Renoprotective Mechanism of Sodium-Glucose Cotransporter 2 Inhibitors: Focusing on Renal Hemodynamics
Article information
Abstract
Diabetic kidney disease (DKD) is a prevalent renal complication of diabetes mellitus that ultimately develops into end-stage kidney disease (ESKD) when not managed appropriately. Substantial risk of ESKD remains even with intensive management of hyperglycemia and risk factors of DKD and timely use of renin-angiotensin-aldosterone inhibitors. Sodium-glucose cotransporter 2 (SGLT2) inhibitors reduce hyperglycemia primarily by inhibiting glucose and sodium reabsorption in the renal proximal tubule. Currently, their effects expand to prevent or delay cardiovascular and renal adverse events, even in those without diabetes. In dedicated renal outcome trials, SGLT2 inhibitors significantly reduced the risk of composite renal adverse events, including the development of ESKD or renal replacement therapy, which led to the positioning of SGLT2 inhibitors as the mainstay of chronic kidney disease management. Multiple mechanisms of action of SGLT2 inhibitors, including hemodynamic, metabolic, and anti-inflammatory effects, have been proposed. Restoration of tubuloglomerular feedback is a plausible explanation for the alteration in renal hemodynamics induced by SGLT2 inhibition and for the associated renal benefit. This review discusses the clinical rationale and mechanism related to the protection SGLT2 inhibitors exert on the kidney, focusing on renal hemodynamic effects.
INTRODUCTION
Diabetic kidney disease (DKD) is one of the most important complications in patients with type 1 and type 2 diabetes mellitus. Diabetes is currently the most common cause of chronic kidney disease (CKD), leading to kidney failure worldwide [1]. In South Korea, nearly 50% of end-stage kidney disease (ESKD) cases are caused by diabetes [2]. Over 30% of people with diabetes have albuminuria or reduced glomerular filtration rate (GFR) [3]. The progress of standard care and the advent of new drugs have substantially decreased the risk of morbidity and mortality in patients with diabetes [4]. Nonetheless, the prevalence of DKD and ESKD is steadily increasing, whereas that of ischemic heart disease and ischemic stroke has gradually decreased over the past decades [5,6]. Given the significantly increased mortality risk related to ESKD [7], preventing DKD with intensive risk factor management is critical. In major clinical trials, the renoprotective effect of renin-angiotensin-aldosterone system (RAAS) inhibitors has been demonstrated in diabetic patients with hypertension [8–10]. However, for approximately 20 years after that, no single treatment that could delay the progression of DKD has been reported [11,12].
Sodium-glucose cotransporter 1 (SGLT1) is expressed mostly in the intestinal tract and less so in the kidney, whereas SGLT2 is predominantly present in the renal proximal tubule; both SGLTs are responsible for the reabsorption of glucose and sodium [13]. SGLT2 is responsible for 80% to 90% glucose reabsorption in the early proximal tubule, whereas SGLT1 reabsorbs the remaining 10% of the filtered glucose [14]. Some studies have indicated that the mRNA levels and activity of SGLTs are increased in patients with diabetes [15,16]. Therefore, strategies to inhibit SGLT2 have been proposed to lower blood sugar levels in diabetic patients [17,18], and several SGLT2 inhibitors have been introduced. In addition to their glucose-lowering effect, SGLT2 inhibitors have beneficial effects on cardiovascular and renal disease risk factors, such as weight loss and blood pressure reduction [19,20]. Indeed, cardiovascular and dedicated renal outcome trials of SGLT2 inhibitors have demonstrated that these drugs inhibit and delay the development and progression of cardiovascular and renal diseases [21–23].
A single effect cannot explain the mechanisms underlying the cardiovascular and renal benefits of SGLT2 inhibitors. Instead, various metabolic and hemodynamic effects appear to be related, and the mechanisms by which SGLT2 inhibitors exert beneficial effects on the renal and cardiovascular systems have been reviewed elsewhere [24,25]. This review discusses the mechanism by which SGLT2 inhibitors exert a renoprotective effect. In particular, we summarize the results of recent studies focusing on changes in renal hemodynamics induced by SGLT2 inhibition.
IMPLICATIONS FROM CARDIOVASCULAR AND RENAL OUTCOME TRIALS OF SGLT2 INHIBITORS
Table 1 summarizes the renal effects of SGLT2 inhibitors in cardiovascular and renal outcome trials. The cardiovascular outcome trial of empagliflozin ((Empagliflozin) Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients [EMPA REG OUTCOME]) demonstrated a significant reduction in the incidence of major adverse cardiovascular events with empagliflozin versus placebo in patients with type 2 diabetes mellitus and established cardiovascular disease (CVD) [26]. In this study, pre-specified renal endpoints, incident or worsening nephropathy (progression to macroalbuminuria, doubling of serum creatinine, initiation of renal replacement therapy, or death from renal disease) also significantly decreased by 39% in the empagliflozin group [27]. Thereafter, in the Canagliflozin Cardiovascular Assessment Study (CANVAS) of canagliflozin and the Dapagliflozin Effect on Cardiovascular Events (DECLARE) trial of dapagliflozin, the risk for prespecified renal endpoints was also significantly reduced in the treatment group compared to placebo by 30% and 39%, respectively [28,29]. Although these studies have limitations in that kidney outcomes were measured as a secondary endpoint, the results exceeded the magnitude of the beneficial effects of angiotensin receptor blockers on the progression of DKD, which was demonstrated by the Reduction of Endpoints in NIDDM with the Angiotensin II Antagonist Losartan Study (RENAAL) and Irbesartan Diabetic Nephropathy Trial (IDNT) (16% and 19%, respectively) [8,10]. Subsequent dedicated renal outcome trials, namely the Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE) and Dapagliflozin And Prevention of Adverse outcomes in Chronic Kidney Disease (DAPA CKD) studies, confirmed the effects of canagliflozin and dapagliflozin on primary renal endpoints in advanced CKD patients [10,30]; the risk of renal endpoints was significantly reduced in the CREDENCE study by 30%, and in the DAPA CKD study by 39%. In particular, the DAPA CKD study was conducted in patients with advanced CKD with or without diabetes, and the renoprotective effect of dapagliflozin was observed in both diabetic and non-diabetic patients [31].
The cardiovascular and renal outcome trials described above have consistently shown favorable results for kidney outcomes of SGLT2 inhibitors; therefore, it can be said that SGLT2 inhibitors have a protective role in the DKD progression as a class effect. The results of meta-analysis and subgroup analyses of these studies have several important clinical implications. First, the renal benefit of SGLT2 inhibitors is consistent regardless of CKD stage and albuminuria [21,22,29,30,32,33]. Notably, a similar renal benefit was seen even in patients with stage 1 or 2 CKD or normoalbuminuria as in those with higher stages of CKD or increased albuminuria. The absolute risk reduction for adverse renal events was even greater in patients with normal estimated glomerular filtration rate (eGFR) than in patients with advanced CKD [21,22]. This suggests that SGLT2 inhibitors can be applied at an earlier stage of DKD, significantly affecting early changes in the diabetic kidney, including glomerular hyperfiltration. Second, an initial decline in eGFR (initial “dip”) was observed after SGLT2 inhibitor use in most trials [34,35]. This pattern is similar to the transient eGFR decrease often observed after RAAS inhibitor use, suggesting significant changes in renal hemodynamics after SGLT2 inhibitor treatment initiation. As described later, decreased renal blood flow or renal vascular resistance appears to be responsible for this phenomenon. The initial dip in eGFR is a reversible process. It has been reported that the degree of decline is associated with subsequent kidney function preservation in some [34,36], but not in all trials [37]. In addition, many clinical trials have reported a low risk of acute kidney injury related to SGLT2 inhibitors [22,38], which indirectly shows that the initial dip occurs within a physiological range. Third, it has been demonstrated that SGLT2 inhibitors have beneficial effects not only on renal outcomes but also on heart failure outcomes [39–41]. Given that both CKD and heart failure have reduced effective circulating volume, it has been suggested that SGLT2 inhibitors can induce significant and beneficial changes in systemic hemodynamics [42].
HEMODYNAMIC ALTERATIONS IN DIABETIC KIDNEY DISEASE
The mechanism by which glomerular hyperfiltration occurs in patients with diabetes is not fully understood, but the structural, vascular, and tubular changes associated with hyperglycemia and diabetes seem to be involved [43]. In addition, changes in various growth factors and cytokines in response to hyperglycemia and hyperinsulinemia induce renal hypertrophy [44,45], which is recognized as a causative factor for the occurrence of hyperfiltration.
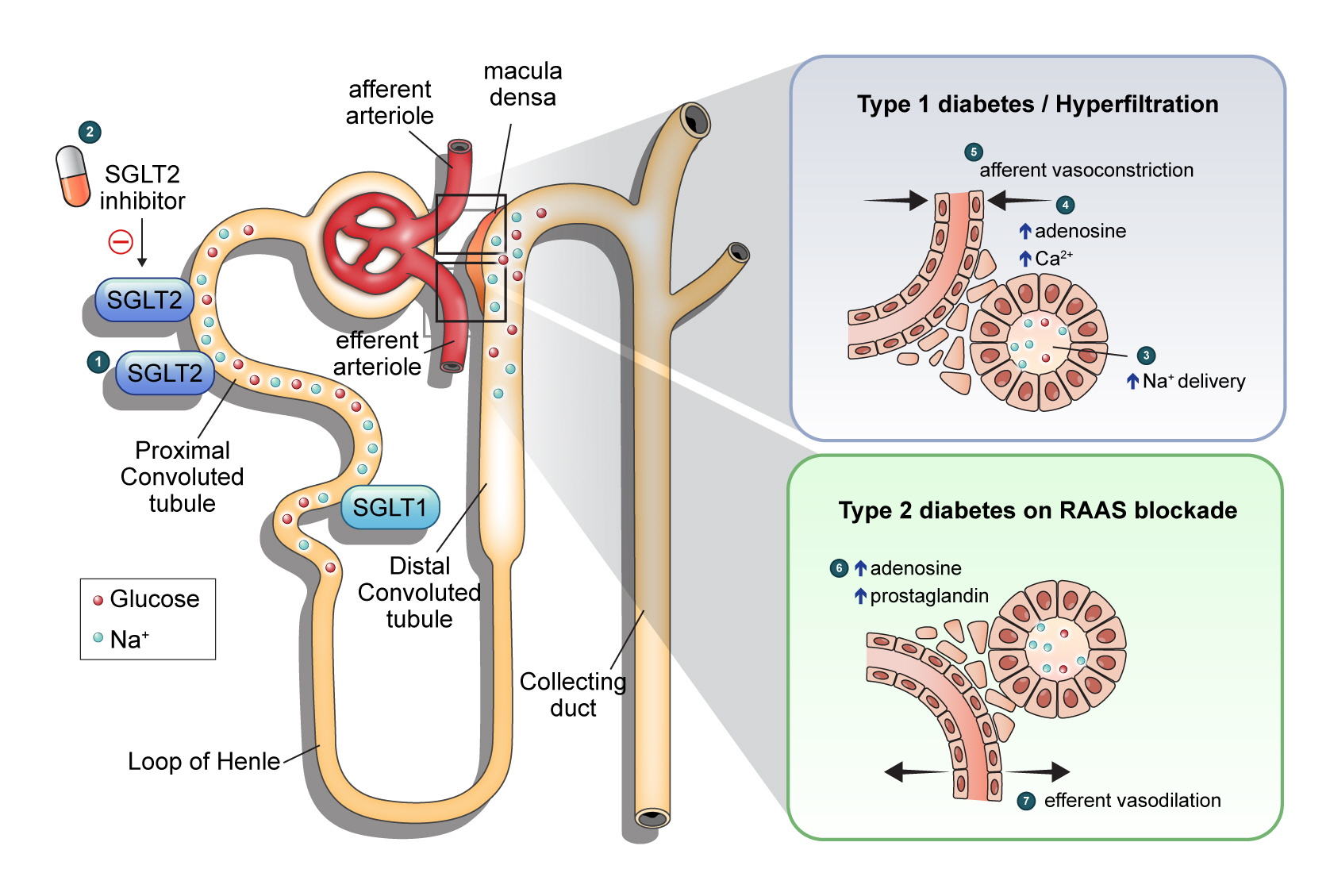
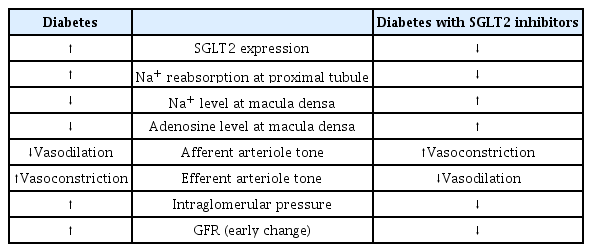
Hyperfiltration is generally regarded as an early hemodynamic change in patients with diabetes [46] and is found in up to 70% of patients with type 1 and type 2 diabetes mellitus [43]. Afferent renal vasodilation, subsequent glomerular hypertension, and an increased filtration fraction (the ratio between GFR and effective renal plasma flow) have been observed in patients with early diabetes and experimental models of diabetes [47,48]. Tubuloglomerular feedback (TGF) is an important mechanism explaining this phenomenon [49]. During intravascular volume contraction or decreased circulating plasma volume, sodium delivery to the macular densa decreases. This leads to vasoconstriction of the efferent arteriole by renin/angiotensin release and vasodilation of the afferent arteriole by reduced adenosine formation. As a result, intraglomerular pressure is restored, and GFR increases. Therefore, sodium delivery to the distal nephron and the subsequent changes in renin, angiotensin, and adenosine production is central to the TGF model [50].
Under physiologic condition, glucose filtered from the glomerulus is reabsorbed almost completely in the proximal tubule [14]. In patients with diabetes, glucose filtration is increased by hyperglycemia, leading to increased sodium and glucose reabsorption via SGLT upregulation [51]. Increased SGLT1 and SGLT2 expression have been observed in diabetic mouse models and human studies [15,16]. This is supported by the fact that the threshold of glucosuria in diabetic patients is approximately 60 mg/dL higher than that in normal subjects [52]. Consequently, the amount of sodium reaching the macular densa at the juxtamedullary apparatus decreases, which is recognized as a decrease in the effective circulating volume [53,54]. This induces changes in glomerular capillaries, leading to hyperfiltration and glomerular hypertension [55]. In detail, when sodium is transferred to the tubular epithelial cells of the macular densa, the conversion of adenosine triphosphate (ATP) to adenosine diphosphate by the Na+/K+-ATPase decreases, and consequently, the production of adenosine decreases. Adenosine induces arteriolar vasoconstriction through the adenosine type 1 (A1) receptor at the afferent arteriole. Therefore, a decrease in adenosine production inhibits this process and causes relative afferent arteriolar vasodilation. In contrast, a reduction in sodium delivery to the distal tubule in the diabetic kidney induces intrarenal RAAS activation, which results in efferent arteriolar vasoconstriction [55,56]. Taken together, the effects of hyperglycemia in the diabetic kidney influence both afferent and efferent arterioles, resulting in intraglomerular hypertension and hyperfiltration.
EFFECTS OF SGLT2 INHIBITORS ON RENAL HEMODYNAMICS
According to clinical studies, SGLT2 inhibitors reduce glycosylated hemoglobin by 0.4% to 1.0% in patients with type 2 diabetes mellitus under various trial settings [57]. In addition, SGLT2 inhibitors also control risk factors for CVD and CKD by reducing systolic blood pressure by 2 to 5 mm Hg and body weight by 2 to 4 kg [19,20]. However, compared to glucagon-like peptide-1 receptor agonists, which had a greater effect on reduction in glycosylated hemoglobin and weight loss, SGLT2 inhibitors showed superiority in ESKD prevention [57,58]. This finding implies that the effects of SGLT2 inhibitors on metabolic changes and cardiovascular risk factors do not fully explain their renoprotective effects.
TGF restoration is an important mechanism for explaining renal hemodynamic changes induced by SGLT2 inhibitors. SGLT2 inhibitors normalize the decreased distal sodium delivery to the macular densa in the diabetic kidney by inhibiting sodium reabsorption in the proximal tubule [56]. This increases adenosine production and consequently induces vasoconstriction of the afferent arteriole. As a result, the intraglomerular pressure is reduced, and hyperfiltration is improved. This has been demonstrated in several animal studies and patients with type 1 diabetes mellitus. In streptozotocin-induced diabetic rats, dapagliflozin decreased single-nephron GFR, accompanied by decreased proximal solute reabsorption and increased distal tubule chloride [59]. In patients with type 1 diabetes mellitus with hyperfiltration, empagliflozin treatment significantly reduced renal blood flow and hyperfiltration, accompanied by a decrease in plasma nitric oxide and an increase in renal vascular resistance [60]. In an animal model of type 1 diabetes mellitus, in vivo imaging demonstrated that empagliflozin reduced hyperfiltration via afferent arteriole constriction. In this study, an A1 adenosine receptor blocker counteracted the action of empagliflozin, supporting the hypothesis that SGLT2 inhibition affects renal hemodynamic function via adenosine production [61]. These studies showed that TGF restoration by SGLT2 inhibition does work, but there are limitations in that the studies were conducted primarily under the conditions of type 1 diabetes mellitus and hyperfiltration.
Of note, most large-scaled renal outcome trials have demonstrated the renoprotective effects of SGLT2 inhibitors in patients with type 2 diabetes mellitus and advanced stage of CKD. A possible explanation for the role of hyperfiltration in patients with advanced CKD is that hyperfiltration still occurs at the single nephron level in the late stage of kidney disease progression [62]. This is considered a compensatory mechanism for the reduced nephron number [63]. On the other hand, obesity, which is frequently found in patients with type 2 diabetes mellitus, also contributes to increased tubular sodium reabsorption by increasing abdominal pressure and compression of loops of Henle [64,65]. Therefore, even in advanced CKD, SGLT2 inhibitors may show hemodynamic effects similar to those observed in early CKD stages.
A recent study on patients with type 2 diabetes mellitus provided new insights into the hemodynamic effects of SGLT2 inhibitors [66]. In this study of 44 patients with type 2 diabetes mellitus, dapagliflozin decreased the measured GFR, and filtration fraction compared to gliclazide but did not increase renal vascular resistance. In addition, dapagliflozin treatment increased urinary adenosine and prostaglandin concentrations. Adenosine, a vasoconstrictor in the afferent arteriole, had a conflicting effect (vasodilation) on the efferent arteriole in the presence of RAAS blockade [67]. This means that SGLT2 inhibitors have different renal hemodynamic effects in type 2 diabetes mellitus patients on top of RAAS blockers compared to type 1 diabetes mellitus patients. In other words, efferent arteriole vasodilation through prostaglandin release could be a novel mechanistic explanation. The patients in this study had a baseline mean eGFR of 85 mL/min/1.73 m2 and diabetes duration of 9.8 years, and about 70% of participants were treated with RAAS inhibitors, which suggested that SGLT2 inhibitors may have a different renohemodynamic effect in patients with advanced type 2 diabetes mellitus without hyperfiltration. Another study, including 101 patients with type 2 diabetes mellitus, showed that treatment with empagliflozin and linagliptin versus metformin and insulin glargine did not increase the resistance of afferent arterioles. Still, it decreased efferent arterioles’ resistance and was accompanied by reduced GFR [68]. Table 2, Fig. 1 summarizes renal hemodynamic changes following SGLT2 inhibition.

Hemodynamic changes following sodium-glucose cotransporter 2 (SGLT2) inhibition in the kidney. ① SGLT2 is responsible for 80% to 90% glucose reabsorption in the early proximal tubule, and SGLT1 reabsorbs the remaining 10% of the filtered glucose under physiologic conditions. ② Under hyperglycemic condition, glucose filtration is increased, which leading to increased sodium and glucose reabsorption via SGLT2 upregulation. SGLT2 inhibitor primarily blocks the action of SGLT2, ③ resulting in increased delivery of sodium and glucose to the distal renal tubule. ④ In type 1 diabetes mellitus or hyperfiltration state, increased sodium delivery to the tubular epithelial cells of the macular densa induces adenosine production which activates adenosine A1 receptor, triggering an increase in cytosolic Ca2+. ⑤ Restoration of tubuloglomerular feedback ultimately results in afferent arteriolar vasoconstriction. ⑥ In type 2 diabetes mellitus on renin-angiotensin-aldosterone system (RAAS) blockade, SGLT2 inhibition may act on renal arterioles in a different way. SGLT2 inhibitors increased production of adenosine and prostaglandin ⑦ which resulting in efferent vasodilation on RAAS blockade.
CONCLUSIONS
Recent evidence clearly shows that SGLT2 inhibitors prevent or delay DKD progression. The renoprotective effects of SGLT2 inhibitors appear throughout the course of CKD, from the early to the late stages of the disease. In type 1 diabetes mellitus or under hyperfiltration conditions, restoration of TGF induced by SGLT2 inhibition reduces glomerular hypertension via afferent arteriole vasoconstriction. Recent studies have suggested that the renal hemodynamic effect of SGLT2 inhibitors in older patients with type 2 diabetes mellitus may differ from that in type 1 diabetes mellitus or under hyperfiltration conditions. However, although the route is likely to differ, intraglomerular pressure reduction by SGLT2 inhibition has been consistently observed. This change in renal hemodynamics is one of the important mechanisms explaining the renoprotective effect of SGLT2 inhibitors. It is expected that more evidence in the future will provide a better understanding of the true renal effects of SGLT2 inhibitors.
Notes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
FUNDING
This study was supported by the National Research Foundation of Korea (NRF) (Grant No. 2021R1C1C1005674).
ACKNOWLEDGMENTS
None