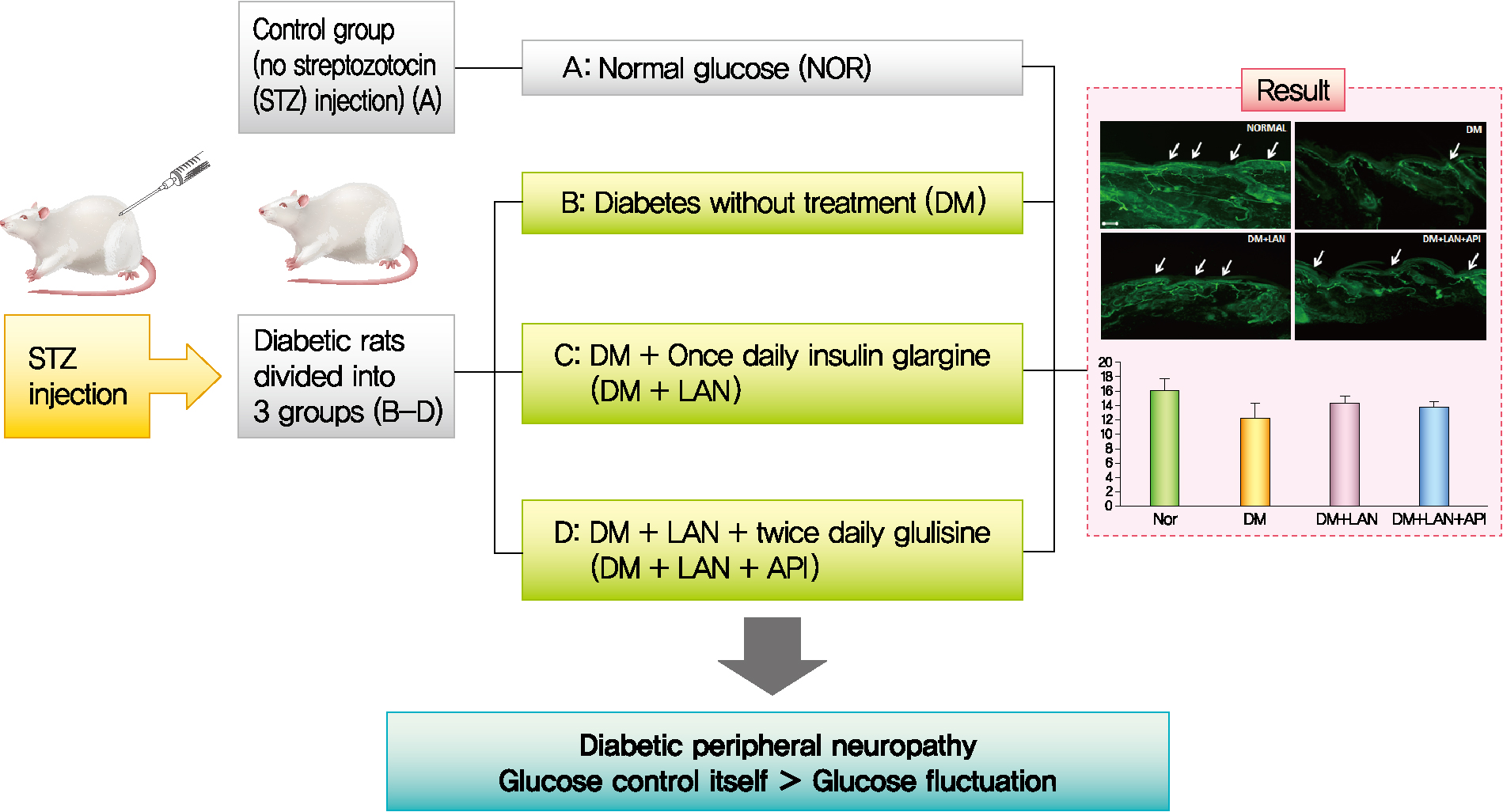
Influence of Glucose Fluctuation on Peripheral Nerve Damage in Streptozotocin-Induced Diabetic Rats
Article information
Abstract
Background
It is unclear whether glycemic variability (GV) is a risk factor for diabetic peripheral neuropathy (DPN), and whether control of GV is beneficial for DPN. The purpose of this study was to investigate the effect of GV on peripheral nerve damage by inducing glucose fluctuation in streptozotocin-induced diabetic rats.
Methods
Rats were divided into four groups: normal (normal glucose group [NOR]), diabetes without treatment (sustained severe hyperglycemia group; diabetes mellitus [DM]), diabetes+once daily insulin glargine (stable hyperglycemia group; DM+LAN), and diabetes+once daily insulin glargine with twice daily insulin glulisine (unstable glucose fluctuation group; DM+Lantus [LAN]+Apidra [API]). We measured anti-oxidant enzyme levels and behavioral responses against tactile, thermal, and pressure stimuli in the plasma of rats. We also performed a quantitative comparison of cutaneous and sciatic nerves according to glucose fluctuation.
Results
At week 24, intraepidermal nerve fiber density was less reduced in the insulin-administered groups compared to the DM group (P<0.05); however, a significant difference was not observed between the DM+LAN and DM+LAN+API groups irrespective of glucose fluctuation (P>0.05; 16.2±1.6, 12.4±2.0, 14.3±0.9, and 13.9±0.6 for NOR, DM, DM+LAN, and DM+LAN+API, respectively). The DM group exhibited significantly decreased glutathione levels compared to the insulin-administered groups (2.64±0.10 μmol/mL, DM+LAN; 1.93±0.0 μmol/mL, DM+LAN+API vs. 1.25±0.04 μmol/mL, DM; P<0.05).
Conclusion
Our study suggests that glucose control itself is more important than glucose fluctuation in the prevention of peripheral nerve damage, and intra-day glucose fluctuation has a limited effect on the progression of peripheral neuropathy in rats with diabetes.
INTRODUCTION
Diabetic peripheral neuropathy (DPN) is a common microvascular complication of diabetes, which leads to disability and decreases quality of life due to pain, sensory loss, gait disturbance, foot ulceration, and amputation [1]. The prevalence of diabetic neuropathy is reported to be 7% within 1 year of diagnosis to 50% for those who have had diabetes for more than 25 years [2].
The pathogenesis of DPN is complicated and is associated with a number of modifiable and non-modifiable risk factors, including the degree of hyperglycemia, duration of diabetes, dyslipidemia, high blood pressure, cigarette smoking, alcohol consumption, and height of the patient [3]. Among these factors, chronic hyperglycemia and the duration of diabetes have been considered to be major risk factors for the development of DPN [4]. Hyperglycemia can cause direct damage to the nerve parenchyma as well as indirect hyperglycemia-induced neuronal ischemia by decreasing neuronal blood flow [4]. However, the exact pathophysiological mechanisms of DPN are not fully understood.
Glycemic variability (GV) is defined as fluctuations in blood glucose levels [5]. The broad definition of GV considers the intraday glycemic excursions, including episodes of hyperglycemia and hypoglycemia, as well as blood glucose fluctuations that occur at the same time on different days [6]. In recent years, GV has been proposed to play an additional role in the development of diabetic neuropathy, independent of chronic hyperglycemia, by contributing to oxidative stress that leads to neural damage [7]. Most studies examining the effect of GV on diabetic neuropathy have focused on autonomic neuropathy [8,9]. Conversely, the association of GV with peripheral nerve dysfunction has not been explored systematically. Therefore, the aim of the present study was to demonstrate the effects of GV on peripheral nerve dysfunction, evaluated as the sensory and morphological parameters of peripheral nerve assessment and levels of oxidative stress markers, on inducing glucose fluctuations in streptozotocin (STZ)-induced diabetic rats with different insulin administration methods.
METHODS
Animals
Male Sprague-Dawley rats (6 weeks old, weighing approximately 160 to 180 g) were purchased from Orient Bio (Seongnam, Korea). Prior to the experiments, rats were allowed to adapt to their new environment for 1 week. During the study period, the rats were kept under standard conditions (24°C±2°C, 53%±2% humidity, and a 12-hour light/dark cycle) with free access to standardized diet and water. All experiments and protocols were in conformance with the regulations of the Institutional Rat Care and Use Committee, Jeonbuk National University Hospital (CUH-IACUC-141104-8).
Materials
To induce diabetes, a single intraperitoneal injection of 60 mg/kg STZ (Sigma Chemical, St. Louis, MO, USA) in citrate buffer (0.09 mol/L, pH 4.5) was administered to the rats. The control group received an equal volume of vehicle-sodium citrate buffer (pH 4.5). Blood samples were collected from the tail vein after overnight fasting and before insulin administration, around 8:00 AM, and blood glucose was measured using Accu-Chek Performa (Roche Diabetes Care, Mannheim, Germany). Rats whose blood glucose levels were above 350 mg/dL 1 week after STZ administration were considered diabetic.
Experimental design
Thirty-six rats were randomly assigned to four groups: rats with normal glucose levels that received sodium citrate buffer vehicle (normal glucose group [NOR], n=10); diabetic rats that did not receive treatment (diabetes mellitus [DM], n=8); diabetic rats who were administered subcutaneous injections of 15 to 20 IU/kg insulin glargine (Lantus®, Sanofi-Aventis, Paris, France), once daily at 4:00 PM (DM+LAN, n=9); and diabetic rats who were administered insulin glargine, as described before, and approximately 5 to 10 IU/kg insulin glulisine (Apidra®, Sanofi-Aventis) twice daily every 12 hours, at 8:00 AM and 8:00 PM (DM+LAN+API, n=9). The NOR and DM groups received an equal volume of saline solution at 8:00 AM, 4:00 PM, and 8:00 PM. The DM+LAN group also received saline injections at 8:00 AM and 8:00 PM.
Administration of insulin started 1 month after STZ injections, and this time point was defined as week 0. Body weight was measured every 2 weeks. Evaluation of behavior, plasma anti-oxidant enzymes, and morphometric parameters of cutaneous and sciatic nerves was performed at week 24.
Monitoring of glucose fluctuation
The serum glucose levels were measured to assess the daily glucose and fluctuation pattern eight times on 1 day in week 24. Glycosylated hemoglobin (HbA1c) levels were measured with a commercially available kit (NycoCard, Oslo, Norway) every 3 months, using blood samples collected from the tail vein.
GV was assessed by measuring the standard deviation (SD) and the coefficient of variance (CV; SD divided by mean×100%). The mean and SD of blood glucose levels were calculated according to the 8-point blood glucose measurements made on 1 day in week 24. For long-term GV, HbA1c variability was evaluated using the SD-HbA1c and CV of serial measurements of HbA1c every 3 months, including the HbA1c value obtained on the first day.
Measurements of oxidative stress markers (superoxide dismutase, catalase, and glutathione) in blood
At week 24, all rats were anesthetized via isoflurane inhalation. Isoflurane was delivered by a 0.4 to 0.6 liters per minute (LPM) O2 flow meter (SURGIVET®, Smiths Medical, Plymouth, MN, USA). Blood samples were drawn into tubes of 5 cc after euthanasia by cardiac puncture, and plasma was collected after centrifugation at 1,000 g for 10 minutes with 1 mM ethylenediaminetetraacetic acid (EDTA) at 4°C. Anti-oxidant enzymatic activity was measured using a commercial enzyme assay kit (Cayman Chemical Company, Ann Arbor, MI, USA), following the manufacturer’s protocol.
Behavioral assessment using tactile, thermal, and pressure stimuli
Tactile allodynia was measured at week 24 by applying flexible von Frey filaments (Stoelting, Wood Dale, IL, USA) of 4, 6, 8, 10, and 15 g, perpendicularly to the plantar surface of each hind paw in a series of ascending forces. Each filament was tested five times per paw, and the mechanical threshold was defined as the minimal force that caused at least two withdrawals in three consecutive trials [10].
To assess thermal hyperalgesia threshold, the time of latency was defined as the time period between the zero point, when the animal was placed on a hot plate surface (Ugo Basile, Trappe, PA, USA) at 52°C±0.5°C, and the time when the animal licked its paw or jumped off to avoid thermal pain. The tail-flick test using an infrared radiometer (Jeungdo B&P, Seoul, Korea) was also performed to study heat sensitivity. The time of tail avoidance after contact with radiant heat was measured. A point 5 cm from the rat’s tail end was used to test the heat threshold.
A Randall-Selitto analgesic meter (Ugo Basile, Comerio, Italy) was used to examine the mechanical threshold response according to increased pressure in the rat’s hind limb. The nociceptive threshold was defined as the force (g), upon which the rat pulled back its paw or made a sound.
Morphometric assessment and quantitative comparison of peripheral nerves
For immunohistochemical analysis of intraepidermal nerve fiber density (IENFD), 3×3 mm2 tissues were taken from the dorsum of the hind foot with skin biopsy at week 24 after euthanizing the rats. Sciatic nerves from all experimental rats were immediately dissected. Segments of the sciatic nerve were obtained, immersed in a fixative (in a solution of 2.5% paraformaldehyde and glutaraldehyde), and incubated overnight at 4°C. Subsequently, these specimens were embedded in paraffin and processed to resin blocks, before cutting sections of 1 µm and staining with toluidine blue. The procedures used for immunohistochemical analysis were the same as those described in previous studies [11].
Skin tissue specimens were fixed with periodate-lysine-paraformaldehyde (2% paraformaldehyde, 0.075 M lysine, 0.05 M phosphate buffer pH 7.4, and 0.01 M sodium periodate) solution for 24 hours. After thoroughly rinsing in phosphatebuffered saline (PBS) containing 20% glycerol and 0.1 M phosphate buffer for 48 hours at 4°C, tissue specimens were covered in a cryoprotectant and Tissue-Tek (OCT compound, Miles, Elkhart, IN, USA). Sections of 40 μm thickness were cut perpendicular to the dermis with a sliding microtome (Leica CM 1510®, Leica Microsystems AG, Wetzlar, Germany) and washed twice in PBS for 10 minutes at room temperature. Samples were then sequentially processed with 0.25% potassium permanganate for 15 minutes, PBS for 2 minutes for washing, and 4.5% oxalic acid for 2 minutes. Samples were then transferred into microcentrifuge tubes containing Dako Protein Block Serum-Free® (Dako, Carpinteria, CA, USA) supplemented with 1% normal goat serum as a blocking buffer. After 30 minutes of blocking at room temperature, specimens were washed with PBS twice for 10 minutes and then incubated overnight with primary antibody at a 1:1,000 dilution, rabbit anti-protein-gene-product 9.5 (PGP 9.5) (Bio-Rad, Poole, UK) at 4°C. The antibodies were diluted in antibody diluent (Dako) supplemented with 1% goat serum. After complete washing, secondary antibody (goat anti-rabbit immunoglobulin G-fluorescein isothiocyanate [IgG-FITC] at a 1:200 dilution, Vector, Peterborough, UK) was loaded for 1 hour at room temperature in a dark room. Sections were then washed with PBS twice for 10 minutes, placed on slides, and mounted with fluorescent mounting media (Dako).
Photomicrographs of the intraepidermal nerve fiber (IENF) were taken using a digital camera (Axiocam HRC®, Carl Zeiss, Goettingen, Germany) with magnifications of ×100 and ×400. PGP 9.5-immunoreactive nerve fibers in the epidermis of each section were counted [12,13]. Each nerve fiber with branching points inside the epidermis was counted as one fiber in the cutaneous nerves. IENFD, which is the number of IENFs per length (fibers/mm), was considered as the amount of innervation.
For quantification of axon and myelin diameters, six pieces of sciatic nerve tissue were obtained and six sections from each sample were prepared. We took over 30 pictures from each sample and selected 15 pictures with good quality. Using ZEN simplified image analysis program, consisting of an Aiocam 506 camera, myelinated fibers or axonal area represented by the outer or inner border of the myelin sheath was measured in the sciatic nerve, and the mean diameter and area of myelinated fibers were subsequently determined. The average values were calculated and compared between the experimental groups. To avoid any possible bias, experiments were performed by two individual investigators without knowledge of the experimental groups.
Statistical analysis
The data were analyzed using one-way analysis of variance (ANOVA) with Duncan’s post hoc test to compare the experimental groups and expressed as mean±standard error of mean. Statistical significance was accepted at a P value <0.05, with a 95% confidence interval. Statistical analysis was performed using the SPSS version 18.0 software (SPSS Inc., Chicago, IL, USA).
RESULTS
Body weight of experimental animals
The weight gain was not significant in the STZ-induced diabetic groups, whereas body weights in the normal group increased gradually from 373.0±7.1 g at week 0 to 551.3±13.1 g at week 24. The body weights of the normal group were significantly greater than those of the STZ-induced diabetic group at all experimental time points, irrespective of insulin administration (P<0.05). At weeks 12 and 24, the body weights of insulin-administered diabetic groups were significantly higher than of the DM group (P<0.05). However, body weight did not differ significantly between the DM+LAN and DM+LAN+API groups (Fig. 1A).
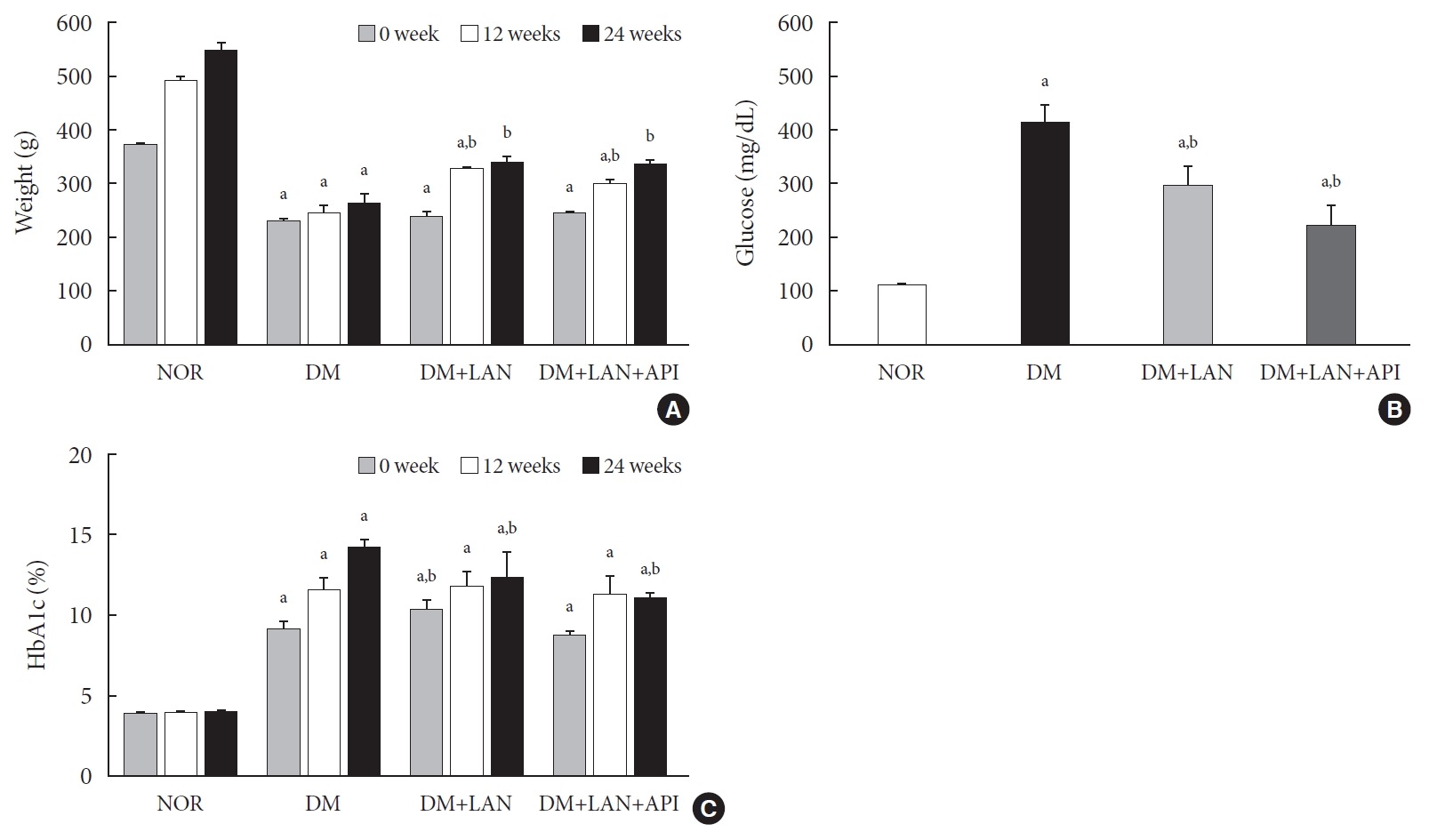
(A) Body weight change, (B) mean blood glucose levels on the first day of week 24, and (C) glycosylated hemoglobin (HbA1c) levels in the experimental groups. Values are presented as mean±standard error of mean. NOR, normal; DM, diabetes mellitus; DM+LAN, DM treated with insulin glargine; DM+LAN+API, DM treated with insulin glargine and glulisine (n=8–10 in each group). aP<0.05 vs. normal, bP<0.05 vs. DM.
Blood glucose levels and HbA1c of animals during experimental period
After STZ injections, average blood glucose levels increased above 400 mg/dL in diabetic rats, while blood glucose levels in control rats did not significantly change throughout the study period. Mean blood glucose levels of the DM group on 1 day of week 24, were significantly greater than those of the normal and insulin-administered groups (P<0.05). There was no significant difference between the insulin-administered groups (Fig. 1B).
HbA1c levels were measured three times at weeks 0, 12, and 24. Significant differences of mean HbA1c levels between the normal and STZ-induced diabetic groups were observed (P<0.05). HbA1c levels at week 24 were significantly lower in the insulin-administered groups than in the DM group (12.4%±0.5% DM+LAN; 11.1%±0.1%, DM+LAN+API vs. 14.3%±0.2%, DM; P<0.05). However, there was no significant difference between the insulin-administered groups (Fig. 1C).
Comparison of glucose fluctuation among experimental groups
On the first day of 24 weeks, 8-point glucose monitoring showed an abrupt drop in the glucose levels after glulisine injection in the DM+LAN+API group as compared to those in the other DM groups (Fig. 2A). Intraday GV, as estimated by the mean SD of blood glucose measured on 1 day, was significantly higher in the DM+LAN+API group than in other groups (P<0.05) (Fig. 2B). All parameters of HbA1c variability were significantly higher in the STZ-induced diabetic groups than in the normal group (P<0.05) (Fig. 2C-E). There was a significant difference in the absolute change in HbA1c between the DM group and insulin-administered groups (P<0.05), with no significant difference between the DM+LAN and DM+LAN+API groups. The degree of SD and CV% in HbA1c level of the DM+LAN group was not different significantly than in the DM and DM+LAN+API groups (Fig. 2D and E), although intraday glucose levels fluctuated the most in the DM+LAN+API group.
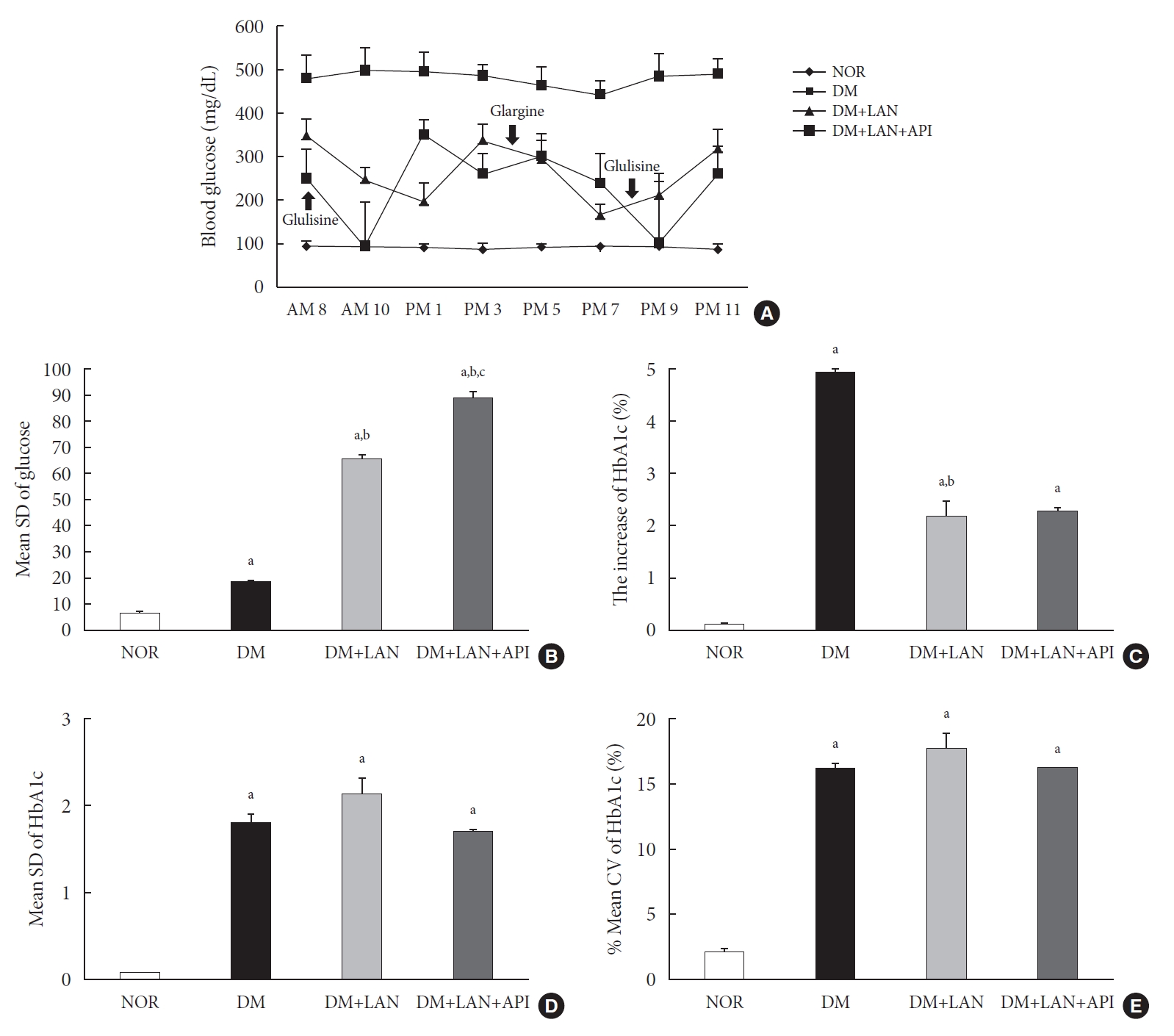
Comparison of glycemic variability indices of the experimental groups: (A) 8-point glucose monitoring on the first day of week 24; (B) mean standard deviation (SD) of blood glucose on the first day of week 24; (C) absolute change in HbA1c (0 to 24th week); (D) mean SD of HbA1c (0 to 24th week); and (E) % coefficient of variation (CV) of HbA1c (0 to 24th week). Values are presented as mean±standard error of mean. NOR, normal; DM, diabetes mellitus; DM+LAN, DM treated with insulin glargine; DM+LAN+API, DM treated with insulin glargine and glulisine (n=8–10 in each group). aP<0.05 vs. normal, bP<0.05 vs. DM, cP<0.05 vs. DM+LAN.
The level of oxidative stress markers in blood of experimental animals
At week 24, the absolute values of superoxide dismutase (SOD) and catalase activities in the DM group were lower than those in the normal and insulin-administered groups. However, these differences were not statistically significant. Glutathione levels were significantly lower in the STZ-induced diabetic groups than in the normal group (P<0.05). In addition, compared to the insulin-administered groups, the DM group exhibited significantly decreased glutathione levels (2.64±0.10 µmol/mL, DM+LAN; 1.93±0.0 µmol/mL, DM+LAN+API vs. 1.25±0.04 µmol/mL, DM; P<0.05). However, no significant difference was observed between the DM+LAN and DM+LAN+API groups (Fig. 3).
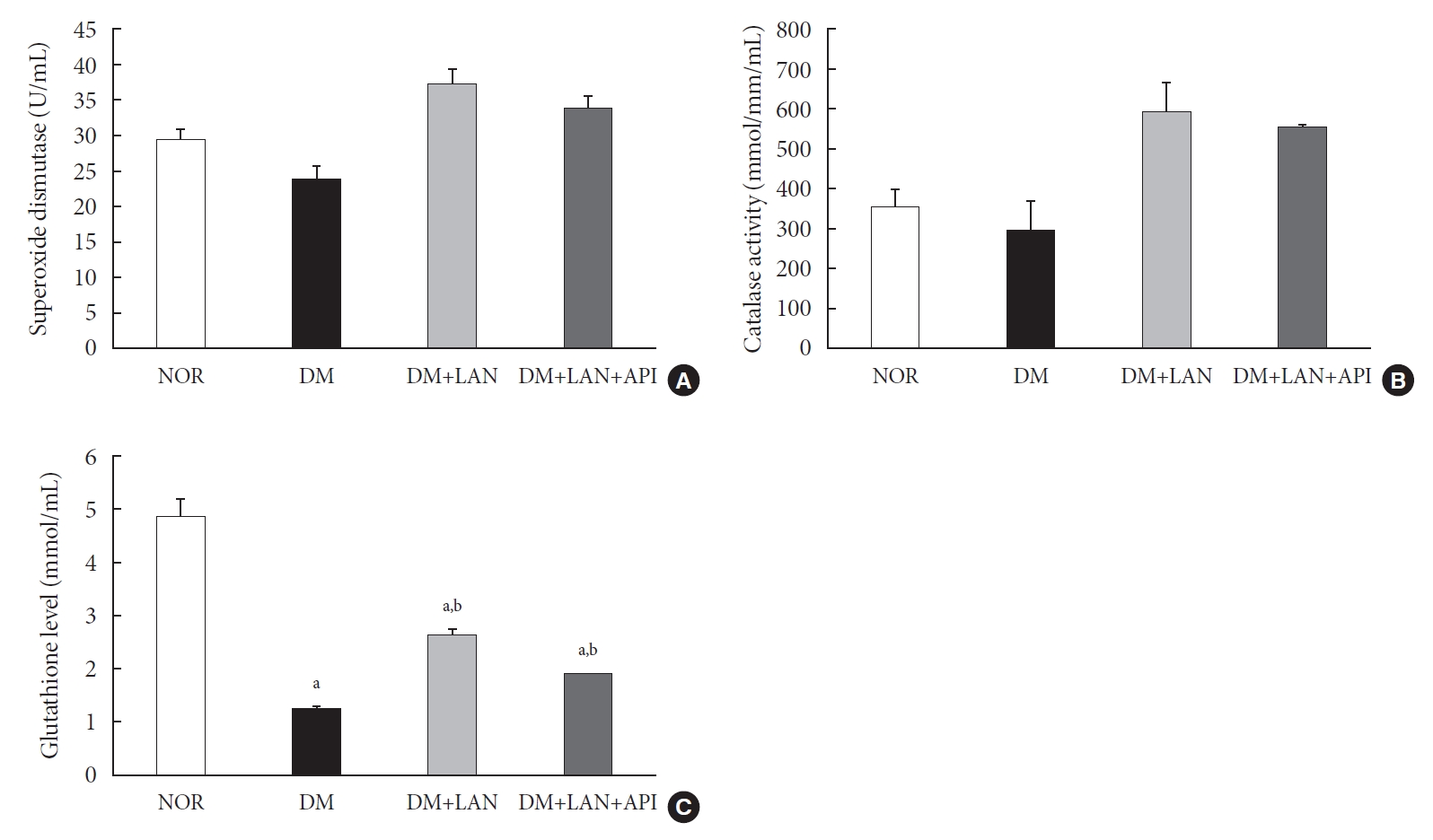
The level of antioxidant enzymes of the experimental groups at week 24. (A) Superoxide dismutase, (B) catalase activities, and (C) glutathione level in blood. Values are presented as mean±standard error of mean. NOR, normal; DM, diabetes mellitus; DM+LAN, DM treated with insulin glargine; DM+LAN+API, DM treated with insulin glargine and glulisine (n=8–10 in each group). aP<0.05 vs. normal, bP<0.05 vs. DM.
The comparison of behavioral responses with tactile, thermal, and pressure stimuli
At week 24, the paw withdrawal threshold was reduced by 28% in the DM group and by 21% in the DM+LAN group, compared to the normal group. The reduced thresholds in both the DM and DM+LAN groups were significantly lower than those in the normal and DM+LAN+API groups (P<0.05). These thresholds were similar between the normal and DM+LAN+API groups (Fig. 4A).
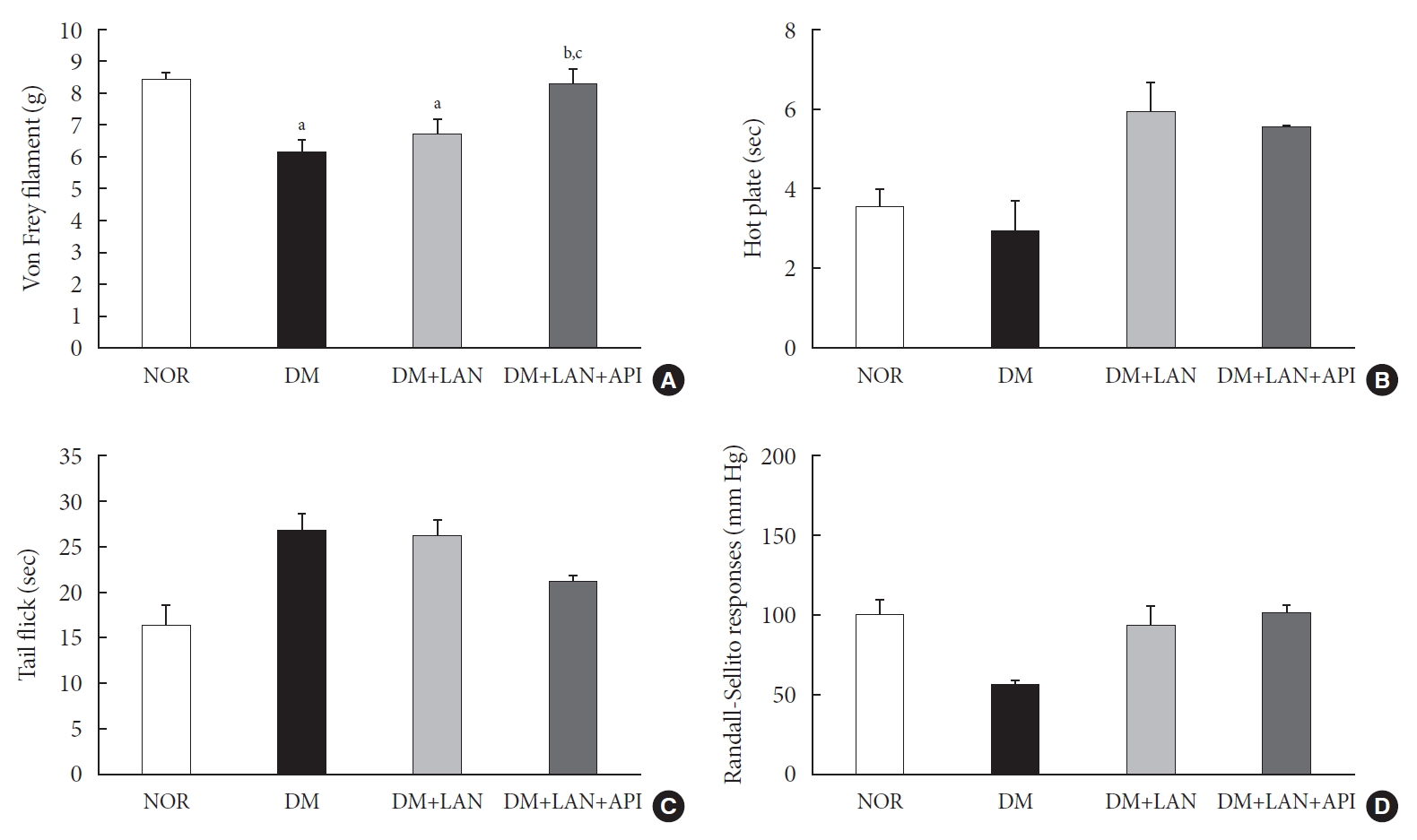
The threshold of responses with diverse sensory tests in the experimental groups at 24 weeks. (A) Von Frey filament response, (B) the responses for hot plate, (C) tail flick test, and (D) Randall-Sellito test. Values are presented as mean±standard error of mean. NOR, normal; DM, diabetes mellitus; DM+LAN, DM treated with insulin glargine; DM+LAN+API, DM treated with insulin glargine and glulisine (n=8–10 in each group). aP<0.05 vs. normal, bP<0.05 vs. DM, cP<0.05 vs. DM+LAN.
The time for paw withdrawal on the hot plate was reduced by 15% in the DM group, 21% in the DM+LAN group, and 17% in the DM+LAN+API group, compared with the normal group (Fig. 4B). The tail flick response latency on the hot plate tended to increase in the STZ-induced diabetic groups compared with the normal group, irrespective of insulin administration. The mean response latency was lower in the DM+LAN+API group than in the DM and DM+LAN groups (21.4±0.5 seconds, DM+LAN+API vs. 26.8±1.9 seconds, DM and 26.3±1.5 seconds, DM+LAN) (Fig. 4C). The response threshold to pressure stimuli in the Randall-Sellito test was reduced by 44% in the DM group compared to the normal group. The threshold in the DM group was lower than that in the DM+LAN and DM+LAN+API groups (Fig. 4D).
However, there were no significant differences in the responses to thermal and pressure stimuli between the DM+LAN and DM+LAN+API groups.
Morphometric and quantitative comparison of the cutaneous and sciatic nerves
Quantitative comparison of the cutaneous peripheral nerves revealed that the mean IENFD was lower in the DM group than in the normal group and the insulin-administered groups at week 24 (16.2±1.6 mm, normal; 12.4±2.0, DM; 14.3±0.9, DM+LAN; 13.9±0.6, DM+LAN+API). However, the differences in the IENFD among the normal and STZ-induced diabetic groups were not statistically significant (Fig. 5A). Fig. 5B shows the morphological finding of PGP 9.5-positive small nerve fibers extending into the epidermis in each group, in which relatively shortened and reduced nerve fibers were observed in the STZ-induced diabetic groups compared to the normal group. The diameters of the myelin sheath and the axonal fibers of sciatic nerves in the STZ-induced diabetic groups had reduced as compared to those in the normal group, irrespective of insulin administration (P<0.05) (Fig. 6A and B). However, the diameters were not significantly different in the DM+LAN and DM+LAN+API groups. The morphological findings of the sciatic nerves according to the different insulin administration methods are shown in Fig. 6C.
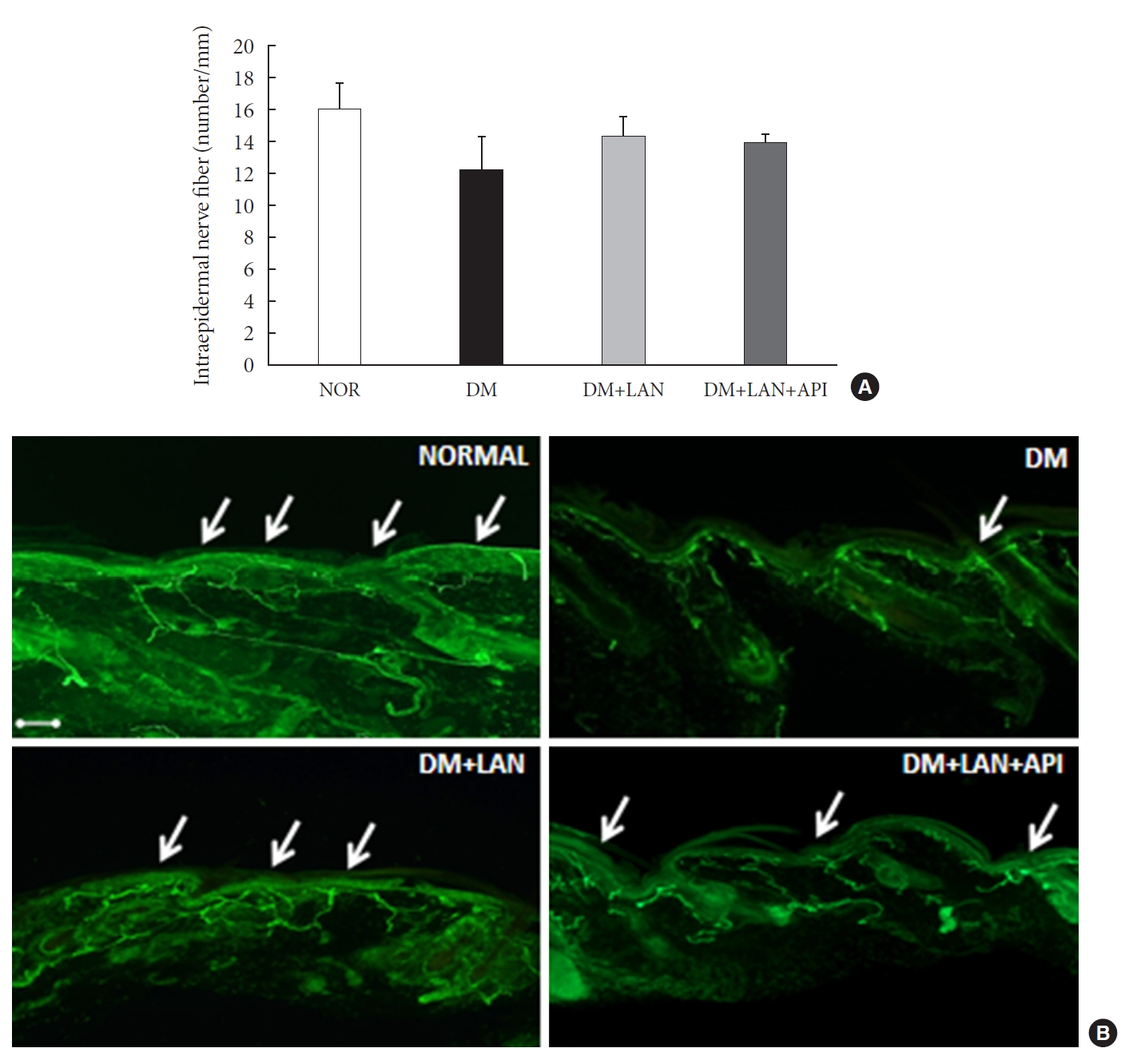
Quantitative comparison of cutaneous nerves with (A) the mean intraepidermal nerve fiber density and (B) immunohistochemistry of cutaneous small nerve fibers of the dorsum (×100). Arrows indicate immunostained small nerve fibers. Bar indicates 100 μm. Values are presented as mean±standard error of mean. NOR, normal; DM, diabetes mellitus; DM+LAN, insulin glargine treated DM; DM+LAN+API, insulin glargine with glulisine treated DM (n=8–10 in each group).

Quantitative comparison of sciatic nerve, including (A) the diameter of myelin sheath, (B) the diameter of axon, and (C) immunohistochemistry of the sciatic nerve of the experimental groups (×1,000). Bar indicates 20 μm. Values are presented as mean±standard error of mean. NOR, normal; DM, diabetes mellitus; DM+LAN, DM treated with insulin glargine; DM+LAN+API, DM treated with insulin glargine and glulisine (n=8–10 in each group). aP<0.05 vs. normal, bP<0.05 vs. DM.
DISCUSSION
In the present study, we induced glucose fluctuation to investigate how GV contributes to the progression of peripheral nerve damage in diabetes. We observed that the GV in the DM+LAN+API group was different from that in the DM and DM+LAN groups. Mean glucose values and HbA1c levels were significantly lower in the insulin-administered groups than in the DM group at week 24. However, these levels were not significantly different in the DM+LAN and DM+LAN+API groups during the experimental period. Although the DM+LAN+API group was exposed to severe intraday glucose fluctuation more frequently than the DM+LAN group, GV indices for HbA1c were not significantly different between the two groups. Therefore, this study suggests that the glucose control state itself is more important than GV, such as glucose fluctuation in peripheral nerve protection in diabetes.
The basic pathogenic mechanism of DPN is complicated and may involve several factors. Long-term hyperglycemia leads to polyol pathway hyperactivity, advanced glycation end-product/receptor for advanced glycation end-product reactions, and increased reactive oxygen species that activate multiple deleterious pathways within nerves [3]. Recent laboratory and animal studies have shown that acute fluctuation or a wide range of glucose levels may worsen hyperglycemia-induced oxidative stress [14,15]. Similarly, two human studies among patients with type 2 diabetes mellitus (T2DM) revealed that oscillating glucose levels resulted in further deterioration of endothelial dysfunction and higher levels of oxidative stress markers compared with sustained hyperglycemia in T2DM [16,17].
Clinical studies have reported that GV may play a role in the development of diabetic microvascular and macrovascular complications [18-21]. A recent study using a continuous glucose monitoring system showed a significant fluctuation in blood glucose values in children with type 1 diabetes mellitus (T1DM), as well as in those with excellent HbA1c values. This suggests that in addition to HbA1c, glucose variability may have predictive value for the development of diabetic complications [22,23]. However, human studies have not consistently shown that GV is harmful. Bragd et al. [18] confirmed the lack of a relationship between short-term GV measured as SD and microvascular complications in patients with T1DM. However, they found that GV was significantly related to the presence of peripheral neuropathy, and was a borderline predictor of its incidence [18]. Even in light of the above considerations, the concept of GV is a complex phenomenon and it is suggested that GV may partially contribute to the development of diabetic complications.
Studies have suggested different methods to assess GV [24-26] and there is no clear consensus in clinical research on the gold standard for measuring GV. Among the most commonly used methods is SD, an index of the dispersion of data around mean blood glucose [24]. The SD of HbA1c can be used to assess long term GV. Several previous studies have shown that HbA1c variability is associated with microvascular complications in diabetes. It is known that annual fasting plasma glucose and HbA1c variability are strongly associated with diabetic nephropathy in T2DM [25]. In another study, it was shown that variability in HbA1c added to the mean value in predicting retinopathy and nephropathy in T1DM [26]. Based on these studies, we assessed mean glucose values with SD and 8-point glucose monitoring as the intraday GV index and SD and %CV of HbA1c as the long-term GV index in this study.
The impact of GV on DPN and its exact pathogenesis have not been fully elucidated. GV has been suggested as an additional risk factor for the worsening of DPN [27,28]. However, most studies assessing the impact of GV on DPN have focused on autonomic neuropathy, especially cardiac autonomic neuropathy (CAN), rather than somatic neuropathy [9,29]. A recent study reported that GV was independently associated with the presence of CAN in patients with T2DM, and HbA1c variability was also independently associated with the severity of CAN [9]. In our study, the results of a diverse set of sensory tests were inconsistent. Only the von Frey filament test showed a significantly decreasing threshold in the DM+LAN group compared with the threshold in the DM+LAN+API group. Interestingly, there was no significant difference in IENFD between the insulin-administered groups, although a significant reduction in allodynia was observed in the DM+LAN group compared to the DM+LAN+API group. In general, functional changes related to sensory processing precede structural changes in small fiber diabetic neuropathy [30]. Therefore, our findings support the observation that the ability of pathologic findings to explain functional changes in all peripheral nerves and sensory manifestations is limited. The findings related to anti-oxidant enzymes in blood were also inconsistent. The levels of the anti-oxidant glutathione in the blood were reduced in the STZ-induced diabetic groups compared to the normal group. There was a significant reduction of the anti-oxidant glutathione in the DM group compared with the insulin-administered groups, but there was no significant difference according to the induction of glucose fluctuation. SOD and catalase activities were not significantly different among the experimental groups. In fact, the observed difference in the levels of glutathione in the blood may not directly reflect the levels in peripheral nerve tissue. For this reason, assessment of oxidative stress and antioxidant levels in peripheral nerve tissue is necessary to establish pathological differences.
In this study, most of the tests used to quantify the amount of peripheral nerve fiber damage did not show significantly different results between insulin administered groups. This could be due to the fact that the induced GV in our study was not enough to cause peripheral nerve fiber damage. Another possible explanation could be that the experimental period was insufficient for inducing GV-associated nerve damage. Furthermore, GV may have relatively smaller effects on peripheral nerves than chronic hyperglycemia. The duration and absolute value of GV involved in the initiation and progression of DPN remains an open question. Finally, it is possible that insulin itself may have affected the peripheral nerves. There is controversy regarding the relationship between insulin therapy and the presence of DPN. Katulanda et al. [31] demonstrated that insulin treatment is a strongly associated risk factor of DPN. A possible explanation for this association could be that insulin use indicates beta cell failure and may reflect a later stage of diabetes or greater severity [31]. However, randomized clinical trials have shown that intensive insulin therapy can prevent or delay the development of DPN compared with conventional insulin therapy [32]. Further studies will be necessary to clarify whether insulin administration is beneficial for peripheral nerves.
This study has a few limitations. First, we did not assess intra-day GV more accurately by repeating the 8-point glucose measurement during the 24 weeks. Although glucose fluctuation was induced in our study, more accurate glucose fluctuations may be required to show that GV alone can influence peripheral neuropathy in diabetes. Second, insulin can also affect the natural course of peripheral nerve damage in diabetes. Therefore, GV induction by different methods, without insulin treatment, may be helpful to exclude the effect of insulin on DPN. Third, serial comparisons of the IENFD and behavioral tests at 0, 12, and 24 weeks with oxidative stress assays might be more helpful to support our suggestion. Fourth, the variable state of hyperglycemia according to insulin treatment may affect the variability of behavioral measurement, although insulin was not administered on the day of the sensory tests. Further investigation on the presence of retinopathy or nephropathy will be helpful to clarify the impact of glucose fluctuation on the microvascular complications in diabetes. Despite these limitations, this study is the first to compare the functional and morphological changes of peripheral nerves occurring due to glucose fluctuations in diabetes.
In conclusion, the results of our experiment suggest that glucose fluctuation induced by insulin administration is relatively less harmful by itself, and the glucose control state is more important than GV in the progression of peripheral nerve damage in diabetes.
Notes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTIONS
Conception or design: T.S.P., H.Y.J.
Acquisition, analysis, or interpretation of data: Y.J.K., N.Y.L., K.A.L., H.Y.J.
Drafting the work or revising: Y.J.K., H.Y.J.
Final approval of the manuscript: Y.J.K., H.Y.J
FUNDING
None
Acknowledgements
The authors would like to thank the Research Institute of Clinical Medicine of Jeonbuk National University–Biomedical Research Institute of Jeonbuk National University Hospital for supporting this study in part through access to experimental facilities.