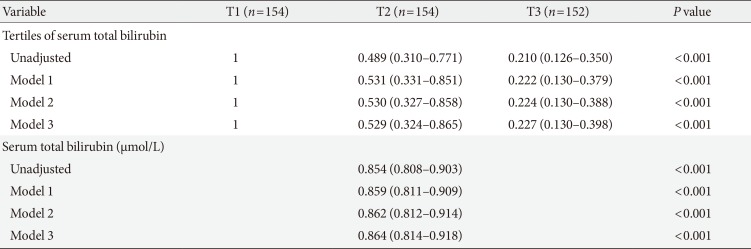
Risk for obstructive CAD according to serum total bilirubin level
As shown in Table 3, the odds ratios for obstructive CAD consistently showed an increasing trend across the total bilirubin tertiles. Serum total bilirubin level as a continuous variable was also inversely associated with the prevalence of obstructive CAD. Serum total bilirubin level, as both a categorical and a continuous variable, showed an independent negative relationship with obstructive CAD after adjusting for age, gender, duration of diabetes, prevalence of hypertension, systolic blood pressure, diastolic blood pressure, and current smoking (model 1). These associations remained significant even after adjusting for BMI, HbA1c, LDL-C, HDL-C, and triglycerides (model 2). Further adjustment for the prevalence of diabetic retinopathy and nephropathy, eGFR, current use of insulin and statins, AST, ALT, and alcohol intake did not significantly affect the results (model 3). In multivariate logistic regression model 3, the odds ratio for the highest versus the lowest tertile of total bilirubin was 0.227 (95% confidence interval [CI], 0.130 to 0.398), and an increment of 1 µmol/L in serum total bilirubin level was associated with a 14.6% reduction in the incidence of obstructive CAD.
Risk associated with a CAC score ≥400 and with each plaque subtype according to serum total bilirubin level
As a function of the serum total bilirubin level, the odds ratio for a CAC score ≥400 was 0.718 (95% CI, 0.641 to 0.804; P<0.001) after full adjustment (Table 4). An elevated serum total bilirubin level also appeared to show an independent negative relationship with the presence of a calcified plaque but did not reach statistical significance (odds ratio, 0.955; 95% CI, 0.901 to 1.011; P=0.114). In addition, the odds ratio for the presence of mixed and noncalcified plaques was 0.886 (95% CI, 0.829 to 0.948; P<0.001) and 0.916 (95% CI, 0.867 to 0.969; P=0.002) after full adjustment, respectively (Table 4).
Comparison of the predictive ability of the FRS alone and FRS plus serum total bilirubin level
ROC curve analysis revealed that the AUC for FRS plus serum total bilirubin level was 0.712 (95% CI, 0.668 to 0.753), which was significantly greater than that of the FRS alone (AUC, 0.654; 95% CI, 0.609 to 0.698; P=0.0028) (Fig. 1).
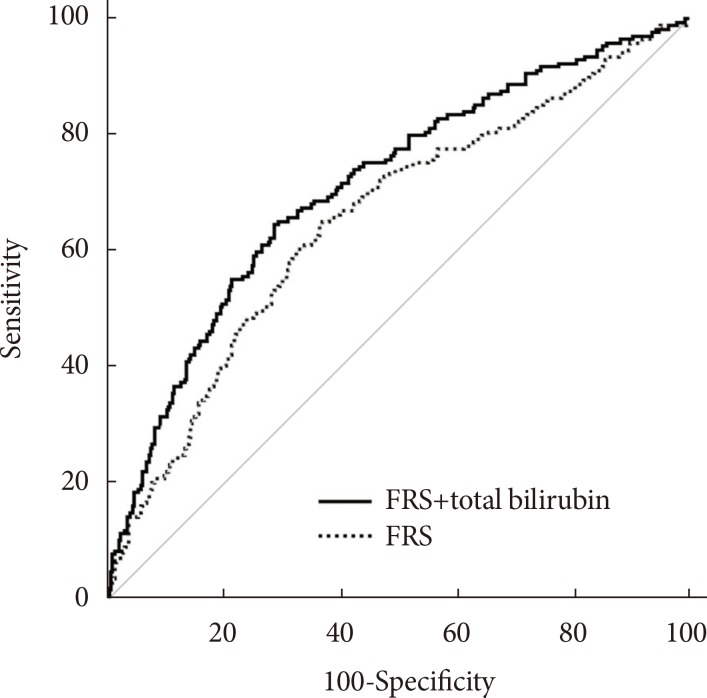
Receiver operating characteristic curves showing the ability of the Framingham risk score (FRS) alone or the FRS plus serum total bilirubin level to predict obstructive coronary artery disease. The area under the curve for the FRS plus serum total bilirubin level was 0.712 (95% confidence interval [CI], 0.668 to 0.753), significantly greater than that of the FRS alone (area under the curve, 0.654; 95% CI, 0.609 to 0.698). P=0.0028 for the comparison between the two areas.
DISCUSSION
Here, we examined whether serum total bilirubin levels can be used as a marker for identifying subjects with obstructive CAD among asymptomatic patients with type 2 diabetes. We found that serum total bilirubin tertiles were inversely associated with the prevalence of obstructive CAD as assessed by CCTA and that serum total bilirubin levels provided additional risk information over the FRS.
Accumulating evidence has shown that serum bilirubin levels are negatively associated with a prevalence of various diseases [10]. Indeed, serum bilirubin makes the largest contribution to total antioxidant capacity within the circulation [7]. Thus, serum bilirubin may be an important protective factor against oxidative damage, preventing the development and progression of oxidative stress-mediated diseases such as CAD. Indeed, several animal studies have shown that bilirubin treatment protects against vascular complications [1819]. Oxidative stress also plays a major role in the pathogenesis of diabetes and its associated complications [20]. A recent Japanese population-based study showed that the serum bilirubin level is inversely associated with the prevalence of diabetic retinopathy [21]. Fukui et al. [22] also reported a negative correlation between serum bilirubin levels and microalbuminuria and arterial stiffness. Furthermore, accumulating evidence consistently shows that lower serum bilirubin levels are associated with a higher risk of CAD [891011]. However, most of these studies were performed using subjects without diabetes, and it has not been verified whether serum bilirubin can serve as a useful biochemical marker for identifying asymptomatic patients with type 2 diabetes at higher risk for obstructive CAD. The cohort examined in the present study was composed exclusively of asymptomatic patients with type 2 diabetes. The results showed that serum bilirubin levels are negatively associated with obstructive CAD in patients with type 2 diabetes.
Oxidative stress plays an essential role in the pathogenesis of atherosclerosis [23]. Specifically, lipid oxidation enhances foam cell formation through oxidized LDL formation and uptake. Importantly, serum bilirubin prevents LDL oxidation more efficiently than other endogenous antioxidants [24]. Therefore, serum bilirubin may protect against atherosclerosis by preventing LDL oxidation. On the other hand, there was no association between serum bilirubin level and LDL-C level, suggesting that bilirubin's protective effect is not due to the lowering of the LDL-C level.
It is suggested that the presence and extent of calcified plaques in coronary arteries are independently associated with CAD events and that taking this into account can increase the predictive power of traditional cardiovascular risk factors in asymptomatic diabetic patients [25]. On the other hand, mixed or noncalcified plaques are thought to be more vulnerable to rupture, which can result in an acute coronary event [26]. Recently, Canpolat et al. reported on the association of serum total bilirubin levels and plaque morphology in Turkish patients suspected to have CAD [27]. They found that serum total bilirubin levels were inversely associated with the presence and extent of noncalcified plaques but not calcified and mixed plaques. By contrast, a recent cross-sectional studies conducted in a Korean population reported that serum total bilirubin level was inversely associated with the presence of calcified plaques [28]. Here, we found a significant and inverse relationship between serum total bilirubin levels and the presence of mixed or noncalcified plaques. In addition, the serum total bilirubin levels were negatively associated with a CAC score ≥400 but not the presence of calcified plaques. The discrepancy between our study and the previously mentioned studies may be explained by differences in the study populations, including ethnicity and the presence/absence of diabetes or CAD symptoms.
Assessing the risk for CAD in asymptomatic patients with type 2 diabetes is an important challenge for clinicians. In 1998, the American Diabetes Association recommended CAD screening for diabetic patients with multiple CAD risk factors [29]. However, subsequent studies demonstrated that an approach guided by the number of CAD risk factors is not successful for the early detection of CAD in diabetic patients because patients with fewer risk factors frequently present with CAD [30]. In addition, when applied to CAD risk assessments in asymptomatic diabetic patients, the effectiveness of predictive models such as the FRS is modest [31]. The present study shows that the serum total bilirubin levels provide additive risk information over the FRS and that this may help to identify asymptomatic patients with type 2 diabetes at higher risk for having obstructive CAD.
This study has several limitations. First, the cross-sectional nature of this study did not allow a causal relationship to be determined. Second, this study was performed at a single university hospital in Korea. There are considerable ethnic genetic differences in bilirubin metabolism between Asian and Caucasian populations [32]. Therefore, our results may not be applicable to other ethnic groups. Third, an elevated serum total bilirubin level indicates liver damage, and it may therefore be significantly affected by underlying liver diseases. Here, we excluded patients with known chronic liver diseases. In addition, we adjusted the results of liver function tests such as AST and ALT to rule out the residual confounding effect of unrecognized underlying diseases. Fourth, in several cross-sectional studies, serum total bilirubin level was found to be negatively associated with the prevalence of ischemic stroke or peripheral arterial disease [3334]. We cannot exclude the possibility that such underlying conditions affected our analysis in evaluating the relationship between serum total bilirubin level and CAD because they were not included in our exclusion criteria and the information about them was not collected carefully. Finally, serum direct and indirect bilirubin levels seem to be different in their anti-inflammatory and antioxidant activities [35]. A recent study reported that direct bilirubin levels are positively correlated with CAD severity assessed by the Gensini score, whereas there is no association between total bilirubin levels and CAD severity [36]. In the present study, we did not measure serum direct bilirubin levels. Future studies are required for evaluating the relationship between serum direct bilirubin level and CAD.
Our findings suggest that serum total bilirubin levels are negatively associated with obstructive CAD among asymptomatic patients with type 2 diabetes. Furthermore, serum total bilirubin levels provide risk information additional to that provided by the FRS. Serum bilirubin assays are inexpensive and widely available. Therefore, the serum total bilirubin may be helpful for identifying asymptomatic patients with type 2 diabetes at increased risk for having obstructive CAD and who require more aggressive screening using CCTA.
ACKNOWLEDGMENTS
This research was supported by the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology (2006-2005412 and 2009-0091988: K.U.L.).
Notes
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.