Prevalence of Depression and Glucose Abnormality in an Urbanizing Rural Population of Bangladesh
Article information
Abstract
Background
Depression and glucose abnormality are increasing in Bangladesh including its rural area. This study was designed to determine the prevalence of depression in an urbanizing rural population of Bangladesh with or without glucose abnormality (including diabetes mellitus [DM], and pre-diabetes which combines impaired fasting glucose and impaired glucose tolerance pre-DM).
Methods
A total of 2,293 subjects aged ≥20 years were investigated. Sociodemographic and anthropometric details, blood pressure, fasting (fasting plasma glucose) and 2 hours after 75 g plasma glucose (2-hour plasma glucose), were studied. Montgomery-Asberg Depression Rating Scale was used to assess depression.
Results
The overall prevalence of DM was 7.9% and pre-DM was 8.6%. Prevalence of depression was 15.31% (n=351; 95% confidence interval [CI], 1.59 to 1.36) with mean depressive score 17.62±3.49. Female were more likely to have depression (17.16%). The 22.35% of male and 29.46% of female with pre-DM and 26.58% male and 36.27% female with DM had depressive symptoms. There was no significant variation in the mean age of different groups (healthy, depressed and with glucose abnormality). Depression was significantly associated with age, marital status, occupation, high physical activity, and low body mass index. The odds ratio (OR) for depression was significantly increased in patients with glucose abnormality compared with those without pre-DM (OR, 2.49; 95% CI, 1.76 to 3.51; P<0.000) and DM (OR, 3.27; 95% CI, 2.33 to 4.60; P<0.000).
Conclusion
Prevalence of depression found alarming in our study area though lesser than previous studies and it is significantly related to glucose abnormality. The study reveals that mental health should get more focused specially along with metabolic diseases.
INTRODUCTION
Depression expected to become the number one cause of disease burden in developing countries. The overall prevalence of depressive disorder in Bangladeshi adult population is 4.6% [1]. Prevalence of psychiatric disorders in rural Bangladesh is 16.5% [2].
Glucose abnormality (includes diabetes mellitus [DM], impaired fasting glucose [IFG], and impaired glucose tolerance [IGT]) is also increasing globally. In rural Bangladesh, prevalence of DM and IFG increased from 2.3% to 6.8% and 4.6% to 5.8% in between 1999 and 2004 [3]. Still population-based study on IGT prevalence in rural Bangladesh is not well documented, only one population based study in 2004, IGT prevalence in rural Bangladesh was found 2.0% [3]. Because of its widespread prevalence and potentially debilitating impact, glucose abnormality has become an international and national priority area of health concern.
Depression has been found to be associated with lower quality of life, poorer diabetes self-care, impaired glycaemic control and an increased risk of developing diabetes-related complications [4].
To our knowledge, data on depression in the general population of South Asia is inadequate, particularly for pre-diabetes mellitus (pre-DM; combines IGT and IFG) and DM patients. Among urban South Indians (Chennai) the overall prevalence of depression was 14.3%, and an increasing prevalence of depression was seen with increasing grades of glucose abnormality: normal fasting glucose (13.1%), IFG (15.7%), and DM (19.7%) (trend w2¼ 57.1, P<0.001) [5]. In a rural community of Pakistan the prevalence of depression was 14.7% amongst diabetic subjects [6]. The available data regarding the prevalence of depression in subjects with glucose abnormality of Bangladesh are also limited. Pointing out the risk factors is a vital need for better outcome and lower treatment cost for both the diseases. Depression and glucose abnormality are proved to be related risk factors for each other.
Feeling that need a group of researchers from Bangladesh Diabetic Association has been studying in a particular rural area from 1999 with 5 years interval. According to that chronicle in 2004 the study revealed, 29.0% of male and 30.5% of female participants with DM and 6.0% of male and 14.6% of female subjects without DM had depressive symptoms [7]. Like other developing countries, Bangladesh has undergone marked economic and epidemiologic transition in recent years. Increasing urbanization has been founded with a sedentary lifestyle, higher calorie food intake and stressful condition, which might have contributed to the increasing prevalence of glucose abnormality [89] as well as change in depressive symptoms. This study was designed to investigate the status of depression along with glucose abnormality in that particular area in 2009, following almost the same procedure.
METHODS
Ethics
The protocol was approved both by Ethical Review Committee of both Oslo University, Norway and Diabetic Association of Bangladesh. The procedure was adopted in order to avoid selection bias. Verbal orientation of the objectives and the procedure were read aloud to the subjects, including their right to refuse and withdraw at any stage of the study or to bar their data from analyses. Verbal consent was received from all subjects prior to inclusion in the study. All information and data collected were deemed confidential. All subjects received a hardcopy of their own biochemical results.
Study area and population
This study was part of a large longitudinal epidemiological study on DM and other non-communicable diseases in rural Bangladesh which has been described previously [3]. The current cross-sectional study was done in 2009 in an urbanizing rural area named "Chandra," 40 km north from the capital city 'Dhaka.' Some structural changes occurred during the last 10 years due to territorial expansion of Dhaka city which modified the rural area to urbanizing. Ten villages were randomly selected with a population of approximately 20,000 with aged ≥20 years. For this study, 3,000 individuals were randomly selected and among them 2,293 participated (842 male and 1,451 female) for whom all the variables were available. Pregnant women and those with a diagnosed acute physical or severe mental illness were excluded.
Survey for glucose abnormality and blood sample collection
Once selection procedure was completed, participants were requested to visit a nearby field centre after an overnight fast of 8 to 12 hours. Then 8 mL of venous blood was collected from each participant. Fasting plasma glucose (FPG) and glycosylated hemoglobin (HbA1c) were determined from fasting blood sample. All subjects other than those with known DM (n=40) were then given an oral glucose solution (75 g oral glucose in 250 mL of water) to drink. Another 3 mL of venous blood was collected after 2 hours to determine 2-hour post-oral glucose. The samples for plasma glucose test were collected in a tube containing sodium fluoride and potassium oxalate (1:3) and were centrifuge immediately after collection. Separated plasma samples were sent to laboratory of Bangladesh Institute of Research and Rehabilitation for Diabetes, Endocrine and Metabolic Disorders in ice jell packed cooling boxes and stored at -70° until laboratory assays was done. Quality control on the blood glucose measurement was checked by measuring the 2-hour plasma glucose (2hPG) values using the glucose oxidase method in every 10th case. The intra-assay coefficient of variation was 1.24% at a mean of 5.86 mmol/L, and the interassay coefficient of variation was 2.10% at a mean of 5.23 mmol/L. Plasma glucose was measured by the glucose oxidase method using Dimension RxL Max (Siemens Healthcare Diagnostics Ltd., Camberley, UK) on the same day. HbA1c estimated by high performance liquid chromatography, VARIANT Hemoglobin A1c Program (Bio-Rad 10 system; Bio-Rad Laboratories, Hercules, CA, USA).
Diagnosis of various degree of glucose abnormality: DM and pre-DM
DM: DM was diagnosed if FPG value was ≥7.0 mmol/L and/or 2hPG was ≥11.1 mmol/L. Pre-DM: IFG, IGT and IFG+IGT are categorized as pre-DM in the study.
IFG was defined if FPG was between 6.1 and 7 mmol/L and 2hPG was <7.8 mmol/L. IGT was defined if FPG was <6.1 mmol/L and 2hPG was between 7.8 and 11.1 mmol/L. Combine IFG and IGT was defined if FPG values was between 6.1 and 7 mmol/L and 2hPG was between 7.8 and 11.1 mmol/L. For screening diagnostic criteria of World Health Organization (WHO) was used [10].
Procedures and anthropometric measurements
During the 2-hour waiting period for oral glucose tolerance test, the respondents were interviewed for sociodemographic information using a pretested questionnaire [11]. Education was counted by the years in academic institutions. Financial status was measured upon the expenditure of the subjects.
The rural population in Bangladesh is primarily labourers or farmers, both occupations requiring a high level of manual labour. Leisure time physical activity was recorded by interview. Finally, reported hours were dichotomized as 'low' for those who reported leisure time physical activity of <2 hours per week and 'high' for those reporting physical activity of >2 hours per week.
Smoking, chewing tobacco, consuming 'hukka' and alcohol habit was classified as either current or non/ex-consumer. Their anthropometric measurements including height, weight, hip, and waist girth were recorded. Anthropometric measurements were taken with light clothes and without shoes. For height, the subject stood in erect posture vertically touching the occiput, back, hip, and heels on the wall while gazing horizontally in front and keeping the tragus and lateral orbital margin in the same horizontal plane. Waist circumference was measured by placing a plastic tape horizontally midway between 12th rib and iliac crest on the mid-axillary line. Similarly, hip was measured by taking the extreme end posteriorly and the symphysis pubis anteriorly. Cutoff values for general obesity for both sexes were a body mass index (BMI) of ≥23 kg/m2, cutoff values for central obesity including waist circumference for man and women were ≥90 and ≥80 cm, respectively [1213].
Blood pressure measurement
In addition, blood pressure (BP) was measured in both sitting and standing position using standard cuffs for adults fitted with sphygmomanometer minimized variation in measurement. Prior to the measurement, 10-minute rest was assured. Two readings were taken 5 minutes apart, and the mean of the two was taken as the final blood pressure reading of the individual. Subjects found with raised BP were identified and BP was measured twice with 7 days interval within 2 consecutive weeks. Hypertension (HTN) was defined as a systolic blood pressure (SBP) of ≥140 mm Hg and/or diastolic blood pressure (DBP) of ≥90 mm Hg in all three episodes of measurements or current treatment with antihypertensive medication [14].
Assessment of depressive symptoms
In 1979, Montgomery and Asberg [15] developed a depression rating scale with 10 items questionnaire. Since its development, the Montgomery-Asberg Depression Rating Scale (MADRS) has been widely used all over the world, including Bangladesh. The questionnaire was translated into local language 'Bangla' and administered to all individuals [11]. Fieldworkers, who had received 1 week training in using this questionnaire prior to the survey, filled it out. The score of each item can range from 0 to 6; hence, the sum for the 10 items can range from 0 to 60. In general, MADRS scores are categorized in four groups: 0 to 12 (healthy), 13 to 19 (mild depression), 20 to 34 (moderate depression), and 35 to 60 (severe depression) [14]. Based on previous experiences [711] and limited samples it was decided to use only two groups 0 to 12 (healthy subjects) and ≥13 (depressed subjects). Thus the term 'depression' is not a clinical diagnosis but refers to an epidemiological definition of depression, based on a threshold level of symptom scale.
Statistical analysis
All the continuous variables were presented as mean or geometric mean and categorical data as percentage (all at 95% confidence interval [CI]). Age specific and age standardized prevalence by direct standardization method were estimated on the basis of 2001 census data before performing statistical tests [16]. Logistic regression models were used to examine the statistical difference of proportions and estimate the odds ratio (OR) adjusted for age and potential confounding factors. Multiple logistic regression analyses were also used to study the associations. The estimation of standardized prevalence was performed with STATA version 11 (Stata Corp., College Station, TX, USA) and the other analyses were carried out with PASW version 18 (SPSS Inc., Chicago, IL, USA).
RESULTS
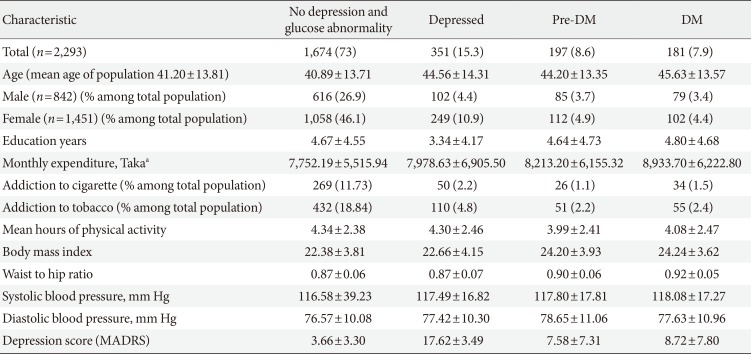
Prevalence of depression was 15.3% (n=351; 95% CI, 1.59 to 1.36) with mean depressive score 17.62±3.49. The overall prevalence of DM and pre-DM were 7.9% and 8.6%. One thousand and 674 subjects (73.0%) had no symptom of depression or glucose abnormality.
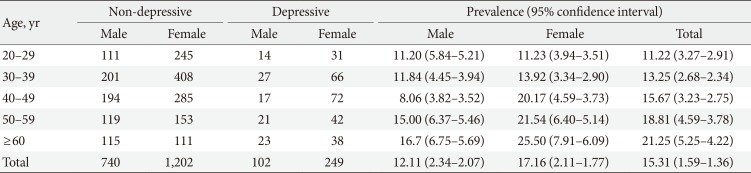
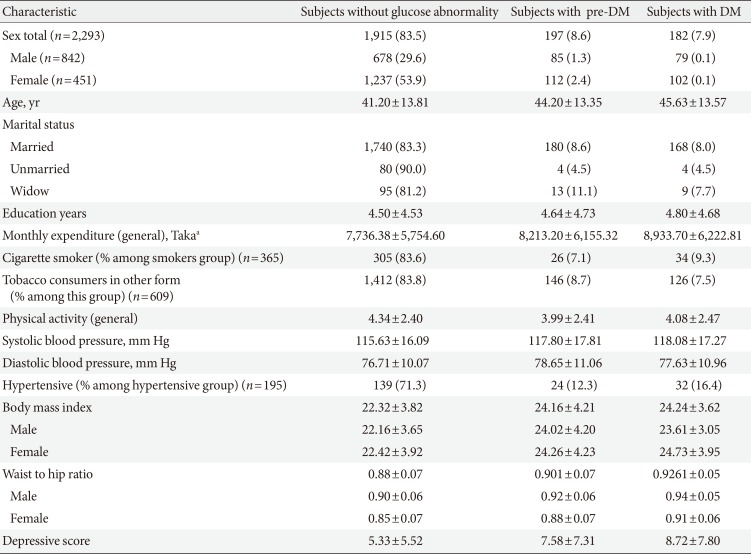
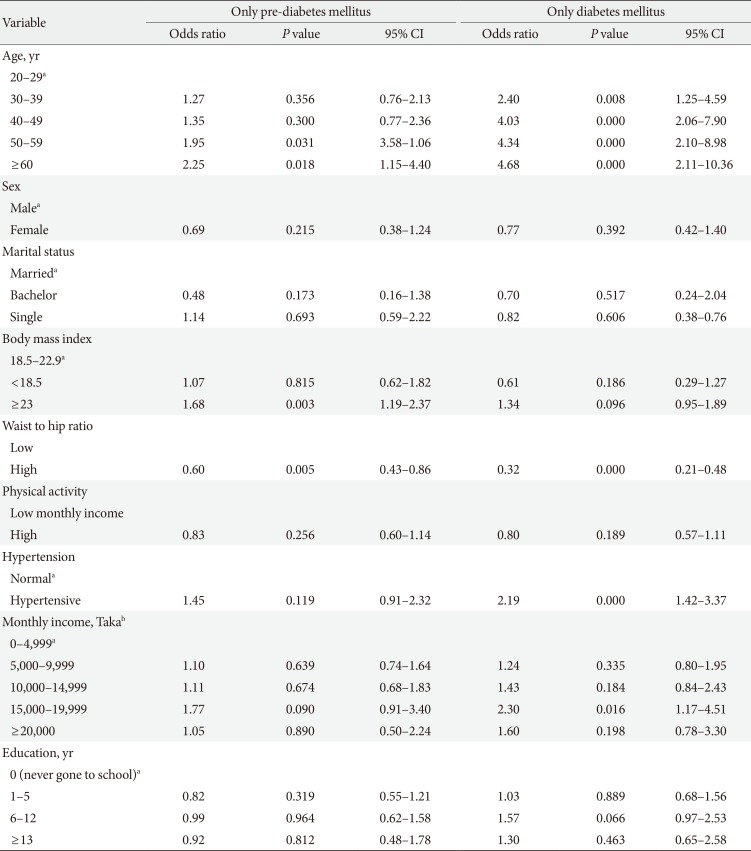
Mean age of the population was 41.81±13.82. There was no significant variation in the mean age of different groups (healthy, depressed and with glucose abnormality) (Tables 1 and 2). Female were more likely to have depression (17.2%). The 22.3% of male and 29.5% of female pre-DM subjects and 26.6% male and 36.3% female DM subjects had depressive symptoms. Depression was more common in people who were single (not unmarried) due either to death of spouse or separation (27.3%). The mean level of education found lowest in depressed group (3.34±4.17). Prevalence of depression among the pensioner was the highest (38.5%), whereas the rate in unemployed group was 15.9% and lowest in the group of businessperson 10.1%. The study showed that 73.0% people were practiced with high level of physical activity and among them 15.3% were depressed, 8.1% were prediabetic and 7.3% were diabetic. On the other hand people with low physical activity (27.0%) were suffering more from pre-DM (10.0%) and DM (9.5%) but did not diverge much in case of depression (15.3%). Mean BMI of the group of depressed (without glucose abnormality) people was the lowest among all the groups (21.92±3.88) but it increased with glucose abnormality (22.66±4.15). The pre-DM and DM patients, with and without depressive symptoms had higher level of mean waist to hip ratio (WHR) than the subjects with only depression and healthy subjects eventually. Overall prevalence of HTN was only 15.5%. In the healthy group people were least hypertensive (14.5%) but HTN elevated with depression, pre-DM, and DM. The mean SBP or DBP did not vary much among the groups (Tables 1,2,3).

Prevalence of subjects with or without depression and varying degrees of glucose tolerance with associated risk factors

Clinical and biochemical characteristics of the subjects with depression, glucose abnormality compared with healthy subjects

Comparison between depressed and non-depressed group for different sociodemographic, clinical, and biochemical factors
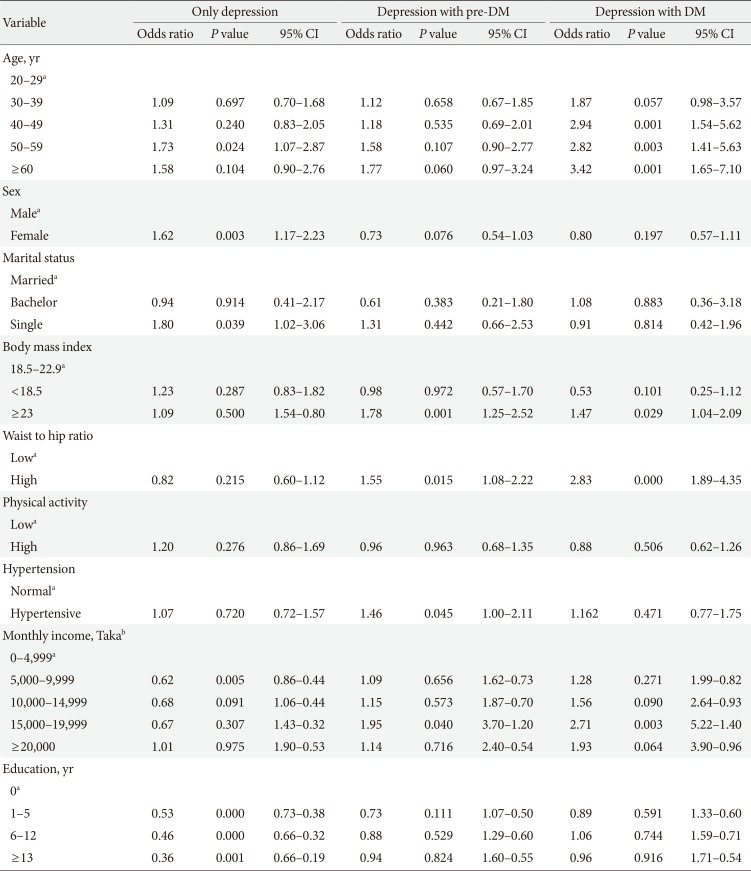
Mean level of income (monthly expenditure), education level, physical activity, BMI, and WHR found lower in depressed population, compared to pre-DM and DM group, and they were more addicted to smoking and tobacco consumption (Table 2). The prevalence rate of depression increased with age for both sex (except for male in the age group of 40 to 49) (Table 4). BMI, WHR, and physical activity had strong and linear association with DM and pre-DM when other socioeconomic factors were adjusted (Tables 5 and 6). Financial status and education level may have some relationship with depression and glucose abnormality which was not very clear (Tables 5 and 7). The OR for depression was significantly increased in patients with pre-DM and DM compared with those without pre-DM (OR, 2.49; 95% CI, 1.76 to 3.51; P<0.0001) and DM (OR, 3.27; 95% CI, 2.33 to 4.60; P<0.0001) (Table 5).

Comparison between pre-DM, DM, and 'without glucose abnormality group' for different sociodemographic, clinical, and biochemical factors
Age had a steady and significant relationship with depression, pre-DM, and DM. In case of female subjects the OR increased with depression but vice versa in glucose abnormality, when the other variables like BMI, WHR, and physical activity were adjusted. Marital status showed a significant relationship with depression (Table 7).
DISCUSSION
The overall prevalence rate of depression (15.3%) was not unexpected but quite alarming. It was lower than the previous study (29.7%) [8], but much higher than the statistical information (4.6%) from National Institute of Mental Health, Bangladesh [1]. This result was also higher than another study (8%) in a similar geographical area near Dhaka in 2007 [2].
This discrepancy between the studies may be due partly to change of socioeconomic status of that population. Our population was recruited from a rural (urbanizing) area of Bangladesh where poverty was common but recent development of that particular area might have some influence to reduce the overall prevalence of depression within these 5 years. though mean monthly income was lower with depressed population which assumes that depression is related to poverty any steady association could not be stablished between of socioeconomic strata with depressive symptoms while combined with glucose abnormality.
Studies from both the East and West had explored higher prevalence of depression in women than men. The explanation for this assumed to be that, women are influenced by biological factors including hormones, psychological attributes, unfavourable family experiences, harsh sociocultural role, and poor social support [17181920]. Female subjects were affected disproportionately by poverty in the local culture. In the previous study, the prevalence of depression among male and female was 28.4% and 29.6% [7]. Our data found the rate reduced around half of that but the gender proportion seems to be broadened.
As it had been shown in earlier studies, 'extreme age' is usually associated with depression, pre-DM, and DM, our study also supported this [2021]. The difference in prevalence of depression widened in the older age strata in general.
The significant predictors of depressive symptoms were age, gender and the comorbid state of glucose abnormality. Less known is the increased risk for depression: individuals with DM have a 2-fold increased risk for depression, affecting approximately one in every five DM patients [2021]. Controlling the potential confounding factors, glucose abnormality remained the strongest risk factor for depressive symptoms in our study (P=0.0001). The prevalence rate was more than 3- and 2-fold higher in diabetic and prediabetic subjects respectively. The finding of a high prevalence of symptoms of depression in prediabetic (26.4%) and diabetic (32.0%) subjects theoretically was neither unexpected nor incongruous with previous studies. This result was also comparable with a study from neighbouring country India (where the prevalence rate of depression was 27.0% among DM and 11.1% in non-DM subjects) [22] and studies from round the world (prevalence of depression among DM subjects) like Korea (22.6%) [23], Iran (71.8%) [24], Greece (33.0%) [25], South Australia (24.0%) [26], and South Asian people in United Kingdom (20.6%) [27].
Several studies proved glucose abnormality and HTN have some association and our data also supported that. Study found higher BMI and WHR among subjects with impaired glucose regulation and it was consistent with previous two studies conducted in this population [328]. The pre-DM and DM groups had higher mean BMI level (labelled as overweight according to WHO cutoff point for Asian people) and the depressive group had lower mean BMI in comparison with both healthy and diseased. Higher BMI and WHR were found to be protective factors against depression. It was also observed in previous studies in Bangladesh [7] and Pakistan [6]. The findings regarding BMI were also an important point to notice the current risk of non-communicable diseases in our population.
The interesting thing is this; the study also confirmed the trend of glucose abnormality which was upward in rural (urbanizing) Bangladesh. Like previous studies it also showed that male subjects are more prone to glucose abnormalities according to all age group [3]. Improvements in the mean BMI score (22.63±3.9) and the mean financial status (US $112.80±83.62) was predicted to be the cause, though it was not confirmed. In the present study, it was confirmed that the WHR, a measure indicative of central obesity, is a major risk indicator of glucose abnormality which was also evident in previous studies in Bangladesh [28]. Despite the fact that Asians have lower BMI than Western counterparts, some Asian countries have a similar or even higher prevalence of DM than Western countries. This finding is explained by the observation that Asian populations are more prone to abdominal obesity with increased insulin resistance compared with their Western counterparts. Therefore, central obesity is a useful marker as a risk of DM, especially in individuals with normal BMI values [29].
Percentage of people with both diseases was only 3.5% which was also lower than the previous study (16.9%) [7]. The reason behind these was likely to be the statistics of depression. The percentage of depressive symptoms decreased by these years. The probable reason may be the development of infrastructure of that particular area, increment of financial status and the different pattern of presenting depressive problems. Ten villages under our study project had expansion in their small (36.3%) and medium (46.2%) trade, electric supply (3.5%), sanitation (4.1%) and road and transport facilities [11]. The health services facilities were also improved through some Non-governmental Organizations and other satellite clinics. These findings once again prove that depression in context of country like Bangladesh is also related with poverty and inferior social structure.
Along with the previous findings this study data emphasis that depressive symptoms in this culture are common, especially in those with glucose abnormality. Psychotherapy may be necessary in addition to lifestyle changes to prevent the exponential increase in the occurrence of glucose abnormality. In addition, a common approach including psychiatric treatment in glucose abnormality care may be necessary to achieve improved glycaemic control in this population.
The strength of the study was that, it was a large, well-performed study. So far this was one of the recent studies reported from the urbanizing rural population in Bangladesh. Bias was taken care of by random sampling.
The limitation of the study was that, the prevalence of depression among adolescents was not included. The increased prevalence in the present report is not likely to be the true prevalence of depression and glucose abnormality in overall urbanizing rural population of Bangladesh. The reliability and validity of this approach (MADRS) may be psychiatrically weak as we used the epidemiological tool, otherwise it was healthy.
In conclusion, as it is stated that half of the people with DM are still undiagnosed it was really important to find out the curve of prevalence of glucose abnormality in each sociodemographic strata in our country. The rising prevalence of glucose abnormality in the present study indicates the environmental factors may encompass a strong role for the rising prevalence of glucose abnormality in urbanizing rural population in a developing country like Bangladesh. Despite limitations, the present study offered the insight into the prevalence of depression in a large rural population with and without glucose abnormality in the Bangladesh which represents a significant impediment to the interests of public health. In summary we report the prevalence of depression in the urbanizing rural area near Dhaka is 15.3% which is startling and female gender, age, education, low income, and marrital status are associated with depression in this population. Therefore, is a clear need to increase mental health services and to integrate this with general health services in Bangladesh. These issues may be of particular concern in South Asian communities and of course in Bangladesh, who are at increased risk for both diabetes and depression along with their adverse outcomes associated with comorbidity [3031]. Further research is modestly recommended to develop the strategy for the prevention and treatment of the condition. Moreover, the health policy needs to address the issue of depression with particular references to the sociao economic factors for depression such as lack of education.
Notes
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.