Differentiation of Microencapsulated Neonatal Porcine Pancreatic Cell Clusters in Vitro Improves Transplant Efficacy in Type 1 Diabetes Mellitus Mice
Article information
Abstract
Background
Neonatal porcine pancreatic cell clusters (NPCCs) have been proposed as an alternative source of β cells for islet transplantation because of their low cost and growth potential after transplantation. However, the delayed glucose lowering effect due to the immaturity of NPCCs and immunologic rejection remain as a barrier to NPCC’s clinical application. Here, we demonstrate accelerated differentiation and immune-tolerant NPCCs by in vitro chemical treatment and microencapsulation.
Methods
NPCCs isolated from 3-day-old piglets were cultured in F-10 media and then microencapsulated with alginate on day 5. Differentiation of NPCCs is facilitated by media supplemented with activin receptor-like kinase 5 inhibitor II, triiodothyronine and exendin-4 for 2 weeks. Marginal number of microencapsulated NPCCs to cure diabetes with and without differentiation were transplanted into diabetic mice and observed for 8 weeks.
Results
The proportion of insulin-positive cells and insulin mRNA levels of NPCCs were significantly increased in vitro in the differentiated group compared with the undifferentiated group. Blood glucose levels decreased eventually after transplantation of microencapsulated NPCCs in diabetic mice and normalized after 7 weeks in the differentiated group. In addition, the differentiated group showed nearly normal glucose tolerance at 8 weeks after transplantation. In contrast, neither blood glucose levels nor glucose tolerance were improved in the undifferentiated group. Retrieved graft in the differentiated group showed greater insulin response to high glucose compared with the undifferentiated group.
Conclusion
In vitro differentiation of microencapsulated immature NPCCs increased the proportion of insulin-positive cells and improved transplant efficacy in diabetic mice without immune rejection.
INTRODUCTION
Type 1 diabetes mellitus (T1DM) is a metabolic disease caused by destruction of the β-cells in the pancreas. Although exogenous insulin can be used to control blood glucose levels in patients with T1DM, controlling blood glucose levels in an ideal range for the prevention of diabetic chronic complications has been rarely achieved in patients with T1DM [1]. Furthermore, it is very hard to prevent life-threatening hypoglycemia in some cases [2]. Islet transplantation has been proposed as a promising method to cure T1DM. However, a single islet transplantation requires at least two donor pancreases to achieve insulin independence [3]. Due to the huge discrepancy in the number of donors and recipients for islet transplantation, islet transplantation has not been widely performed in clinical practice. Therefore, the study of xenotransplantation using pigs has drawn increased attention to resolve the shortage of donor pancreas supply.
Neonatal porcine pancreatic cell clusters (NPCCs) represent a potentially inexpensive and easily available source of β cells compared with adult pig islets and have already been performed in human clinical studies [4,5]. However, NPCCs are composed of around 50% of ductal cells and only 20% of endocrine cells [6,7]. In addition, as they are immature, it usually takes two to three months to normalize the blood glucose levels with a sufficient number of NPCCs transplantation [8,9]. Some studies have induced differentiation of NPCCs before transplantation to address these limitations. Hassouna et al. [10] reported that differentiation of NPCCs by chemicals improved blood glucose levels compared to the control group in diabetic mice. Other studies have reported improved glycemic control by differentiating NPCCs [9], but immune rejection issues still persisted.
The failure of graft tissues and cells due to immune rejection such as instant blood-mediated inflammatory reaction occurs via T cell-mediated rejection in the absence of immunosuppressive regimens [11]. Paradoxically, exposure to immunosuppressive drugs such as dexamethasone and sirolimus (rapamycin) has been reported to damage the engrafted islets and also inhibit differentiation into β-cells from precursor cells in the NPCCs [12,13]. Previously, we demonstrated successful allogeneic and xenogeneic islet transplantation using microcapsules as immune barriers without immunosuppressants [14]. In this study, we induced the differentiation of microencapsulated NPCCs in vitro and compared their transplantation efficacy with undifferentiated NPCCs in diabetic mice.
METHODS
Isolation of NPCCs
Neonatal porcine pancreases were procured from 3-day-old piglets (XP Bio, Anseong, Korea). The procedure for NPCC isolation was previously described by Yoon et al. [15]. Briefly, piglets were anesthetized with a mixture of rompun and ketamine (1:5 ratio). Pancreas was surgically removed, followed by cold ischemia in M199 (Thermo Fisher Scientific, Waltham, MA, USA). The pancreas was minced into less than 1 mm3 for 15 minutes, followed by enzymatic digestion with 1.5 mg/mL Collagenase P (Roche, Basel, Switzerland), dissolved in M199 for 16 minutes in a shaking water bath (37°C, 160 rpm). Digested tissues were filtered through a 500 µm cell strainer and washed using HBSS (0.25% bovine serum albumin, Sigma-Aldrich, St. Louis, MO, USA) three times. After filtration, pancreatic tissues were centrifuged for 2 minutes at 1,000 rpm. The NPCCs were then cultured in Ham’s F-10 media (Sigma-Aldrich) containing HEPES, D-glucose, L-glutamine, nicotinamide, CaCl2, IBMX, 1× anti/anti, 10% fetal bovine serum (Thermo Fisher Scientific) at 37°C, 5% CO2 for 5 days. Full media change was performed 24 hours later, and half the media was changed on day 3.
Microencapsulation of NPCCs
Microencapsulation was used as a method described by Park et al. [16]. First, 2% alginate (Pronova, Sandvika, Norway) dissolved in Ca2+ free Krebs-Ringers-HEPES buffer (KRH buffer) was transferred into the cell pellet (10,000 islet equivalent [IEQ]/mL). NPCCs were suspended in an alginate solution. The cell-mixed alginate was dropped into 10 mM BaCl2 solution to form a crosslink via a syringe pump (11 Plus Syringe Pump, Harvard apparatus, Hopkinton, MA, USA). Microencapsulated NPCCs were washed in cold HBSS (Welgene, Gyeongsan, Korea) and cultured in Dulbecco’s Modified Eagle Medium (DMEM)/F-12 (Thermo Fisher Scientific). After microencapsulation, NPCCs were stabilized in basal media for 2 days.
Differentiation of microencapsulated NPCCs and chemical treatment
DMEM/F-12 media was used as basal media for both groups. Maturation of microencapsulated NPCCs was achieved by supplementing the media with 10 µM activin receptor-like kinase 5 inhibitor II (ALK5iII, Tocris, Bristol, UK), 1 µM triiodothyronine (T3, Sigma-Aldrich), and 20 ng/mL exendin-4 (Ex-4, Prospec, Rehovot, Israel). They were cultured for 2 weeks. Media change was performed every other day.
Transplantation of microencapsulated NPCCs in mice
BALB/c mice (Orient Bio, Seongnam, Korea) served as recipients. Diabetes was induced by intraperitoneal (IP) injection of 180 mg/kg streptozotocin (Sigma-Aldrich) dissolved in 0.01 M citric acid. Mice with blood glucose levels >300 mg/dL were used in the experiment. Transplantation of the microencapsulated NPCCs (8,000 IEQ) was achieved by anesthetizing mice via exposure to N2O, O2 and isoflurane, and transplanted via IP injection of capsule using a 16 G angio-catheter. Recipient mice were 10 for the control, 14 for the undifferentiated group, and 14 for the differentiated group. And half of each group was harvested at 4 weeks of transplantation and the other half at 8 weeks of transplantation.
Blood glucose level, intraperitoneal glucose tolerance test, and serum insulin
Blood was drawn from the tail vein of mice and measured once a week for glucose measurements. An intraperitoneal glucose tolerance test (IPGTT) was performed in recipient mice at 4 and 8 weeks post-transplantation. After 15 hours of fast, 2 g/kg glucose was administered to mice, and blood glucose was measured at 0, 30, 60, 90, and 120 minutes.
Recipient blood samples were collected from NPCCs transplanted mice at 4 and 8 weeks post-transplantation. Blood was spun at 13,000 rpm for 10 minutes and blood serum was obtained and stored at –20°C. Serum insulin contents were evaluated by porcine insulin ELISA kit (Mercodia, Uppsala, Sweden) and measured by ELISA Reader (BIO-TEK, Winooski, VT, USA).
Immunohistochemistry of microencapsulated NPCCs and pancreas
Microencapsulated NPCCs and recipient pancreas were harvested from transplanted mice to characterize the cell proportions at 4 and 8 weeks post-transplantation. The microencapsulated NPCCs and pancreas were fixed with 4% (w/v) paraformaldehyde (PFA) and 10% (w/v) formalin, respectively. The fixed samples were embedded in paraffin and cut into 4 µm thickness.
Paraffin sections were deparaffinized and rehydrated, and then boiled to unmask the antigen in 0.01 M citric acid buffer (pH 6.0) for 20 minutes. The samples were then blocked with 1% normal donkey serum to avoid non-specific binding for 30 minutes. The samples were incubated with guinea pig-insulin primary antibody (Abcam, Cambridge, UK), diluted 1:50 for detection of insulin, mouse-CK7 primary antibody (Abcam), and diluted 1:100 for 1 hour at room temperature or overnight at 4°C. Subsequently, the slides were washed with 1× phosphate-buffered saline (PBS) three times and incubated with anti-guinea pig-Rhodamine (Rho) and anti-mouse-fluorescein isothiocyanate (FITC) as secondary antibodies for 1 hour at room temperature in a humid chamber. Samples were washed three times with 1× PBS and the nuclei were stained with DAPI. Microscopic images were acquired using a fluorescence microscope (Axiovert 200, Zeiss, Oberkochen, Germany). To measure the percentage of insulin-positive cells, we counted all the cell clusters by dyeing at least 10 slides with DAB staining kit (Vector Laboratories, Burlingame, CA, USA) following the manufacturer’s instructions. The nuclei were dyed with hematoxylin. The proportion of insulin-positive cells was calculated in terms of the number of cells dyed (brown) divided by the total nuclei (purple).
RNA extraction and real-time polymerase chain reaction
NPCCs were collected at 1, 2, 3, and 4 weeks in vitro and 4- and 8-weeks post-transplantation for RNA extraction and real-time polymerase chain reaction (PCR). Samples were fixed in 4% (w/v) PFA for 15 minutes at 4°C. Following fixation, alginate cross-linked with Ba2+ ion was removed by incubation in 0.1 M tetra-sodium ethylenediaminetetraacetic acid (EDTA) solution (pH 8.0) for 30 minutes at 37°C. The supernatant was removed and TRIzol reagent (Life Technologies, Carlsbad, CA, USA) was added to the cell pellet. Total RNA was extracted according to the manufacturer’s instructions and 1 µg of RNA was synthesized to cDNA using PrimeScript 1st strand cDNA Synthesis Kit (Takara, Kyoto, Japan). The cDNA products were diluted in 200 ng/μL of ultra-pure water, followed by quantitative real-time PCR using the Power SYBR Green PCR Master Mix (Thermo Fisher Scientific). The PCR was run on Step One Plus real-time PCR system (Thermo Fisher Scientific).
Primer sequences were: pig glyceraldehyde 3-phosphate dehydrogenase (GAPDH) 5´-GTCGGTTGTGGATCTGACCT-3´ (forward) and 5´-AGCTTGACGAAGTGGTCGTT-3´ (reverse), pig insulin 5´-CTTCGTGAACCAGCACCTGT-3´ (forward), 5´-TTGGGCGTGTAGAAGAAGCC-3´ (reverse). The quantitative polymerase chain reaction (qPCR) conditions were as follows: 40 cycles of denaturation at 95°C for 15 seconds, followed by annealing at 59°C for 1 minute. The data were analyzed using MiniOpticonTM real-time system (Bio-Rad, Hercules, CA, USA). The relative mRNA levels of insulin gene were normalized to the house keeping gene GAPDH and calculated by the ΔΔCT method.
Acridine orange/propidium iodide for viability test
Cell viability was determined by staining with acridine orange (AO) and propidium iodide (PI). At least 100 islets were stained with AO for live cells and PI for dying cells. Cells were incubated for 15 minutes in a 37°C CO2 incubator. NPCCs were obtained at 1, 2, 3, and 4 weeks in vitro and pre-transplantation. Stained cells were imaged using fluorescence microscopy.
Dithizone staining
To determine the composition of β-cells, NPCCs were stained with dithizone (Sigma-Aldrich) for 5 minutes at room temperature. Dithizone was dissolved in dimethyl sulfoxide (DMSO), followed by PBS supplementation and filtration with a 0.2 µm syringe filter before use.
Glucose-stimulated insulin secretion
To evaluate the function of β-cells, microencapsulated NPCCs were collected at 4 and 8 weeks post-transplantation. NPCCs were incubated in each of the following media for 1 hour: 0 mM glucose, 2.8 mM glucose, and 16.8 mM glucose. Supernatant was collected and stored at –20°C. NPCCs were counted to calculate the cell number.
Statistical analysis
Statistical analyses were performed using GraphPad Prism version 8.00 (GraphPad Software, San Diego, CA, USA). All data are presented as mean±standard error of the mean of at least three independent experiments. The statistical significance was determined via an unpaired t-test. A P value of <0.05 was considered significant.
Ethics statement
All procedures and the care of animals were approved by the Institutional Animal Care and Use Committee (IACUC) of the Catholic University of Korea, Seoul, Korea (Approval No. 2020-0141-05).
RESULTS
In vitro differentiation of microencapsulated NPCCs
Pancreatic and duodenal homeobox 1 (Pdx1) gene expression in the differentiated group was maintained until 3 weeks of culture while it was gradually downregulated over time in the undifferentiated group (Fig. 1A). Pdx1 mRNA levels in the differentiated group were 2.17-, 3.28-, and 2.81-fold greater at 2, 3, and 4 weeks of culture, respectively, compared with the undifferentiated group. Consistently, insulin mRNA levels were significantly higher in the differentiated group than in the undifferentiated group up to 3 weeks of in vitro culture (P<0.05), with the highest level (5.22-fold) at 2 weeks (Fig. 1B).
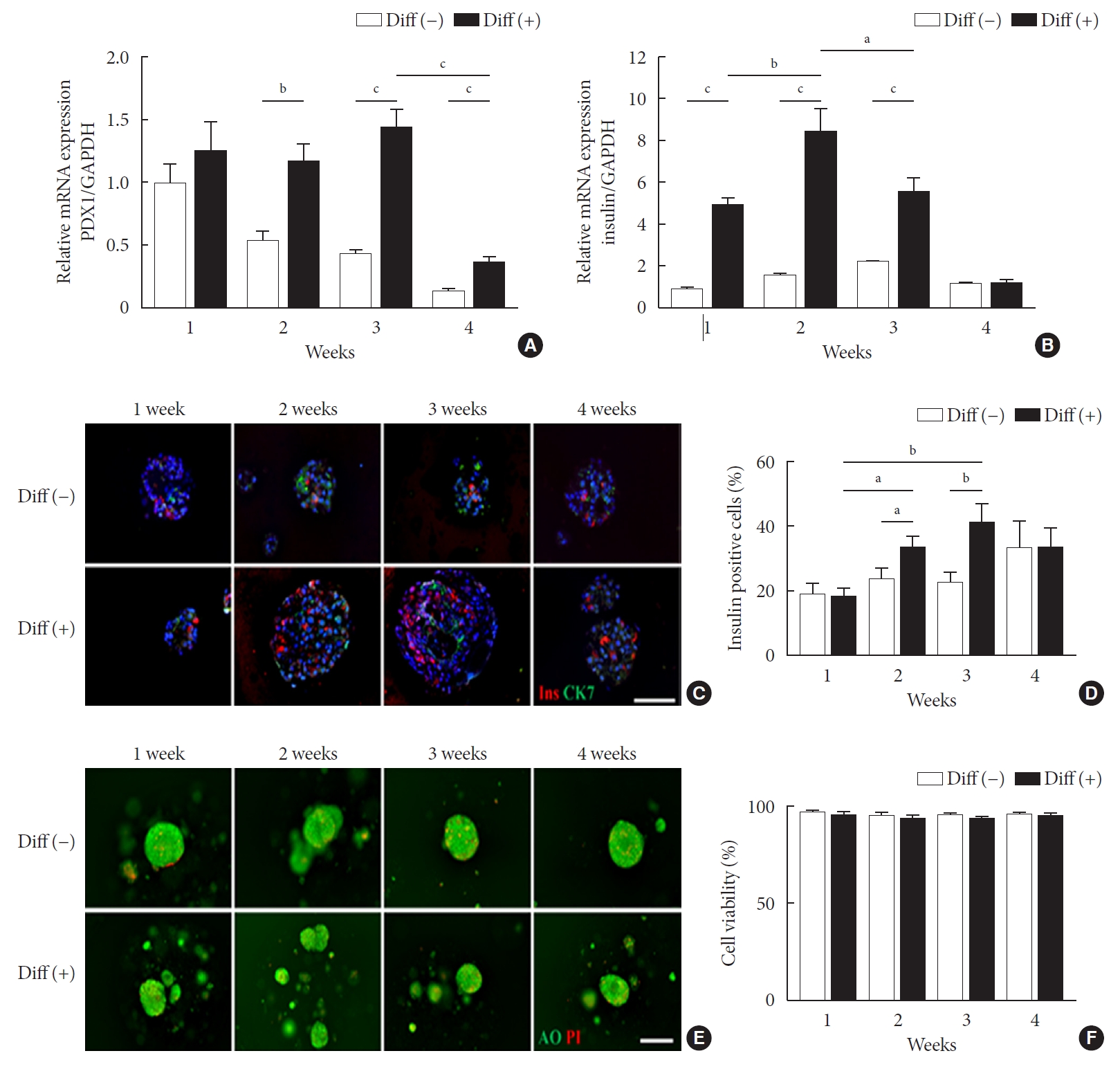
Characterization of differentiated neonatal porcine pancreatic cell clusters (NPCCs) for 4 weeks in vitro culture. Diff (–) and Diff (+) NPCCs were cultured in basal media or basal media supplemented activin receptor-like kinase 5 inhibitor II (ALK5iII), triiodothyronine (T3), and exendin-4 (Ex-4). Microencapsulated NPCCs were analyzed at 1, 2, 3, and 4 weeks in vitro. (A) Relative pancreatic and duodenal homeobox 1 (Pdx1) mRNA expressions. (B) Relative insulin mRNA expressions. (C) Immunofluorescence staining of insulin (red) and CK7 (green). The nuclei were stained with 4´,6-diamidino-2-phenylindole (DAPI, blue; scale bar, 100 µm; ×200). (D) The percentage of insulin-positive cells was counted on DAB-stained slides. (E) Staining by acridine orange (AO, green) and propidium iodide (PI, red) shows cell viability (scale bar, 100 µm; ×200). (F) The cell viability was calculated by PI-positive cells/total cells. Values are presented as mean±standard error of the mean (n=3). Diff (–), undifferentiated NPCC; Diff (+), differentiated NPCC; GAPDH, glyceraldehyde 3-phosphate dehydrogenase. aP<0.05, bP<0.01, cP<0.001.
To determine whether microencapsulated NPCCs differentiated into β-cells in vitro, the composition of NPCCs was assessed via immunostaining with guinea pig anti-insulin (red) and mouse anti-CK7 (green) antibodies. As shown in Fig. 1C and D, insulin-positive β-cells were significantly increased between 2 and 3 weeks in the differentiated group compared with the undifferentiated group (1.41-fold and 1.82-fold higher at 2 and 3 weeks of culture, respectively, both P<0.05). In contrast, CK7-positive ductal cells were continuously decreased over time in both groups (Fig. 1C).
To investigate whether ALK5iII, T3, and Ex-4 treatments with microencapsulation affected cell viability, the microencapsulated NPCCs were stained with AO/PI. Microencapsulated NPCCs in both groups showed more than 95% viability during 4 weeks of culture (Fig. 1E and F), which suggested that cell viability was not compromised by exposure to differentiating chemicals or microencapsulation procedures.
Transplantation of microencapsulated NPCCs with and without differentiation in diabetic BALB/c mice
Fig. 2A presents the experimental scheme for in vivo experiments. Microencapsulated NPCCs were divided into control (control for culture days), undifferentiated (control for differentiation), and differentiated groups. After a stabilization period of 2 days, the control group was cultured in basal media for another 5 days before transplantation. The undifferentiated and differentiated groups were cultured in basal and differentiation media, respectively, for 2 weeks before transplantation (Fig. 2A). In the differentiated group, blood glucose levels started to decrease from 3 weeks of transplantation and normalized at 7 weeks in all mice (Fig. 2B). In contrast, blood glucose levels in the control and undifferentiated groups did not decrease until 8 weeks of transplantation. The mean blood glucose levels at 8 weeks of transplantation were 392.8±49.83, 366.7±21.84, and 196.5±21.92 mg/dL in the control, undifferentiated, and differentiated groups, respectively.
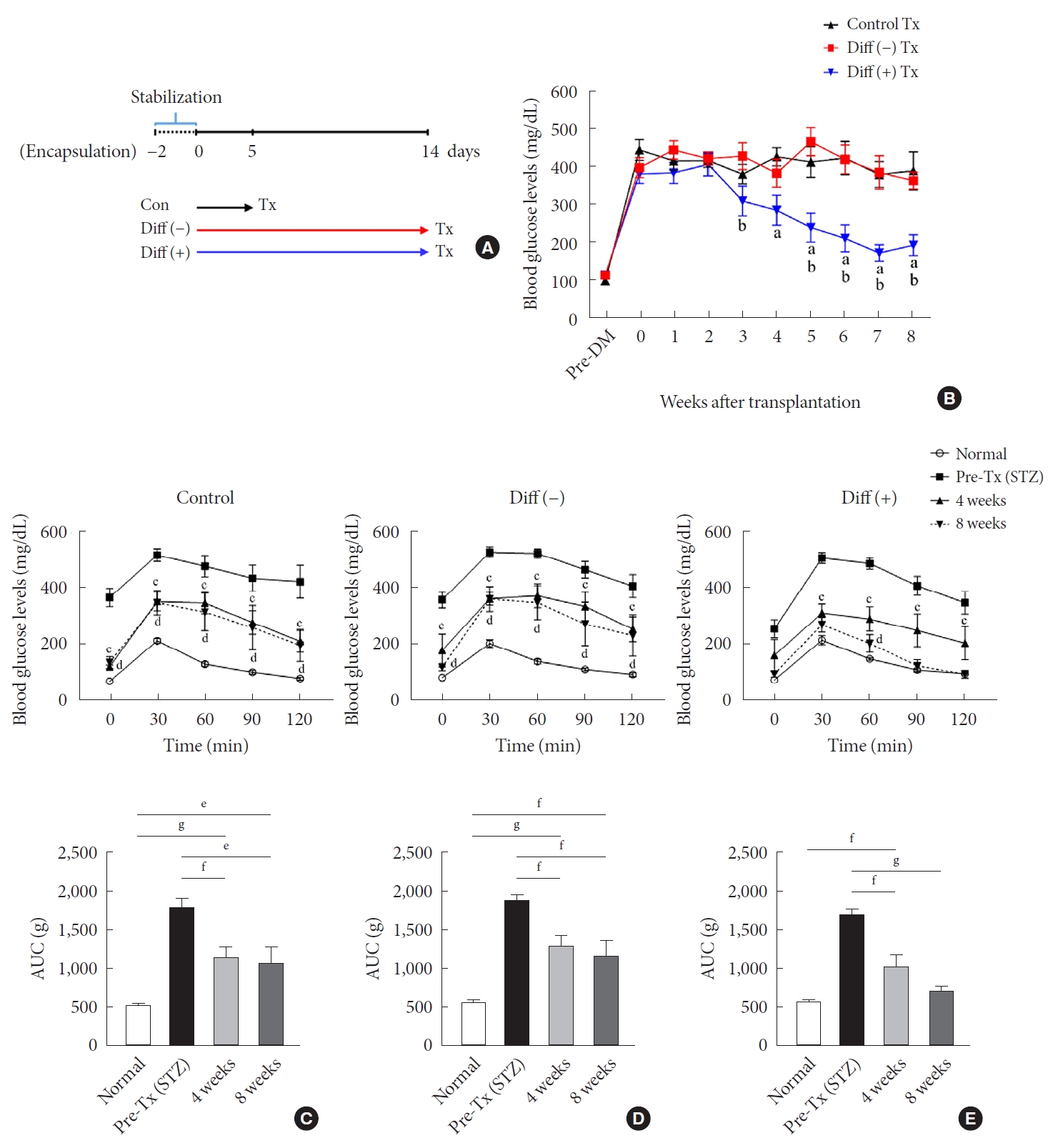
Transplantation (Tx) into diabetic mice and follow-up for 8 weeks post-Tx. (A) Scheme of the in vivo experimental design. (B) Blood glucose levels of recipient mice transplanted with 8,000 islet equivalent (IEQ) of neonatal porcine pancreatic cell clusters (NPCCs) into intraperitoneal (n=10 for control, n=14 for Diff [–], n=14 for Diff [+]). Intraperitoneal glucose tolerance test (IPGTT; top) and area under the curve (AUC; bottom) for (C) control, (D) Diff (–), and (E) Diff (+). Values are presented as mean±standard error of the mean. DM, diabetes mellitus; STZ, streptozotocin. aP<0.05 for control vs. differentiated group, bP<0.05 for undifferentiated group vs. differentiated group, cP<0.05 for normal vs. 4 weeks, dP<0.05 for normal vs. 8 weeks, eP<0.05, fP<0.01, gP<0.001.
IPGTT and serum insulin assays were performed 4 and 8 weeks after transplantation. In the control group, glucose tolerance was significantly improved at 4 and 8 weeks of transplantation without reaching normal levels (Fig. 2C). There was no difference in the levels of glucose tolerance between 4 and 8 weeks. Similar results were observed in the undifferentiated group (Fig. 2D). In the differentiated group, glucose tolerance improved further compared with the other two groups, resulting in near-normal glucose tolerance at 8 weeks of transplantation (Fig. 2E). A slight difference in glucose tolerance was found among all groups 4 weeks after transplantation (Supplementary Fig. 1A and C). The control and undifferentiated groups were similar at 8 weeks after transplantation and the differentiated group showed a lower level than the others, without statistical significance (Supplementary Fig. 1B and D). However, the basal serum insulin levels in the differentiated group were significantly higher than in the other groups at 8 weeks after transplantation (Supplementary Fig. 2).
Comparison of dithizone and immunofluorescence staining results of retrieved microencapsulated NPCCs with and without differentiation
In all groups, microcapsules harvested at 4 and 8 weeks after transplantation exhibited intact morphology without any surface fibrosis, and NPCCs were well preserved within the microcapsule (Fig. 3A). Consistently, all microencapsulated NPCCs were positive for dithizone staining regardless of groups. Over time, NPCCs stained dark red more frequently, especially in the differentiated group (Fig. 3A). This finding suggests that the NPCCs containing immature endocrine cells differentiated into β-cells in vivo.

Dithizone staining and cell composition of neonatal porcine pancreatic cell clusters (NPCCs) after transplantation (Tx). NPCCs pre-Tx and grafts 4, 8 weeks post-Tx were harvested and analyzed. (A) Dithizone staining (red) (scale bar, 200 µm; ×100). (B) Immunofluorescence staining of insulin (red) and CK7 (green) in NPCCs pre-Tx, grafts 4 and 8 weeks post-Tx. The nuclei were stained with 4´,6-diamidino-2-phenylindole (DAPI, blue; scale bar, 100 µm; ×200). (C) The percentage of insulin-positive cells was counted on DAB-stained slides. Values are presented as mean±standard error of the mean. aP<0.05, bP<0.01.
Immunohistochemistry showed an increase in the number of insulin-positive cells with time in all groups (Fig. 3B and C). However, the differentiated group contained notably higher levels of insulin-positive cells than the other two groups before and after transplantation. Before transplantation, insulin-positive cells in the differentiated group were around 1.5-fold higher than in the other two groups. At 8 weeks after transplantation, approximately 70% of all NPCCs were insulin-positive in the differentiated group, which was 1.67- and 1.56-fold greater than in the control and undifferentiated group, respectively.
Assessment of β-cell function in retrieved microencapsulated NPCCs
As shown in Fig. 4A, insulin mRNA levels in grafts increased over time in all groups. In the control group, insulin mRNA levels varied insignificantly between pre-transplantation and the 4th week after transplantation but increased in the 8 weeks post transplantation. In the case of the undifferentiated group, insulin gene expression was upregulated at weeks 4 and 8 after transplantation compared with before transplantation; however, the expression at week 8 after transplantation was not higher than the level at week 4. Insulin mRNA in grafts derived from the differentiated group was substantially higher than in the other two groups, both at 4 and 8 weeks following transplantation. By week 4, the insulin mRNA of the differentiated group was 8.8-fold that of control and 1.2-fold higher than in undifferentiated group. By week 8, the insulin mRNA of the differentiated group was 9.7-fold higher than in the control and 4.6-fold higher than in the undifferentiated group. After 8 weeks of transplantation, the mRNA of the differentiation group increased 5-fold in 4 weeks (Fig. 4A).
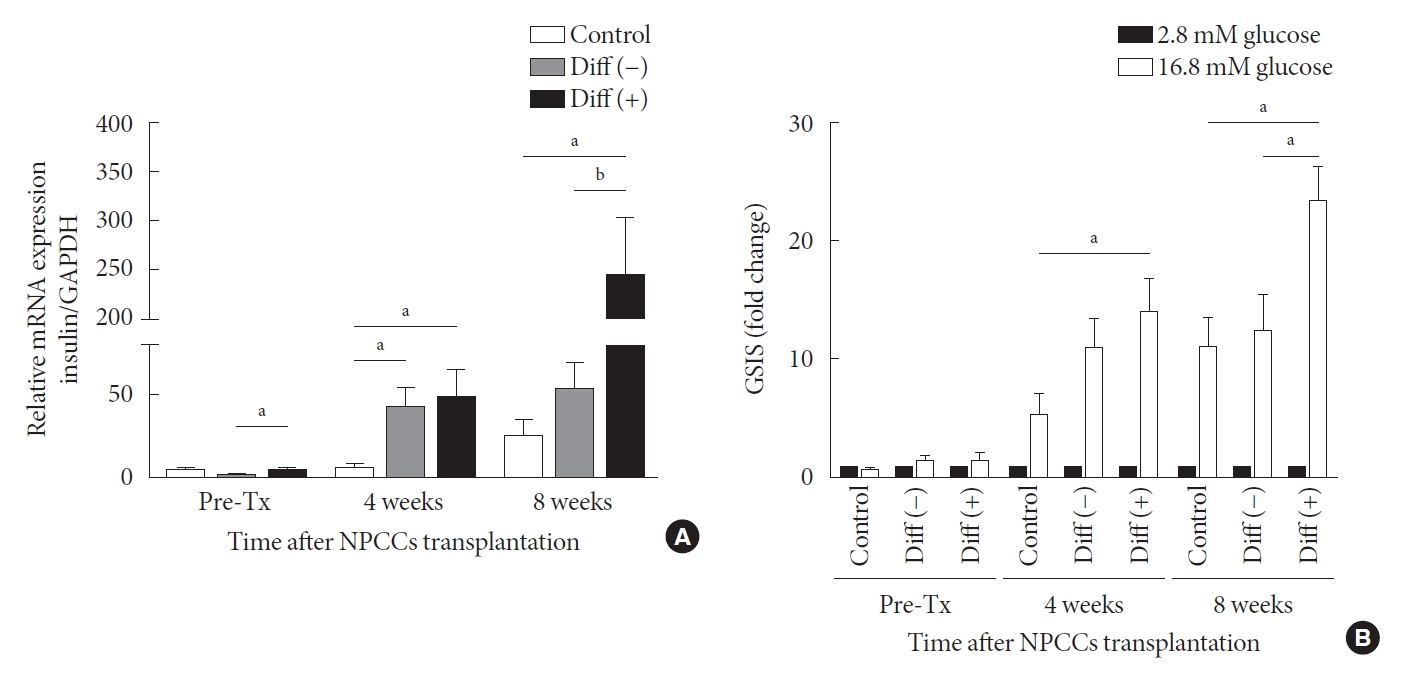
Gene expression and function test of neonatal porcine pancreatic cell clusters (NPCCs) harvested from recipient mice. (A) Insulin mRNA expression of pre-transplantation (Tx) NPCCs, grafts 4 and 8 weeks post-Tx. (B) Insulin secretion of pre-Tx NPCCs and grafts from recipient mice 4 and 8 weeks post-Tx. Values are presented as mean±standard error of the mean. Diff (–), undifferentiated NPCC; Diff (+), differentiated NPCC; GAPDH, glyceraldehyde 3-phosphate dehydrogenase. aP<0.05, bP<0.01.
Glucose-stimulated insulin secretion (GSIS) is a key metabolic test for evaluating graft function and it is critical to determine whether graft response to high glucose. Insulin secretion does not differ before transplantation among all groups. However, at 4 weeks post-transplantation, graft insulin secretion in the differentiated group was higher than in the control and undifferentiated group (Fig. 4B). After 8 weeks of transplantation, graft insulin secretion in the differentiated group was nearly twice that of the control and the undifferentiated groups. The data indicate that ALK5iII, T3, and Ex-4 induce the maturation of β-cells, increasing their responsiveness to glucose.
DISCUSSION
In this study, we demonstrated that microencapsulated NPCCs were efficiently and rapidly differentiated into functional β-cells by chemicals treatment for 2 weeks in vitro. Further, in vitro differentiation of microencapsulated NPCCs significantly improved glycemic control with a marginal number of clusters, resulting in near normal glucose tolerance at 7 weeks of transplantation in immune-competent diabetic mice. These findings suggest that in vitro differentiation of microencapsulated NPCCs can overcome both the delayed improvement of glycemic control and the xenogeneic immune reaction induced by NPCCs.
NPCCs are a mixture of cellular clusters composed of duct cells, bipotent duct progenitor cells, endocrine progenitor cells, immature β-cells, and β-cells [17,18]. Although NPCCs have the potential of expansion and differentiation, their use as an alternative source of β-cells is limited by their immature nature. Previous studies have reported that it takes 12 to 20 weeks to lower the blood glucose level after transplantation of NPCCs into diabetic mice [19,20]. Therefore, efforts to accelerate the differentiation of NPCCs entailed co-culture with other cells, such as mesenchymal stem cells and Sertoli cells, or chemicals such as necrostatin and glucagon-like peptide 1 [9,21,22]. In this study, we used ALK5iII, T3, and Ex-4 to facilitate the differentiation of NPCCs into β-cells. These small molecules, which are associated with functional maturation of β-cells, are used in the final stages of the protocol to generate insulin-producing β-like cells. ALK5iII, a TGF-β inhibitor, prevents the dedifferentiation of β-cells and upregulates the expression of β-cell-specific genes such as MafA, Nkx6.1, and Pdx1 [23]. T3 plays an important role in the development of pancreas, including endocrine cell differentiation via the induction of the MafA gene [24]. Alk5iII, T3 are used in the step of differentiation from ‘pancreatic progenitor to endocrine progenitor’ and from ‘endocrine progenitor to β-cells.’ ALK5iII and T3 induce differentiation to the endocrine lineage from the pancreatic progenitor stage [25,26]. Ex-4 is a glucagon-like peptide 1 (GLP-1)-related peptide derived from lizard venoms and binds to the GLP-1 receptors. Ex-4 has been reported to stimulate differentiation of β-cells from ductal progenitor cells and it has also been reported to promote β-cell proliferation [27]. In rodents, Ex-4 stimulates β-cell proliferation, increases β-cell mass and islet size via epidermal growth factor receptor 1 (EGFR) [27,28]. Especially, in pre-weaned porcine islets, Ex-4 effectively improved the islet yield and function [29]. Taken together, Alk5iII, T3, and Ex-4 are thought to have affected the differentiation stage from the pancreatic progenitor cell (bipotent trunk cell) to β-cell and also promoted β-cell proliferation.
In general, insulin-positive cells are rarely observed in NPCCs. In this study, we showed a significant increase (up to 40%) of insulin-positive cells after in vitro differentiation. Similar to our results, Hassouna et al. [10] showed that the proportion of insulin-positive cells increased approximately 40% with stepwise in vitro differentiation. However, they reversed hyperglycemia at 18 weeks of transplantation, while we normalized blood glucose levels at 7 weeks post-transplantation with a marginal number of NPCCs. In addition, they did not show any temporal variation in normal glucose levels between control and differentiation groups. Lopez-Avalos et al. [20] attempted to induce the maturation of microencapsulated NPCCs using growth factors in vitro, but failed to improve transplantation efficacy compared with control group. Our data showed that the differentiation of microencapsulated NPCCs can be induced by chemical treatment.
During in vitro differentiation, both insulin mRNA expression and insulin-positive cells significantly increased, indicating the growth capacity of NPCCs. β-Cell expansion in NPCCs occurs via neogenesis, in which endocrine cells differentiate into β- or α-cells during pancreatic development, as well as the proliferation of pre-existing β-cells [30]. In this study, CK7-positive cells decreased, while insulin-positive cells increased over time, which suggests the possible differentiation of pancreatic ductal cells into endocrine cells. The number of insulin-positive cells and insulin mRNA levels in the differentiated group gradually increased until 2 to 3 weeks of in vitro culture but decreased at week 4. Similarly, Montanari et al. [31] reported that the insulin content of NPCCs cultured in vitro increased until 2 weeks and then decreased thereafter. In addition, a sustained decrease in insulin secretion occurred during 3 weeks of in vitro culture of NPCCs. Taken together, these results suggest that long-term in vitro culture of NPCCs may have a negative effect on β-cell differentiation and the maximal maturation in vitro might occur within 2 weeks.
To prevent immune rejection, which is a major obstacle of xenotransplantation, we used microencapsulation with alginate. The surface of the microcapsule consists of a passive permeable membrane, which facilitates the diffusion of nutrients, oxygen and small peptides such as insulin, while effectively preventing the entry of immune cells and related proteins such as immunoglobulin G [32]. Besides, microencapsulation also maintains cell morphology, enabling long-term cell culture, and effective retrieval after transplantation [33]. We demonstrated the successful transplantation of microencapsulated NPCCs into immune-competent diabetic mice. Furthermore, we showed improved transplantation efficacy with a marginal dose of NPCCs (8,000 IEQ) via in vitro differentiation, compared with the undifferentiated group. We used a marginal dose of NPCCs (8,000 IEQ) to maximize the effects induced by in vitro differentiation. Omer et al. [34] demonstrated that an adequate number of microencapsulated NPCCs (10,000 IEQ) could reverse diabetes within a month. We showed better graft function via GSIS test (20-fold at 8 weeks vs. 3-fold at 20 weeks after transplantation), and possibly comparable or better results with 10,000 IEQ of NPCCs. Notably, there was no difference in increased insulin secretion in response to high glucose after transplantation between groups with and without differentiation (both two-fold higher than pre-transplantation levels). Therefore, pre-transplant differentiation status might be important in determining graft patency after transplantation.
As studies involved in NPCCs use different cell numbers, sites, and techniques for transplantation, further studies are needed to compare the accuracy of in vitro differentiation of NPCCs. We observed central necrosis in a few cells at 16 weeks of transplantation (data not shown), which may be attributed to insufficient oxygen levels at the center of the cell cluster. Thus, clinical applications underscore the need for further studies to overcome hypoxia and investigations over a longer period of time.
To the best of our knowledge, this is the first study to induce the differentiation of microencapsulated NPCCs using chemicals in vitro. Supplementation of ALK5iII, T3, and Ex-4 increased β-cell mass and insulin mRNA in vitro and induced functional maturation in vivo after transplantation. The differentiated NPCCs rapidly reversed hyperglycemia and improved transplantation efficacy without immune response in diabetic mice.
SUPPLEMENTARY MATERIALS
Supplementary materials related to this article can be found online at https://doi.org/10.4093/dmj.2021.0202.
Intraperitoneal glucose tolerance test (IPGTT) and area under the curve (AUC). (A) IPGTT in 4 weeks after transplantation (n=4 for control [Con]; n=7 for Diff [–]; n=6 for Diff [+] group). (B) IPGTT in 8 weeks after transplantation (n=6 for Con; n=6 for Diff [–] ; n=5 for Diff [+] group). (C) AUC of IPGTT in 4 weeks after transplantation. (D) AUC of IPGTT in 8 weeks after transplantation. Diff (–), undifferentiated neonatal porcine pancreatic cell cluster (NPCC); Diff (+), differentiated NPCC.
Serum insulin levels from BALB/c mice at 4 and 8 weeks of transplantation (n=6, 6 for empty capsule transplantation; n=5, 4 for control group; n=5, 4 for undifferentiated group; n=4, 3 for differentiated group, 4 and 8 weeks of transplantation, respectively). Diff (–), undifferentiated neonatal porcine pancreatic cell cluster (NPCC); Diff (+), differentiated NPCC. aP<0.05.
Notes
CONFLICTS OF INTEREST
Kun-Ho Yoon was the publisher of the Diabetes & Metabolism Journal from 2020 to 2021. He was not involved in the review process of this article. Otherwise, there was no conflict of interest.
AUTHOR CONTRIBUTIONS
Conception or design: G.J.C., J.W.K., K.H.Y.
Acquisition, analysis, or interpretation of data: G.J.C., H.S.P., M.J.K., Y.H.Y., M.R.
Drafting the work or revising: G.J.C., E.Y.L., K.H.Y.
Final approval of the manuscript: G.J.C., K.H.Y.
FUNDING
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2019R1I1A1A01059818), Cooperative Research Program for Agriculture Science and Technology Development Rural Development Administration, Republic of Korea (PJ01345301).
Acknowledgements
None
