Reproductive Life Span and Severe Hypoglycemia Risk in Postmenopausal Women with Type 2 Diabetes Mellitus
Article information
Abstract
Background
Estrogen promotes glucose homeostasis, enhances insulin sensitivity, and maintains counter regulatory responses in recurrent hypoglycemia in women of reproductive age. Postmenopausal women with type 2 diabetes mellitus (T2DM) might be more vulnerable to severe hypoglycemia (SH) events. However, the relationship between reproductive factors and SH occurrence in T2DM remains unelucidated.
Methods
This study included data on 181,263 women with postmenopausal T2DM who participated in a national health screening program from January 1 to December 31, 2009, obtained using the Korean National Health Insurance System database. Outcome data were obtained until December 31, 2018. Associations between reproductive factors and SH incidence were assessed using Cox proportional hazards models.
Results
During the mean follow-up of 7.9 years, 11,279 (6.22%) postmenopausal women with T2DM experienced SH episodes. A longer reproductive life span (RLS) (≥40 years) was associated with a lower SH risk compared to a shorter RLS (<30 years) (adjusted hazard ratio [HR], 0.74; 95% confidence interval [CI], 0.69 to 0.80; P for trend <0.001) after multivariable adjustment. SH risk decreased with every 5-year increment of RLS (with <30 years as a reference [adjusted HR, 0.91; 95% CI, 0.86 to 0.95; P=0.0001 for 30–34 years], [adjusted HR, 0.80; 95% CI, 0.76 to 0.84; P<0.001 for 35–39 years], [adjusted HR, 0.74; 95% CI, 0.68 to 0.81; P<0.001 for ≥40 years]). The use of hormone replacement therapy (HRT) was associated with a lower SH risk than HRT nonuse.
Conclusion
Extended exposure to endogenous ovarian hormone during lifetime may decrease the number of SH events in women with T2DM after menopause.
INTRODUCTION
Reproductive life span (RLS), which is the time period between menarche and menopause, reflects the total period of life in which a woman is exposed to sex hormones. Unlike progesterone, the endogenous ovarian hormone estrogen has protective roles in female bone health, cardiovascular protection, and metabolic homeostasis [1,2]. Therefore, RLS represents not only the years during which women can conceive, but also the period in which women benefit from estrogen exposure. RLS has been related to decreased morbidity, decreased mortality, and cardioprotection [3,4].
Severe hypoglycemia (SH), a hypoglycemic episode requiring the assistance of another person to administer treatment [5], is a troublesome and serious complication in patients with type 2 diabetes mellitus (T2DM) [6]. Patients with long-term T2DM who experience recurrent hypoglycemia with insulin or sulfonylurea treatment usually exhibit blunted or impaired counterregulatory responses to hypoglycemia and have an increased risk of SH [5]. Although the incidence rate of SH is low in T2DM [7], its occurrence can be fatal due to the risk of loss of consciousness, falls, seizures, motor vehicle accidents, coma, and death [8]. Therefore, the identification of risk factors and establishment of preventive strategies for SH in patents with T2DM are urgently required.
The effect of endogenous ovarian hormone on glucose metabolism or insulin action in women with T2DM has not been fully elucidated. Estrogen promotes glucose homeostasis, enhances insulin sensitivity [9], and maintains corticosterone secretory responses in recurrent hypoglycemia [10]. Thus, the beneficial effect of estrogen on glucose or insulin, or its counterregulatory action in women of reproductive age might disappear after menopause in women with T2DM. Moreover, postmenopausal women with T2DM who are at high risk for SH might be more vulnerable to SH events [11,12]. However, the relationship between RLS and SH occurrence in T2DM has not been investigated.
Thus, we aimed to clarify the association between RLS and SH in T2DM and the potential effect of hormone replacement therapy (HRT) on the occurrence of SH in postmenopausal T2DM using a National Health Insurance Service (NHIS) claim database.
METHODS
Data source and study setting
In this study, we used a single-insurer system managed by the government, the Korean NHIS claim database, which provides mandatory comprehensive medical care to 97% of the Korean population and medical aid to 3% of the population with the lowest incomes [13]. The NHIS also provides free biennial national health screenings to all Korean individuals aged ≥40 years and all employees regardless of age, as well as annual health screenings for physical laborers. In addition, the NHIS maintains the National Cancer Screening Program, which includes biennial screening for breast and cervical cancers in every woman aged ≥40 and ≥20 years, respectively. The screening program for breast and cervical cancers includes a self-administered questionnaire about reproductive factors such as parity, duration of breastfeeding, age at menarche, age at menopause, whether the participant had undergone hysterectomy, history of HRT use and its duration, and history of oral contraceptive (OC) use and its duration. All participants were asked to answer and submit the self-administered questionnaire on the day of examination.
Based on an approximate participation rate of 70% [14], the NHIS maintains an extensive health information database that consists of a qualification database (e.g., age, sex, income, region, and type of eligibility), a claim database including diagnosis statements defined by the International Classification of Disease 10th revision (ICD-10), prescription statements, a health check-up database, and death information [15].
Study population
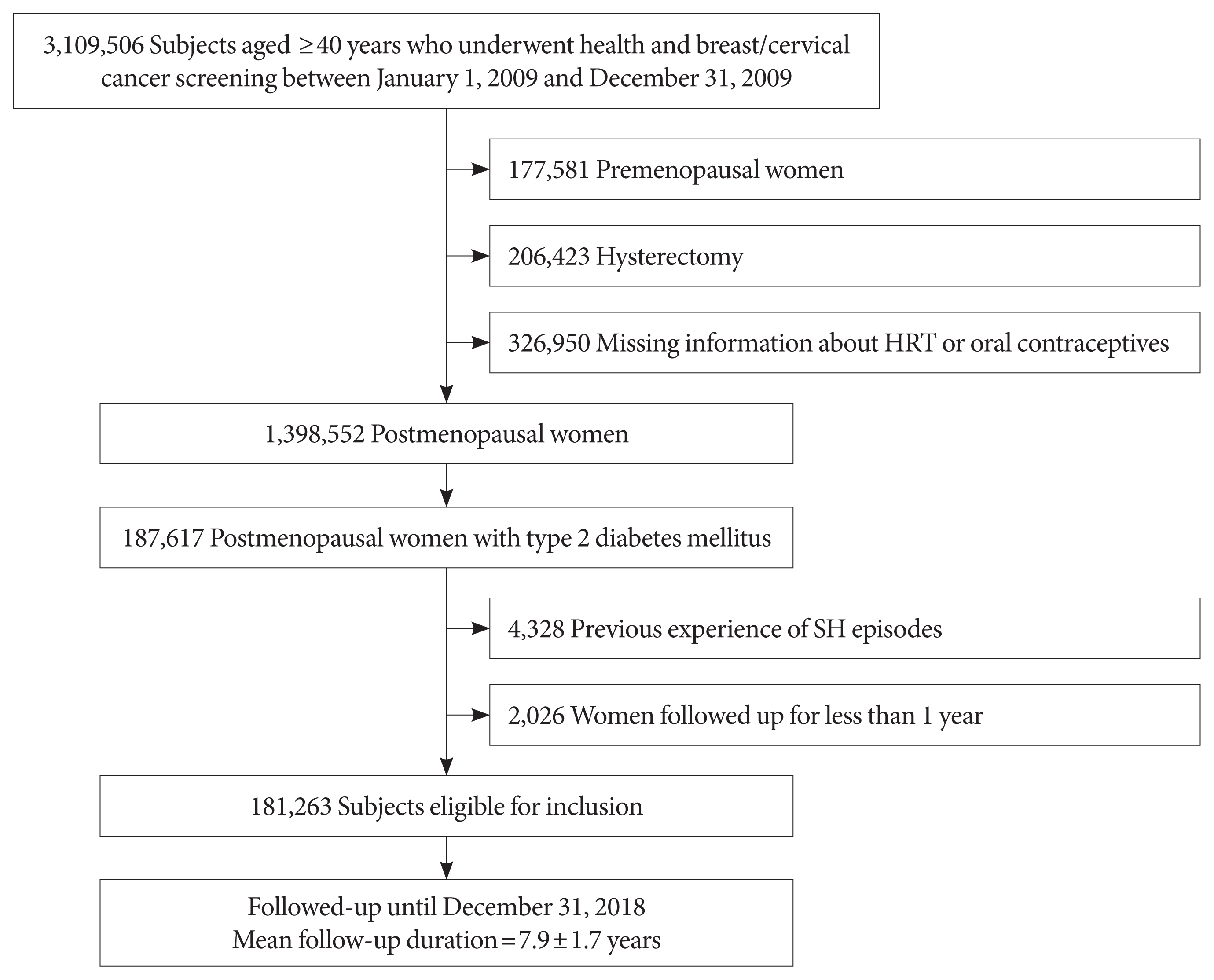
Among the 3,109,506 women aged ≥40 years who simultaneously underwent both health and breast and cervical cancer screening from January 1, 2009 to December 31, 2009, we identified 1,725,502 postmenopausal women and excluded those who reported having a hysterectomy because most did not know whether they also had oophorectomy. We also excluded 326,950 women with missing information about whether they used HRT or OCs.
Of 1,398,552 postmenopausal women, 187,617 women were classified as having T2DM (1) if they had at least one claim with a diagnosis of diabetes based on ICD-10 (E11–14) and were prescribed at least one antidiabetic drug at any time over a 1 year period, or (2) if they were newly diagnosed with diabetes showing a fasting plasma glucose ≥126 mg/dL during a health examination [15].
After excluding those who had experienced SH episodes according to ICD-10 codes prior to the health examination date, 181,263 eligible postmenopausal women with diabetes aged ≥40 were enrolled as the study population (Fig. 1). The cohort was followed up from baseline to the date of SH episode or time of death or until the end of the study period (December 31, 2018), whichever came first. This study was approved by the Institutional Review Board of Catholic Medical Center (VC19ZESI0004). The review board waived written informed consent because the data were public and anonymized under confidentiality guidelines.
Exposure: reproductive factors
The ages at menarche in the Korean population were categorized into ≤12, 13–14, 15–16, and ≥17 years groups. Menopause was defined as having 12 months of consecutive amenorrhea without apparent cause, such as pregnancy or lactation [16], while the age at menopause was categorized into <40, 40–44, 45–49, 50–54, and ≥55 years. Early menopause was defined as menopause before the age of 45 years.
The RLS, which reflects endogenous estrogen exposure during an individual’s entire life, was estimated by the total reproductive period, which was defined as the age at menarche subtracted from the age of menopause and categorized as <30, 30–34, 35–39, or ≥40 years. A shorter period of total reproductive years was defined as ≤30 years.
Parity was categorized as never, 1, or ≥2 children. Total lifetime breastfeeding history was categorized as never, <6, 6–11, and ≥12 months. The duration of HRT was categorized as never, <2, 2–5, or ≥5 years. The duration of OC use was categorized as never, <1, or ≥1 year.
Study outcome: SH case ascertainment
The primary outcome was newly diagnosed SH during the follow-up period. According to ICD-10 based diagnostic clinical codes for SH [17–20], postmenopausal women with T2DM who had any claims for E1163−, E1463–, E160–, E161–, and E162–from inpatient, outpatient, or emergency room since the date of the health examination were classified as having experienced SH episodes. Only the first SH episodes within the observation period were included.
Covariates
Body mass index (BMI) was calculated as the patient’s weight in kilograms divided by square of the patient’s height in meters. Information on each individual’s lifestyle was obtained from the National Cancer Screening Program self-questionnaire. Smoking status was classified into non-, ex-, and current smokers. Individuals who consumed at least 30 g of alcohol per day were defined as heavy alcohol consumers [21]. Regular exercise was defined as performing >30 minutes of moderate physical activity at least five times per week or >20 minutes of strenuous physical activity at least three times per week [17]. Comorbidities including hypertension, dyslipidemia, and cancer were based on ICD-10 codes from past medical history [22]. The estimated glomerular filtration rate was calculated using the Modification of Diet in Renal Disease formula. An estimated glomerular filtration rate <60 mL/min/1.73 m2 was considered indicative of chronic kidney disease. The use of insulin, the use of insulin secretagogues such as sulfonylureas and glinides, and the simultaneous use of three or more oral hypoglycemic agents for glycemic control were defined using claims data. Blood samples for the measurement of serum fasting glucose and lipid levels were drawn after an overnight fast. The hospitals where these health examinations were performed were certified by the NHIS and subjected to regular quality control [13].
Statistical analysis
Continuous variables are presented as mean±standard deviation, and categorical variables are presented as numbers and percentages. Incidence rates of SH were expressed as the number of events per 1,000 person-years. Using Cox proportional hazards regression analysis, we calculated hazard ratios (HRs) and 95% confidence intervals (CIs) for the associations between various reproductive factors and the incidence of SH. Proportional hazards assumptions were evaluated using Schoenfeld residuals with the logarithm of the cumulative hazards function based on Kaplan–Meier estimates for reproductive factors. We did not detect any significant departures from proportionality in hazards over time.
Potential confounders or effect modifiers were identified a priori based on literature review and presumed causal relationships among the covariates. Model 1 was non-adjusted, whereas Model 2 was adjusted for age at menarche, age at menopause, number of parities, duration of breastfeeding, duration of HRT, duration of OC use, alcohol consumption, smoking, regular exercise, income, BMI, hypertension, dyslipidemia, use of three or more oral hypoglycemic agents, insulin treatment, and use of insulin secretagogues such as sulfonylureas and glinides. Model 3 was adjusted for RLS. Tests for linear trend across categories of reproductive factors were performed by modeling an ordinal variable for each category of reproductive factors. Statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
RESULTS
Baseline characteristics
During the mean follow-up of 7.9±1.7 years, 11,279 of 181,263 postmenopausal women with diabetes had a newly identified SH event, corresponding to an incidence rate of 7.9 per 1,000 person-years. The baseline characteristics of the study population according to SH incidence are shown in Table 1. Compared with the patients without SH events, patients with SH events were older (64.33±7.83 years vs. 68.89±7.24 years), had a lower BMI (25.23±3.39 kg/m2 vs. 24.99±3.52 kg/m2), had a higher systolic blood pressure (129.88±16.32 mm Hg vs. 131.41± 16.99 mm Hg), participated in fewer regular physical activities (18.68% vs. 13.57%), were more often on insulin treatment (11.46% vs. 25.90%), were more often on treatment with insulin secretagogues such as sulfonylureas (81.26% vs. 61.19%) and glinides (6.04% vs. 3.63%), had been taking three or more oral hypoglycemic agents (24.32% vs. 42.11%), had more comorbidities such as hypertension (69.90% vs. 81.16%) and chronic kidney disease (17.68% vs. 32.31%), had a more frequent past history of stroke (4.20% vs. 2.55%), heart disease (10.67% vs. 7.45%), and cardiovascular disease (14.24% vs. 9.63%) in analysis, except for those who did not respond, had a lower estimated glomerular filtration rate (79.73±28.68 mL/min/1.73 m2 vs. 71.80±25.82 mL/min/1.73 m2), higher fasting blood glucose level (137.96±43.13 mg/dL vs. 141.86±56.47 mg/dL), lower cholesterol level (202.74±49.14 mg/dL vs. 200.50±55.46 mg/dL), and lower low-density lipoprotein level (119.18±80.25 mg/dL vs. 115.45±109.06 mg/dL) (P<0.0001 for all).
With regard to reproductive factors, differences were observed between the two groups. Compared with the patients without SH events, those with SH events showed a later mean age at menarche (16.58±1.82 years vs. 16.82±1.77 years), and an earlier mean age at menopause (50.09±4.32 years vs. 49.45± 4.61 years), resulting in a shorter mean RLS (33.51±4.71 years vs. 32.63±4.98 years) (P<0.0001 for all). The proportion of HRT use was higher in patients without SH events than in patients with SH events (12.26% vs. 7.00%) (Table 1).
Risk of SH events according to reproductive factors
Menstrual history
A longer RLS of 40≥ years was associated with a lower risk of SH events (adjusted HR, 0.74; 95% CI, 0.68 to 0.81; P for trend <0.001) than a short RLS of <30 years after adjusting for age, BMI, hypertension, dyslipidemia, fasting glucose level, current smoking, alcohol consumption, regular exercise, use of insulin or insulin secretagogues such as sulfonylureas and glinides, multiple oral hypoglycemic agent use, chronic kidney disease, HRT, and OC use (Table 2). The inverse association between longer RLS and risk of SH event was more evident in younger women (aged <65 years) than in older women (aged ≥65 years) (P for interaction=0.0032; P for trend=0.0005) (Table 3 and Supplementary Fig. 1). In addition, patients aged ≥55 years at menopause had a lower risk of SH events than those aged <40 years at menopause (adjusted HR, 0.73; 95% CI, 0.65 to 0.83; P for trend <0.001) after multivariable adjustment (Table 2). The incidence rate of SH and its adjusted HR tended to decrease with increasing RLS (P for trend <0.0001) (Fig. 2). The cumulative incidence of SH for up to 8 years according to reproductive factors is shown using Kaplan–Meier curves (Fig. 3). The cumulative incidence of SH events increases with increasing years of age at menarche and decreasing years of age at menopause and RLS (all log-rank P<0.0001).
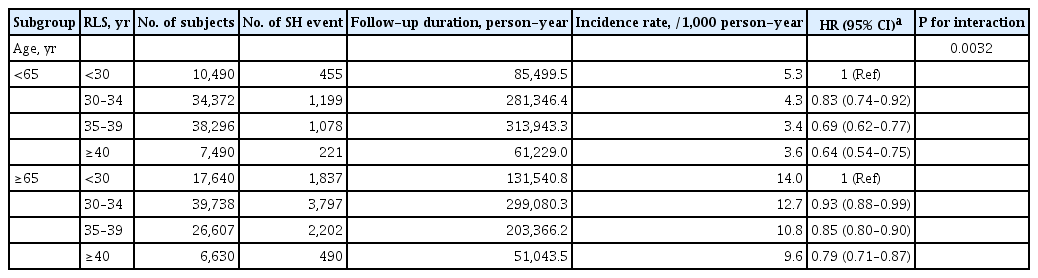
Subgroup analysis for hazard ratios of severe hypoglycemia by reproductive factors for subgroup aged <65, and ≥65 in postmenopausal women with type 2 diabetes mellitus
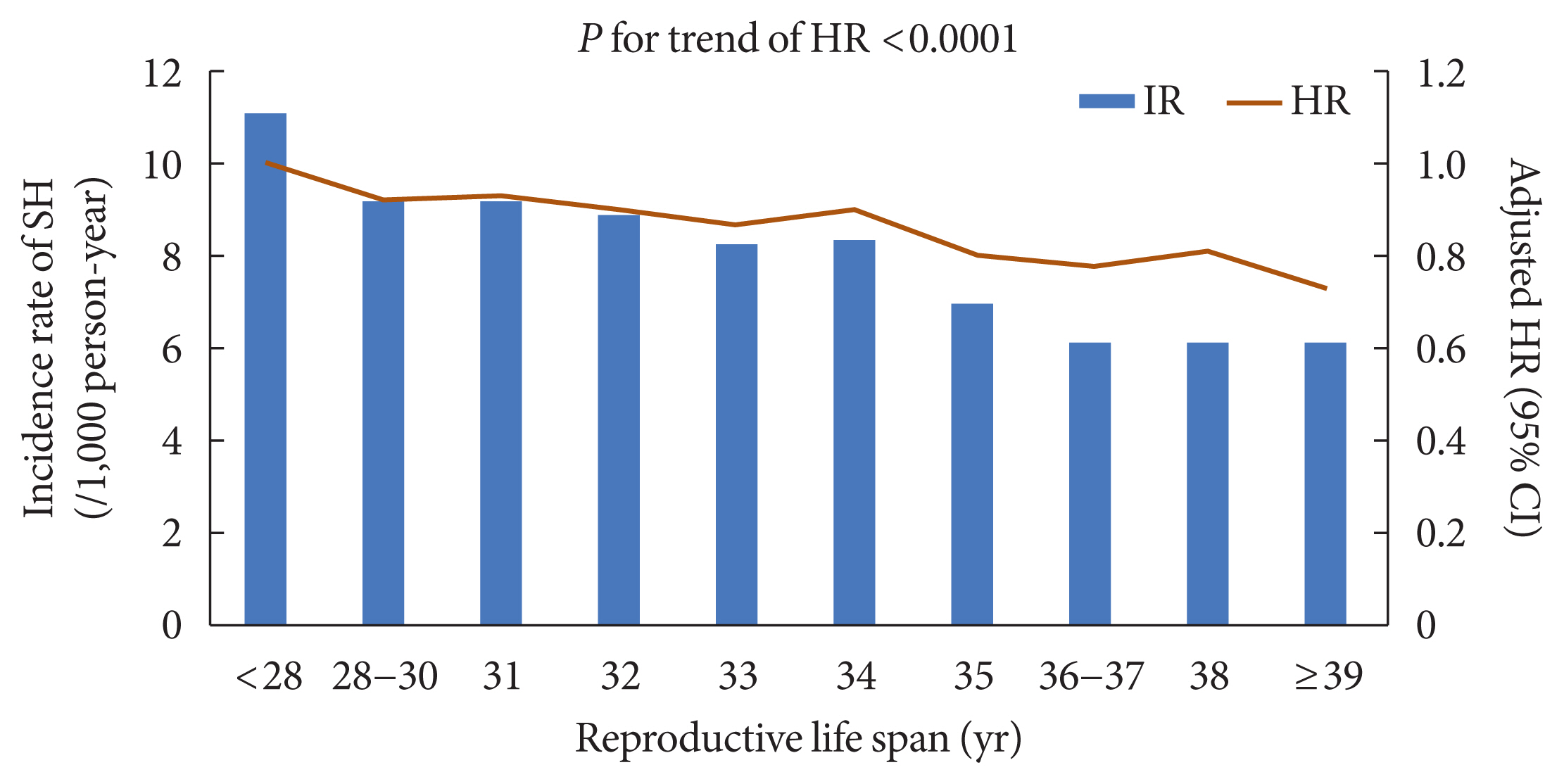
Trend in risk of severe hypoglycemia (SH) event and reproductive life span (RLS). The incidence rate of SH and its adjusted hazard ratio (HR) tended to decrease with increasing RLS (P for trend <0.0001). IR, incidence rate.
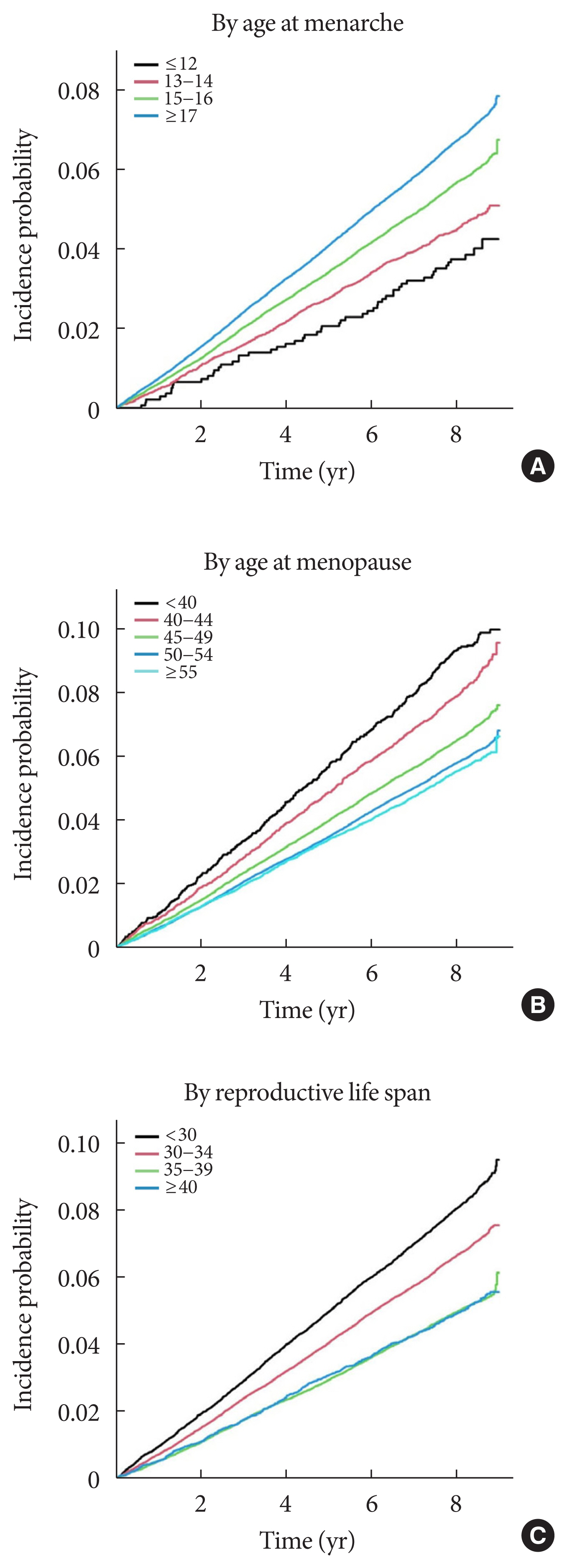
Cumulative incidence of severe hypoglycemia event in postmenopausal women with type 2 diabetes mellitus according to reproductive factors. The cumulative incidence of outcomes according to reproductive factors (A, age at menarche; B, age at menopause; C, reproductive life span) was calculated using Kaplan–Meier curves, and the log-rank test was performed to analyze differences amongst the groups.
Parity and breast feeding
No difference in risk of SH events was observed in the parity groups. Breastfeeding had no effect on the risk of SH events (Table 2).
HRT and oral contraceptive use
Exogenous sex hormone exposure showed variable results; patients of all HRT groups were at lower risk of SH events than were those without HRT after adjustment for multiple variables (adjusted HR, 0.76; 95% CI, 0.69 to 0.83 for the HRT <2 years group; P<0.0001) (adjusted HR, 0.79; 95% CI, 0.68 to 0.92 for the HRT 2 to 4 years group; P=0.0006) (adjusted HR, 0.78; 95% CI, 0.67 to 0.92 for the HRT ≥5 years group; P=0.0006), whereas OC use had no effect on the risk of SH events (adjusted HR, 0.94; 95% CI, 0.88 to 1.01 for OC users <1 year; P=0.151) (adjusted HR, 1.02; 95% CI, 0.95 to 1.10 for OC users ≥1 year; P=0.503) (Table 2).
DISCUSSION
In this nationwide population-based cohort study of postmenopausal women with T2DM, a longer RLS showed an inverse association with SH risk after adjustment for potential confounding factors, including the use of HRT and OCs. The inverse association with SH risk was stronger in younger women (aged <65 years) than in older women (aged ≥65 years), which was verified as a risk factor for SH [23]. These results suggest that extended exposure to endogenous ovarian hormones during the lifetime may be a protective factor against SH events in women with T2DM after menopause. HRT was also inversely associated with a lower risk of SH events in this population, whereas there was no association between OC use and the risk of SH events.
SH is uncommon early in the disease, as the human body physiologically defends against glucagon and adrenaline release and autonomic symptoms drive patients to consume carbohydrates against a further decrease in blood glucose when hypoglycemia occurs. However, these counterregulatory responses to hypoglycemia and the awareness of hypoglycemia are impaired due to increasing durations of diabetes and repetitive hypoglycemic episodes. Consequently, glucagon release is gradually reduced, and sympatho-adrenal responses are activated at lower levels of glucose, increasing the risk of SH [5].
SH was reported to occur every year in 0.1/100 patients with dietary intervention, 0.4 to 0.6/100 patients with sulfonylurea treatment, and 2.3/100 patients with insulin treatment in a randomized controlled trial [24]. More recently, the prevalence of SH was 1.02% to 3.04% in a large cohort study with non-insulin-dependent subjects with T2DM [25]. SH is associated with multiple negative influences [26], and several studies suggest that hypoglycemia is correlated with cardiovascular disease and mortality in T2DM [27,28]. Because of the severity, the prediction and prevention of SH are important for glycemic control in patients with diabetes [6,23].
Regarding counterregulatory action against hypoglycemia, reported were reduced responses of the sympathetic nervous system, growth hormone, and endogenous glucose production in women compared to men with type 1 diabetes mellitus (T1DM) [29]. However, hypoglycemia is prevalent in patients with T1DM regardless of sex [30]. Estrogen has been suggested as a main factor explaining why women with T1DM do not have an increased prevalence of hypoglycemia despite reduced counterregulatory responses [31]. Similar to the observation in T1DM, studies report more blunted counterregulatory actions against hypoglycemia and reduced risk of developing SH in women than in men despite similar glycemic thresholds between the sexes [32]. The responses to hypoglycemia in postmenopausal women with T2DM who were not receiving HRT were comparable to men of the same age with T2DM [33].
Estradiol has been revealed to stimulate hypoglycemia-associated hyperglucagonemia and hypercorticosteronemia in animal studies [10]. Consistent with this finding, another study reported that ovariectomized animals exhibit significantly reduced basal and stress-associated plasma corticosterone levels [34]. Estradiol provides neuroprotection against bioenergetic insults and controls hindbrain metabolic sensor screening and signaling of hypoglycemia-associated neuroenergetic instability [32]. Male rats exposed to recurrent insulin-induced hypoglycemia exhibit diminished Fos immunoreactivity in the hypothalamus [35], yet ovariectomized rats implanted with estradiol, but not oil, exhibit consistent patterns of Fos labeling in the hypothalamus after acute versus repeated hypoglycemia [36]. These results suggest that estradiol sustains or enhances neuronal reactivity to recurring hypoglycemia in the central metabolic structure, supporting the results of the current study showing that extended exposure to endogenous ovarian hormones during the lifetime is a protective factor against SH events in women with T2DM after menopause.
The beneficial effects of HRT on glucose homeostasis have been reported in several studies. Homeostatic model assessment-insulin resistance and abdominal fat were decreased by 13%, and the incidence of T2DM was decreased by 30% with HRT in a meta-analysis of 107 trials evaluating HRT effects on women without T2DM [37]. In women with T2DM, homeostatic model assessment-insulin resistance was further decreased by 36%, and fasting glucose was decreased with HRT [37], although these effects of HRT against T2DM do not last after therapy discontinuation [38].
Estrogen, known to have insulin-sensitizing properties and to be protective for pancreatic beta cells [39], has beneficial effects on glucose homeostasis in HRT by affecting insulin-mediated glucose uptake, insulin signaling, and glucose transport in adipose tissue and muscle [9]. Estrogen also increases the number of insulin receptors and specific insulin binding in a tissue-specific and dose-dependent manner [9]. Animal studies suggest multiple mechanisms by which estradiol may improve insulin-mediated glucose uptake [9]. Progestogens, in contrast, have been associated with insulin resistance. The properties of insulin resistance are different among the progestogens. Progestogens with androgen or glucocorticoid activities such as levonorgestrel or medroxyprogesterone acetate have insulin resistance and may blunt the beneficial effect of estrogen on insulin resistance [40]. Conversely, nonandrogenic progestogens such as norethindrone acetate and dydrogesterone show neutral effects on glucose homeostasis and do not blunt estrogen action on insulin resistance [40]. Therefore, whether HRT increases insulin sensitivity or resistance seems to be decided according to the type of progestogen added and the dose of estrogen.
The effect of HRT on glycemic control seems to depend on the timing of treatment relative to menopause. As suggested for cardiovascular health outcomes [41], a timing hypothesis regarding HRT was suggested for the insulin-mediated glucose disposal rate based on the findings that the insulin-mediated glucose disposal rate was improved with estradiol treatment in early postmenopausal women within 6 years since time of menopause, whereas it was worsened with estradiol treatment in late postmenopausal women ≥10 years after time of menopause [42]. Although the mechanisms for different estrogen effects on glucoregulatory insulin action according to time since menopause is not clearly determined, it is suggested that increasing duration of estrogen deficiency may lead to changes in estrogen receptor (ER) expression [42]. ER alpha (ERα) and beta (ERβ), to which estrogen binds with equal affinity, are two isoforms of ERs. The relative expression of each receptor varies in each tissue and changes with the duration of estrogen deficiency [43]. Furthermore, estrogen binding to ERα seems to be beneficial to glucose homeostasis, whereas estrogen binding to ERβ seems to be harmful [44]. Therefore, the composition of ERα and ERβ in glucoregulatory tissues may determine the effect of estrogen action on whole-body glucose disposal [9].
Differences among the effects of different HRT regimens on diabetes have not been clearly identified, and differences in HRT effects on diabetes according to HRT initiation timing or duration have not been established. Data on the effect of HRT on the counterregulatory action against hypoglycemia are lacking; however, the results of investigations on the effect of HRT on menopausal women with diabetes suggest that HRT has a favorable effect rather than a harmful influence on glycemic control in women with T2DM [42,45,46]. Furthermore, the findings of an experimental study in which ovariectomized female rats with estradiol implants showed consistent neuronal reactivity to repeated hypoglycemia [36] suggest a potential mechanism by which HRT positively influences postmenopausal women with diabetes.
This study has several limitations. First, reproductive factors were reliant on self-reported recall. Age at menarche was shown to be recalled with high reproducibility [47], and age at menopause was also reproducible but more discrepant with increasing time since menopause [48]. Second, although women who experienced surgical menopause were not excluded from the study population, this type of menopause was not assessed because it was infeasible to identify all women who underwent bilateral oophorectomy with the limited period of claims data. This limitation was partially overcome by excluding women with a reported history of any type of hysterectomy. Third, because of the limited information with claims data and self-reported questionnaires, glycemic control status such as glycated hemoglobin or duration of diabetes of study participants was not reflected in analyzing SH risk. Fourth, the end points were defined based on the claims data. The precision is not guaranteed to be 100%; however, the methodology using claims data has been demonstrated in previous studies. Fifth, further research is needed on the stronger inverse association of SH risk with RLS in younger women aged <65 years, because there were overlaps of CI between subgroup aged <65 years and aged ≥65 years in RLS 30 to 34 and RLS ≥40 respectively, though these results were significant (P for interaction=0.0032, P for trend=0.0005). Sixth, clarification of the effects of HRT on the risk of SH was not possible because the regimen and timing of HRT were not provided as primary data. However, though various HRT regimens with more individualized approaches for postmenopausal women emerged after the Women’s Health Initiative trial [49–52], estrogen plus progestogen treatment remain a general regimen for postmenopausal women with a uterus when the present’s study populations and their contemporaries were being treated with HRT [49,53,54]. Finally, this study showed only the results of individuals of Asian ethnicity.
Nevertheless, to the best of our knowledge, this study is the first report to assess the association between RLS and the risk of SH events in postmenopausal women with T2DM. We evaluated each reproductive factor and history of exogenous hormone treatment. Early menopause and premature menopause, which are related to shorter RLSs, were also identified as risk factors, whereas HRT was identified as a protective factor against SH events in postmenopausal women with T2DM.
In conclusion, a longer RLS, which meant a longer lifetime exposure to endogenous sex hormones, was inversely associated with risk of SH in postmenopausal women with T2DM. HRT was also related to a reduced risk of SH events in this population. Perimenopausal women with T2DM with vasomotor symptoms could benefit from HRT as it improves vasomotor symptoms and reduces the potential high risk of future occurrence of SH. Due to the risk of thromboembolism, a major vascular adverse effect of long-term HRT, further studies are needed for patients with high risk of cardiovascular disease. Identifying a possible SH-preventing mechanism of HRT and confirming the risk of SH events according to different regimens, timing, and duration of HRT would be challenges for future research.
SUPPLEMENTARY MATERIALS
Supplementary materials related to this article can be found online at https://doi.org/10.4093/dmj.2021.0135.
Subgroup analysis for hazard ratios (HRs) of severe hypoglycemia by reproductive life span for subgroup aged <65, and ≥65 in postmenopausal women with type 2 diabetes mellitus. The inverse association of reproductive life span (RLS) with severe hypoglycemia (SH) risk was more evident in women below 65 years.
Notes
CONFLICTS OF INTEREST
Yong-Moon Park was statistical advisors of the Diabetes & Metabolism Journal from 2020 to 2021. Seung-Hyun Ko was editorial board member of the Diabetes & Metabolism Journal from 2020 to 2021. They were not involved in the review process of this article. Otherwise, there was no conflict of interest.
AUTHOR CONTRIBUTIONS
Conception or design: K.H., S.H.K.
Acquisition, analysis, or interpretation of data: S.K., Y.M.P., K.H., S.H.K.
Drafting the work or revising: S.K., D.J.K., Y.J.C., J.N., K.H.
Final approval of the manuscript: S.K., Y.M.P., D.J.K., Y.J.C,, J.N., K.H., S.H.K.
FUNDING
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (Grant Number: HI18C0275).
ACKNOWLEDGMENTS
None