Increased Risk of Hospitalization for Heart Failure with Newly Prescribed Dipeptidyl Peptidase-4 Inhibitors and Pioglitazone Using the Korean Health Insurance Claims Database
Article information
Abstract
Background
We assessed the association of dipeptidyl peptidase 4 inhibitors (DPP4i) with hospitalization for heart failure (HF) using the Korean Health Insurance claims database.
Methods
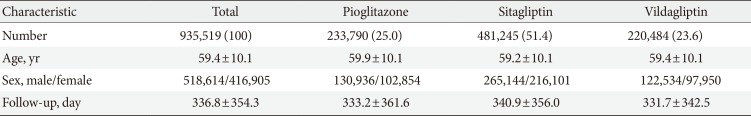
We collected data on newly prescribed sitagliptin, vildagliptin, and pioglitazone between January 1, 2009 and December 31, 2012 (mean follow-up of 336.8 days) to 935,519 patients with diabetes (518,614 males and 416,905 females) aged 40 to 79 years (mean age of 59.4 years).
Results
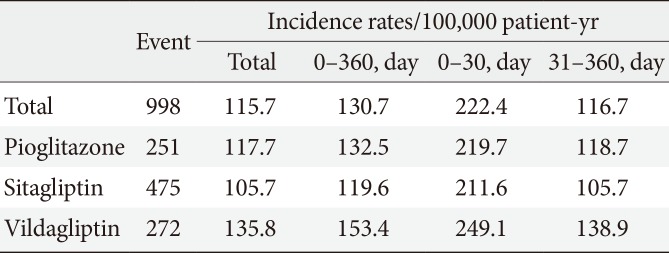
During the study, 998 patients were hospitalized for primary HF (115.7 per 100,000 patient-years). The incidence rate of hospitalization for HF was 117.7 per 100,000 per patient-years among patients on pioglitazone, 105.7 for sitagliptin, and 135.8 for vildagliptin. The hospitalization rate for HF was greatest in the first 30 days after starting the medication, which corresponded to a significantly higher incidence at days 0 to 30 compared with days 31 to 360 for all three drugs. The hazard ratios were 1.85 (pioglitazone), 2.00 (sitagliptin), and 1.79 (vildagliptin). The incidence of hospitalization for HF did not differ between the drugs for any time period.
Conclusion
This study showed an increase in hospitalization for HF in the initial 30 days of the DPP4i and pioglitazone compared with the subsequent follow-up period. However, the differences between the drugs were not significant.
INTRODUCTION
A major development in the pathogenesis of cardiovascular diseases (CVDs) is the understanding that patients with diabetes mellitus are more likely to develop coronary heart diseases and even more pronounced morbidity and mortality than nondiabetic patients [1]. CVD is best prevented in patients with diabetes by controlling the underlying condition, i.e., hyperglycemia. However, about half of patients with diabetes still do not achieve or maintain sufficient glycemic control to avoid substantial long-term morbidity [234].
New types of glucose-lowering agents are required for type 2 diabetes mellitus (T2DM) because a multivariable mix of genetic and environmental factors combine to create a progressive and highly heterogeneous natural history, with at least eight major organ systems being implicated [5]. However, increased risk of CVD, such as myocardial infarction [6] and heart failure (HF) [7], caused by thiazolidinediones (TZDs) has generated concerns of cardiovascular (CV) safety in developing novel drugs for T2DM. The Food and Drug Administration, therefore, mandated that all new glucose-lowering medications undergo a thorough CV safety assessment before market approval [8]. As a consequence, large-scale trials focused on longer-term CV outcomes have been initiated with most of the newer drugs. Recent results from large outcome trial of saxagliptin in patients with prior CVD or multiple CV risk factors (saxagliptin assessment of vascular outcomes recorded in patients with diabetes mellitus-thrombolysis in myocardial infarction [SAVOR-TIMI 53]) showed that saxagliptin did not increase or decrease the rate of ischemic events. However, more patients in the saxagliptin group than in the placebo group were hospitalized for HF [9]. This unexpected result caused controversy and uncertainty about the use of other dipeptidyl peptidase 4 (DPP-4) inhibitors (DPP4i) [10], even though their longterm CV outcome studies are not finished or planned yet.
Therefore, we aimed to assess the association of DPP4i (sitagliptin and vildagliptin) and TZD (pioglitazone) with the HF hospitalization using the Korean Health Insurance claims database.
METHODS
The study was performed in accordance with the Declaration of Helsinki and the guidelines for Good Pharmacoepidemiology Practices. All study protocols were approved by the Institutional Review Board (IRB) of Dong-A Medical Center. Informed consent was waived by the IRB.
Study population
Patients were identified from the Korean Health Insurance Review and Assessment Service database (HIRA), which contains medical claims data for the entire Korean population [11] as a result of the National Health Insurance System (NHIS). Patients pay an average of 30% of the total medical costs related to almost all diseases. Healthcare providers submit reports concerning the medical services performed to the HIRA for a review of the medical costs incurred. These reports contain diagnosis codes in accordance with the International Classification of Diseases 10th revision (ICD-10) as well as outpatient or inpatient status, drug name, dosage, prescription date, duration, and method of administration. The HIRA provided data with the individual identifiers removed, in accordance with the Act on the Protection of Personal Information maintained by public agencies. Thus, the database included an unidentifiable code representing each individual with the patient's age, gender, diagnosis, and a list of prescribed drugs. The database contained information collected from January 1, 2007 to December 31, 2012. HIRA data before 2007 was deleted and inaccessible in accordance with the Act on the Protection of Personal Information.
Definitions
Diabetic patients between the ages of 40 to 79 who began treatment with one of the three drugs from January 1, 2009 to December 31, 2012 were identified. Use of pioglitazone, sitagliptin, or vildagliptin was defined as receipt of a prescription for the drug during the study period. We only included the patients who were newly prescribed with these drugs by excluding patients with any prior prescription in 2007 or 2008. Pioglitazone, sitagliptin, and vildagliptin were launched in the Republic of Korea in January 2003, December 2008, and February 2009, respectively. Among them, patients who had been hospitalized with a principal discharge diagnosis of HF [12] were identified by using the ICD-10 codes I50. Patients hospitalized with a first diagnosis with HF were recruited by eliminating patients with a prior diagnosis of HF in 2007 or 2008. All hospitalizations for HF that occurred before medication was initiated were also excluded.
Statistical analysis
Data were analyzed using R program version 2.15.3. Values are presented as mean±standard deviation or numbers (%). For all statistical analyses a P value of less than 0.05 (two-sided) was considered to be statistically significant. We used a poisson regression to model the relationship and generate hazard ratios (HRs) and 95% confidence intervals comparing the days 0 to 30 with days 31 to 360 after TZD or DPP4i prescription [13]. A Cox regression model was used to examine whether the specific medication played a role in the hospitalization for HF. Age and sex were included as covariates in this model.
RESULTS
The database contained information regarding 935,519 patients with diabetes (518,614 males and 416,905 females) age 40 to 79 years (mean of 59.4 years) with prescriptions from January 1, 2009 to December 31, 2012 (mean follow-up of 336.8 days). Baseline characteristics are displayed in Table 1. A total of 25.0% patients took pioglitazone, 51.4% took sitagliptin, and 23.6% took vildagliptin. From 2009 to 2012, these subjects had 998 hospitalizations for primary HF (115.7 per 100,000 patientyears). The incidence of hospitalization for HF was 117.7 for pioglitazone, 105.7 for sitagliptin, and 135.8 for vildagliptin per 100,000 patient-years. The incidence according to time interval is depicted in Table 2. The rate of hospitalization for HF was greatest in the first 30 days after medication, and corresponded to a significantly higher incidence at days 0 to 30 compared with days 31 to 360 for all three drugs (Table 3). The HR was 1.85 for pioglitazone, 2.00 for sitagliptin, and 1.79 for vildagliptin. The incidence of hospitalization for HF did not differ among drugs at any time period, as shown in Table 4.
DISCUSSION
We observed a 1.8- to 2.0-fold increase in hospitalization for HF in the initial 30 days of the medication (pioglitazone, sitagliptin, and vildagliptin) compared with the subsequent followup period. We did not find a significant difference between the drugs.
Our finding of significantly higher hospitalization for HF in the first 30 days of treatment is novel and suggests a drug effect on the outcome. We did not include saxagliptin in this study because it was prescribed to too few patients (less than 3%). Instead, we chose sitagliptin and vildagliptin as comparators because they were the first two DPP4i released in the market and have the longest and largest prescription histories. However, studies of their long-term CV outcomes have not been finished or planned. We did not have control group to compare and verify causal relationships. Instead, we have chosen pioglitazone as comparator for the analysis which is known to increase the risk of edema and HF [7]. As expected, pioglitazone increased the risk of hospitalization for HF in 30 days even though doctors may not have prescribed the drug to patients at high risk for HF because TZD is well known risk factor. Sitagliptin and vildagliptin showed similar increase in the first 30 days of medication compared to pioglitazone. In addition, there was no significant difference between sitagliptin and vildagliptin in the key outcome (data not shown). There might be some possibility that hidden HF affected the result of increased hospitalization in the initial 30 days. However, we eliminated those with a prior diagnosis of HF to reduce these possibilities. All these results indicate a class effect and relatively acute drug effect on HF. To date, there is no published data on prescription days before HF hospitalization for SAVOR-TIMI 53. We recommend further analysis of this outcome for the already published long-term CV outcome studies.
The most recent article on sitagliptin found significant increased risk of HF-related hospitalizations among patients with T2DM and HF [14]. The Vildagliptin in Ventricular Dysfunction Diabetes (VIVIDD) trial [15] had more CV deaths in the treatment arm than in the placebo arm. Moreover, there was a statistically significant increase in left ventricular end-diastolic volume and a trend toward increased left ventricular end-systolic volume. In the meantime, a meta-analysis of randomized clinical trials of DPP4i [16] and 20 phase 2 and 3 trials of saxagliptin [17] assessed CV risk. The latter concluded that saxagliptin was not associated with an increased risk of CV, including HF. Furthermore, the former report found that DPP4i treatment reduces the risk of CVD (particularly myocardial infarction) and all-cause mortality in patients with T2DM. However, both studies lack of long-term CV outcome data. Recently, a meta-analysis [18] of DPP4i and HF (including results from SAVOR-TIMI 53) suggested that DPP4i could be associated with an increased risk of HF, without any clear evidence of differences among drugs of the class. The most recent and largest meta-analysis [19] including SAVOR-TIMI 53 data also found the same trend towards increased risk of HF outcomes. Therefore, the findings of our analysis, as well as other recent studies, highlight the need for well-designed trials that rigorously assess for HF in patient with T2DM.
The possible mechanisms underlying the association of DPP4i with HF remain elusive [20]. However, interactions of DPP4i with HF cannot be totally ruled out, since levels of brain natriuretic peptides (BNP), which may be 100 times as high in patients with HF as in patients without HF, are known substrates of the enzyme DPP4 [21]. In addition, DPP4 is not specific to glucagon-like peptide 1 (GLP-1), and has the potential to mediate a wide range of both positive and negative pleiotropic effects independently of GLP-1 [22]. We speculate that reducing inactivated GLP-1, which has positive effects on myocardial function [23], or increasing other vasoactive substrates of DPP4 (e.g., BNP) could have detrimental effects during DPP4i treatment. Moreover, incretin-based therapies (mainly GLP-1 agonists) have been reported to increase heart rate in some studies, especially when used in conjunction with angiotensin-converting-enzyme inhibitor [24]. The increased heart rate may be secondary to the lower blood pressure induced by these agents but may also denote chronic (as opposed to acute, hypoglycemia-induced) activation of potentially adverse neurohormonal systems such as the sympathetic nervous system (SNS) [25]. Chronic SNS activation is a well-established contributor to the pathogenesis and progression HF in susceptible subjects. Lastly, as with all glucose-lowering agents, DPP4i may cause hypoglycemia (particularly when combined with other hypoglycemic agents), which is associated with short-term SNS activation. Whether repeated episodes of hypoglycemia contribute to deterioration in cardiac function, clinical HF, or both is uncertain [20].
The strengths of this study include the large population and the probable inclusion of all hospitalizations for HF because the NHIS is a compulsory and universal health care system in Korea. However, our study had several limitations. The database used was originally designed for billing purposes; therefore, inaccuracy in coding for HF and comorbidities may have occurred. Therefore, misdiagnosis and selective misclassification were possible. The main causes of HF remain unclear, and many important risk factors, clinical and physical findings, symptoms and signs, or disease severity were not available for adjustment or evaluation. We could not adjust for these confounding variables because of lack of data. Further study with full data regarding medications and co-existing illness is needed. We found that the increased hospitalization for HF in the first 30 days of DPP4i treatment was the same as that for pioglitazone. However, we must still be cautious in our interpretations and conclusions because causal inferences cannot be made on the basis of observational studies alone.
In conclusion, the present study shows that the increase in hospitalization for HF at days 0 to 30 for sitagliptin and vildagliptin was the same as that for pioglitazone. The differences between the drugs were not significant. The full spectrum of beneficial and adverse effects of DPP4i will not be known until long-term trials with clinical endpoints are complete. Therefore, we await the results of the ongoing trials with CVD as a main endpoint.
Notes
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.