Maximal Fat Oxidation Rate during Exercise in Korean Women with Type 2 Diabetes Mellitus
Article information
Abstract
Background
The purpose of this study was to determine the appropriate exercise intensity associated with maximum fat oxidation, improvement of body composition, and metabolic status in Korean women with type 2 diabetes mellitus (T2DM).
Methods
The study included a T2DM group (12 women) and a control group (12 women). The groups were matched in age and body mass index. The subjects performed a graded exercise test on a cycle ergometer to measure their maximal fat oxidation (Fatmax). We also measured their body composition, metabolic profiles, and mitochondrial DNA (mtDNA).
Results
The exercise intensity for Fatmax was significantly lower in the T2DM group (34.19% maximal oxygen uptake [VO2 max]) than the control group (51.80% VO2 max). Additionally, the rate of fat oxidation during exercise (P<0.05) and mtDNA (P<0.05) were significantly lower in the T2DM group than the control group. The VO2 max level (P<0.001) and the insulin level (P<0.05) were positively correlated with the rate of fat oxidation.
Conclusion
The results of this study suggest lower exercise intensity that achieves Fatmax is recommended for improving fat oxidation and enhancing fitness levels in Korean women with T2DM. Our data could be useful when considering an exercise regimen to improve health and fitness.
INTRODUCTION
Exercise is recommended for the prevention of type 2 diabetes mellitus (T2DM) and is known to be effective in correcting metabolic defects, including insulin resistance and lipid disorders. T2DM is induced by obesity in many cases, and weight loss from exercise is beneficial during the clinical treatment of this condition [123].
The exercise intensity that causes the highest rate of fat oxidation is referred to as the 'maximal fat oxidation rate' (Fatmax) intensity [1]. It is possible to reproduce measurements of Fatmax using graded exercise calorimetry [4]. This approach can be used to predict the quantity of lipid that will be metabolized during exercise. The technique uses a specific protocol to determine the appropriate exercise level that causes maximum fat oxidation. It has been suggested that the fat oxidation rate during the Fatmax intensity is approximately 2-fold greater than at any other intensity [5]. Thus, exercise at the Fatmax intensity can be useful to control weight in cases of metabolic syndrome. The clinical relevance of Fatmax is also important when considering an exercise prescription for weight loss by efficiently increasing fat oxidation and insulin sensitivity [1]. Thus, the Fatmax intensity is recommended to maximize the beneficial effects of exercise and weight management in type 2 diabetic [1] and obese [23] populations.
Patients with metabolic syndromes, including insulin resistance, hypertension, reduced high density lipoprotein cholesterol (HDL-C) [6], T2DM [7], and obesity [8], exhibit an impaired ability to oxidize lipids relative to control subjects of equivalent body mass index (BMI) [19]. Previous studies demonstrated that Fatmax intensity occurs at approximately 33% to 65% maximal oxygen uptake (VO2 max) in control subjects [1011], but is reduced to 33.4% to 46.1% VO2 max in T2DM patients [10] and to 38% to 53% VO2 max in obese patients [12]. It should be noted that the Fatmax intensity was determined by different means across different conditions. The rate of fat oxidation over a wide range of exercise intensities has most frequently been measured in Western populations [681113]. Asian populations have distinct obesity profiles [14]. Thus, data collected in different study populations may not be directly comparable. Most cases of T2DM are associated with being overweight or obese, and it has been shown that exercise-induced reductions in weight and fat mass can affect blood glucose control in patients with T2DM. Therefore, assessments of individual fat oxidation rates can be used to determine the optimal exercise intensity for weight loss and improved glucose control in patients with T2DM.
The purpose of this study was to investigate the Fatmax intensity in T2DM patients during graded exercise. Additionally, we compared values with age-, sex-, and BMI-matched controls to establish the optimal exercise intensity that caused Fatmax in Korean women with T2DM.
METHODS
Subjects
This study examined 24 women (T2DM group, 12; control group, 12) matched for age and BMI. The participants were selected from a hospital. Diabetes was defined based on data obtained from an oral glucose tolerance test, and the disease was diagnosed using standard criteria [15]. The duration of diabetes was 7.82±2.13 years. There were no subjects with a history of chronic artery disease, cerebrovascular disease or endocrine disorder except T2DM. The patients were not treated with any hormonal substitutions. The Institutional Review Board at the Kangbuk Samsung Hospital in Korea approved the study protocol according to the Declaration of Helsinki. All subjects were examined after obtaining informed consent for the experimental procedures. The exercise protocol and possible risks and benefits were explained to all patients.
Exercise testing and fat oxidation calculations
The subjects performed a graded exercise test to exhaustion on a cycle ergometer (Monark 894E; Monark Exercise AB, Vansbro, Sweden). A breath device (VmaxST; SensorMedics Inc., Yorba Linda, CA, USA) was used to measure gas exchange. Oxygen uptake (VO2) and carbon dioxide production (VCO2) were measured continuously during both the rest and exercise periods. The heart rate (HR) was measured continuously using a Polar Vantage HR monitor (Polar Electro Oy, Kempele, Finland) by telemetry. The test is performed on a cycle ergometer connected to an analyzer. Electrocardiographic monitoring and measurements of VO2, VCO2, and the respiratory exchange ratio were performed during the test. The modified protocol was used by Brandou et al. [16]. The workload for each step was calculated from the theoretical maximal power (Wmax), which represents power corresponding to the theoretical VO2 max [17]. The protocol consisted of a 3-minute warm up at 20% of theoretical Wmax. The warm-up was followed by four 3-minute steady-state workloads at 30%, 40%, 50%, and 60% of Wmax [161819]. The test concluded with a 5-minute passive recovery period. The maximum fat oxidation rate occurs at the point of maximum lipid oxidation induced by the workload. The rate then decreases as carbohydrates (CHO) become the predominant fuel. The Fatmax defines the exercise intensity that elicits Fatmax intensity. The equation for fat oxidation used by Frayn [20] is the following: fat oxidation=1.6946 VO2-1.7012 VCO2.
Blood samples
Blood samples were collected in ethylenediaminetetraacetic acid tubes on ice and were immediately centrifuged at 4℃ for 10 minutes. The plasma was stored at -80℃ until analysis. The serum triglyceride, total cholesterol, and HDL-C levels were measured enzymatically using a chemistry analyzer (Hitachi 747; Hitachi, Tokyo, Japan). The low density lipoprotein cholesterol concentrations were estimated using the Friedewald formula [21]. The plasma glucose levels were analyzed using a conventional commercially available assay on an automatic analyzer (Hitachi 612, Automatic Analyzer; Roche, Basel, Switzerland). The serum insulin level was assessed by radioimmunoassay (Bi-Insulin IRMA kit; Schering CIS bio, Gif-surYvette, France). Insulin resistance was estimated using the homeostasis model assessment estimate of insulin resistance index, which was defined as fasting insulin (µU/mL)×fasting plasma glucose (mmol/L)/22.5 [22]. Mitochondrial DNA (mtDNA) in peripheral leukocytes was extracted from 1 mL of whole blood using the QIAamp Tissue Kit 250 (Qiagen Inc., Valencia, CA, USA). The relative mtDNA was measured by a real-time polymerase chain reaction (RT-PCR). The reactions were performed using a Light Cycler-Fast Start DNA Master SYBR Green I kit from Roche Applied Science (Pleasanton, CA, USA). The forward and reverse primers of the mitochondrial gene were the following: 5'-CCACGGGAAACAGCAGTGATT-3' and 5'-CTATTGACTTGGGTTAATCGTGT GA-3', respectively. After denaturation at 50℃ for 2 minutes, the DNA samples were treated at 95℃ for 10 minutes, 90℃ for 15 seconds, and then 40 cycles at 60℃ for 1 minute. The number of PCR cycles required to reach 20 ng of DNA was defined as the threshold cycle.
Data analysis
The statistical analyses were conducted using SPSS version 16.0 (SPSS Inc., Chicago, IL, USA). All data are presented as the means±standard deviation. Student t-test was used to compare subject demographic and laboratory characteristics. Separate 2×2 (intensity and time) mixed measures analysis of variance, repeated for mode, were used to examine the data for fat oxidation. A Spearman correlation analysis was performed to evaluate the relationship among various parameters. P<0.05 was considered statistically significant.
RESULTS
Our results found that the mtDNA, VO2 max, and Wmax levels in the T2DM group were significantly lower than the control group (P<0.05). The insulin level was also lower in the T2DM group, but the difference was not significant. The glucose level was found to be significantly higher in the T2DM group (P<0.001) (Table 1). The Fatmax intensity was 34.19% of VO2 max in the T2DM group and 51.80% of VO2 max in the control group (95% confidence interval, 44.74 to 52.13; P<0.05). The Fatmax intensity of the T2DM group was significantly lower than the control group (t=-2.140, P<0.05) (Fig. 1A). The fat oxidation rate was 0.382 g/min in the T2DM group and 0.545 g/min in the control group. The fat oxidation rate at the Fatmax intensity in the T2DM group was significantly lower than the control group (t=-2.449, P<0.05) (Fig. 1B).

Comparisons of the demographic and laboratory data of the type 2 diabetes mellitus and the control groups

Comparison of the maximal fat oxidation (Fatmax) intensity (A) and fat oxidation rate at the Fatmax intensity (B) between the type 2 diabetes mellitus (T2DM) and the control groups. aP<0.05.
During graded exercise, the fat oxidation rate of the T2DM group was significantly lower, at 40% (P<0.05), 50% (P<0.05), and 60% VO2 max (P<0.05), than in the control group (Fig. 2).
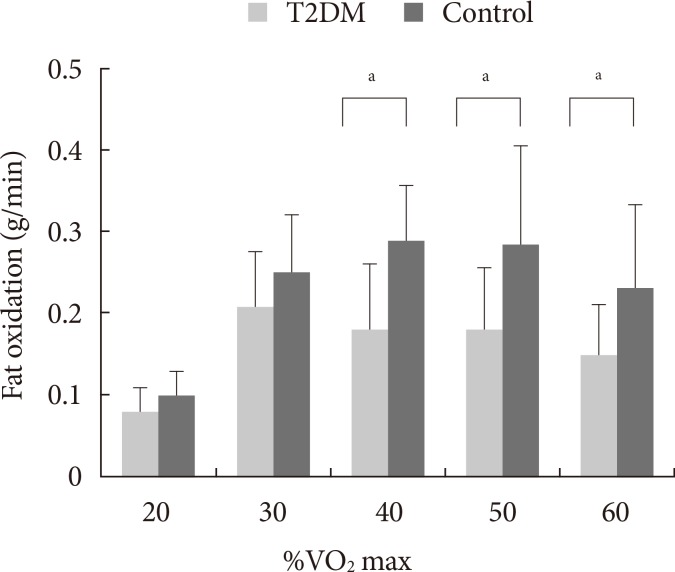
Comparison of fat oxidation in the type 2 diabetes mellitus (T2DM) and the control groups. VO2 max, maximal oxygen uptake. aP<0.05.
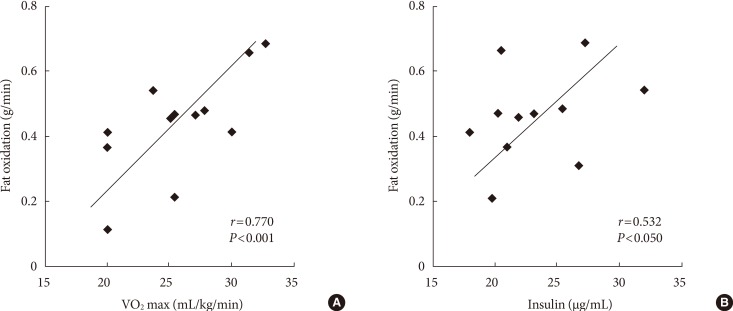
Fig. 3 shows the linear relationships between the fat oxidation rate, VO2 max, and insulin level. There was a positive correlation between fat oxidation and the VO2 max (r=0.770, P<0.001) and insulin (r=0.532, P<0.05) levels. There were no significant differences found in other demographic and laboratory data.
DISCUSSION
This study demonstrates that T2DM patients have significantly reduced fat oxidation compared to control subjects matched in age, sex, weight, height, body fat percentage, and waist circumference. Several studies have reported that fat oxidation decreases in patients with metabolic diseases [1923] and that fat oxidation decreases more with exercise intensity in T2DM patients than in the control groups. Previous studies have also suggested that the range of Fatmax occurs from the 33% to 65% VO2 max exercise intensity recommended for Fatmax [124]. A review and meta-analyses on the use of calorimetry during exercise reported that the exercise intensity corresponding to Fatmax is approximately 55% to 75% of VO2 max intensity in the healthy group [1325]. However, maximum fat oxidation occurs at 25% to 50% of VO2 max intensity in the metabolic disorder group [2627]. Our data showed that Fatmax (34.19% VO2 max) in T2DM was lower than (51.8% VO2 max) in the control group. This result also showed that fat oxidation in patients with T2DM was lower, at 40%, 50%, and 60% VO2 max intensity (P<0.05, each), during incremental exercise compared to controls. Previous studies have reported that Fatmax was at 51.9% VO2 max [28] in the healthy group and 37% VO2 max [29] in T2DM patients. The findings were similar to the results of this study. However, another study reported that Fatmax was 25.3% VO2 max [30] in T2DM and reflects a lower intensity than our T2DM subjects. Differences in Fatmax may be linked to the level of obesity. The BMI of T2DM subjects in previous studies [2829] were similar to our study cases, but the obesity status of T2DM [29] was higher than in our study.
The VO2 max levels in the T2DM group were 15% lower than those in the control group (P<0.05), and the mtDNA levels in the T2DM group were 42% lower than those in the control group (P<0.05). One reason for low fat oxidation during exercise in T2DM patients was the decrease in mitochondrial fatty acid oxidation [29]. The mitochondrial function in patients with T2DM appears to be disturbed, which may decrease the electron transport chain activity [3031] and subsequently disrupt muscle fatty acid uptake [32]. Increases in oxidative stress can increase hyperinsulinemia and mitochondrial DNA damage to cause T2DM [33]. In this study, the lower mtDNA levels in T2DM patients may be due to the lower VO2 max and fat oxidation. Recent studies have shown a similar mechanism. Martin et al. [34] reported that T2DM subjects increased CHO oxidation and lower fat oxidation during exercise. Furthermore, regular exercise increased insulin sensitivity and enhanced mitochondrial fatty acid oxidation [35]. An increased capacity to oxidize fat during exercise in patients with T2DM can reduce their dependence on glucose as a source of energy [36] and can serve as an effective means of weight control [37]. In addition, increased fat oxidation through physical activity improves insulin sensitivity [38]. Although subjects without metabolic disorder receive health benefits with moderate-intensity exercise, low-intensity exercise targeted at the level of Fatmax has been shown to markedly improve impaired glucose tolerance and overweight status [39]. Exercise at the Fatmax intensity affects insulin resistance and fat oxidation and can assist in weight management. Therefore, patients with T2DM need the appropriate exercise intensity to help decrease insulin resistance, fasting glucose levels, and improve weight control.
This study showed a positive correlation between VO2 max and the rate of fat oxidation in T2DM. An individual at a low fitness level can improve their physical activity with low-, moderate- or high-intensity exercise [2537]. Based on the association between VO2 max and the rate of fat oxidation, we suggest that an elevated fitness level in patients with pathological conditions, such as diabetes, could manage their weight by increasing fat oxidation during exercise and improving clinical status. Thus, it would be useful to extend this approach to people with T2DM. Previous studies on the obese and patients with metabolic syndrome have markedly improved physiological and fitness levels after training at Fatmax intensity [2730]. In this study, the Fatmax intensity of T2DM was significantly lower than in the healthy group. Although Fatmax intensity was seen as a low-intensity exercise, this intensity in T2DM patients improved fat oxidation during exercise and reduced obesity. This study suggests that Fatmax intensity exercise in T2DM patients with a low fitness level involves lower-intensity exercise and can increase fitness levels and improve obesity. In addition, the insulin levels are positively correlated with fat oxidation because of the higher fitness level in the control group. Therefore, patients with T2DM at a low fitness level need an exercise program that emphasizes the attaining VO2 max.
Our data showed that patients with T2DM have a Fatmax rate of 34.19% VO2 max, and this intensity is significantly lower than the control group. The Fatmax intensity is positively correlated with VO2 max. Generally, moderate exercise intensity is recommended for these purposes. However, a previous study reported that low exercise intensity may be helpful, especially for women with T2DM and a low fitness level. Therefore, this study suggests that a lower exercise intensity indicated by Fatmax is recommended for improving fat oxidation and enhancing the fitness level in Korean women with T2DM. These results could be useful when considering an exercise regimen to improve patient health and fitness.
Notes
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.