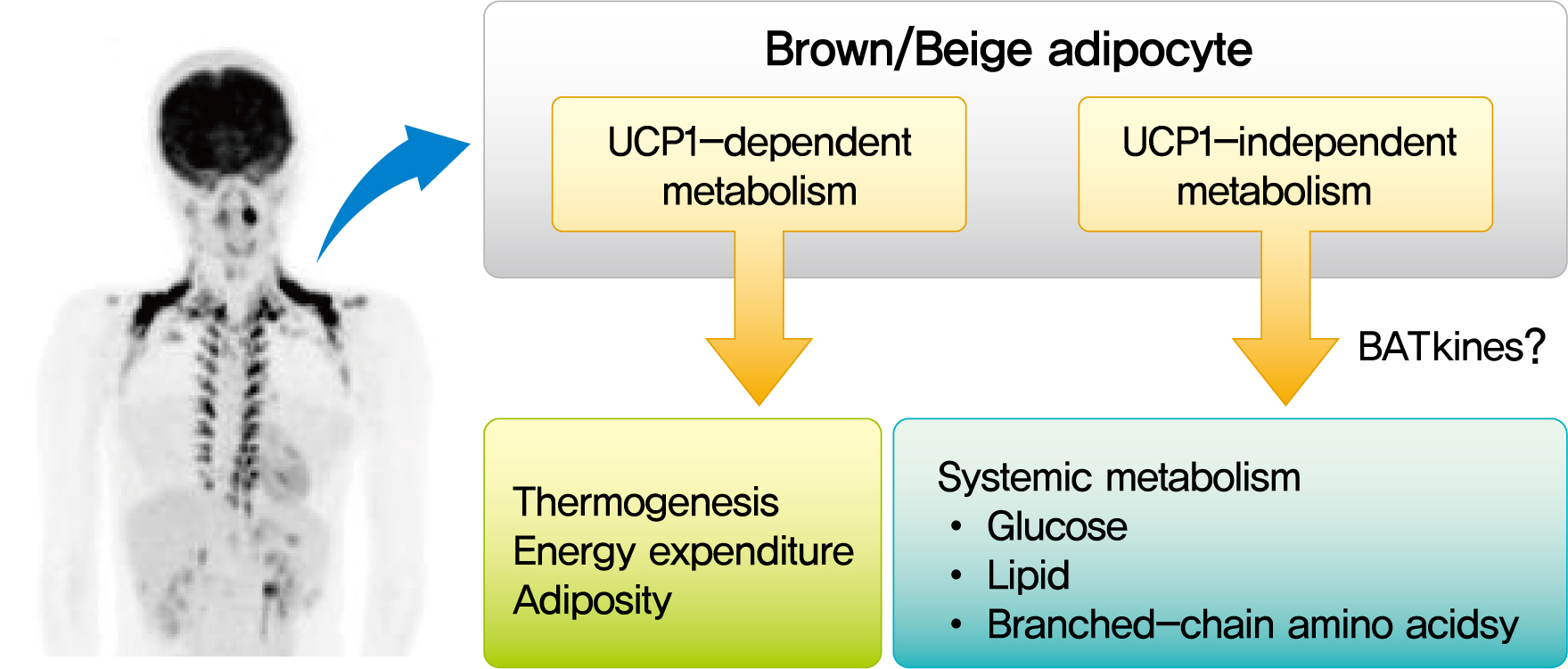
Brown Fat as a Regulator of Systemic Metabolism beyond Thermogenesis
Article information
Abstract
Brown adipose tissue (BAT) is a specialized tissue for nonshivering thermogenesis to dissipate energy as heat. Although BAT research has long been limited mostly in small rodents, the rediscovery of metabolically active BAT in adult humans has dramatically promoted the translational studies on BAT in health and diseases. Moreover, several remarkable advancements have been made in brown fat biology over the past decade: The molecular and functional analyses of inducible thermogenic adipocytes (socalled beige adipocytes) arising from a developmentally different lineage from classical brown adipocytes have been accelerated. In addition to a well-established thermogenic activity of uncoupling protein 1 (UCP1), several alternative thermogenic mechanisms have been discovered, particularly in beige adipocytes. It has become clear that BAT influences other peripheral tissues and controls their functions and systemic homeostasis of energy and metabolic substrates, suggesting BAT as a metabolic regulator, other than for thermogenesis. This notion is supported by discovering that various paracrine and endocrine factors are secreted from BAT. We review the current understanding of BAT pathophysiology, particularly focusing on its role as a metabolic regulator in small rodents and also in humans.
INTRODUCTION
Mammals have two types of adipose tissue, the white adipose tissue (WAT) and brown adipose tissue (BAT), which have opposite physiological roles: WAT is the site to store energy as triglyceride (TG), whereas BAT is a specialized tissue for nonshivering thermogenesis (NST) to dissipate energy as heat [1]. Although BAT research has long been limited mostly to small rodents, the rediscovery of metabolically active BAT using radionuclide imaging technique in adult humans [2-5] has dramatically accelerated the translational studies on BAT in health and diseases, particularly on its role in the regulation of energy balance, body fatness, and substrate metabolism [6,7]. Over the past decade, several remarkable advancements have been made in the field of brown fat biology. First, increasing evidence has been accumulated indicating rodents and humans possess two types of thermogenic adipocytes arising from developmentally distinct lineages: the classical brown adipocytes and the so-called beige or brite cells that are inducible within WAT [8]. Second, in addition to a well-established thermogenic activity of uncoupling protein 1 (UCP1), several alternative molecular mechanisms of NST have been discovered, particularly in beige adipocytes [9]. Third, studies in rodents and humans have revealed that BAT cross talks with some peripheral tissues and controls their functions, systemic homeostasis of energy, and metabolic substrates, suggesting BAT as a metabolic regulator beyond thermogenesis [10]. In fact, various paracrine and endocrine factors are found to be secreted from BAT [11]. In this review, we summarize our current understanding on BAT pathophysiology, particularly focusing on its role as a metabolic regulator in small rodents and humans.
UCP-DEPENDENT AND -INDEPENDENT MECHANISMS OF BAT THERMOGENESIS
The most well-established thermogenic molecule for NST, particularly for cold-induced thermogenesis (CIT), is UCP1 expressed exclusively in BAT. UCP1 has the ability to leak proton gradient across the mitochondrial membrane, and thereby uncouples oxidative phosphorylation to generate heat. When animals are exposed to the cold, temperature sensors (transient receptors potentially channel) on sensory neurons on the body surface transmit information to the brain and increase the activity of sympathetic nerves entering BAT. Noradrenaline released from sympathetic nerve endings stimulates BAT via β-adrenergic receptor (βAR) and triggers cyclic adenosine monophosphate-activated intracellular events, including TG hydrolysis, fatty acid (FA) oxidation, and UCP1 activation (Fig. 1).
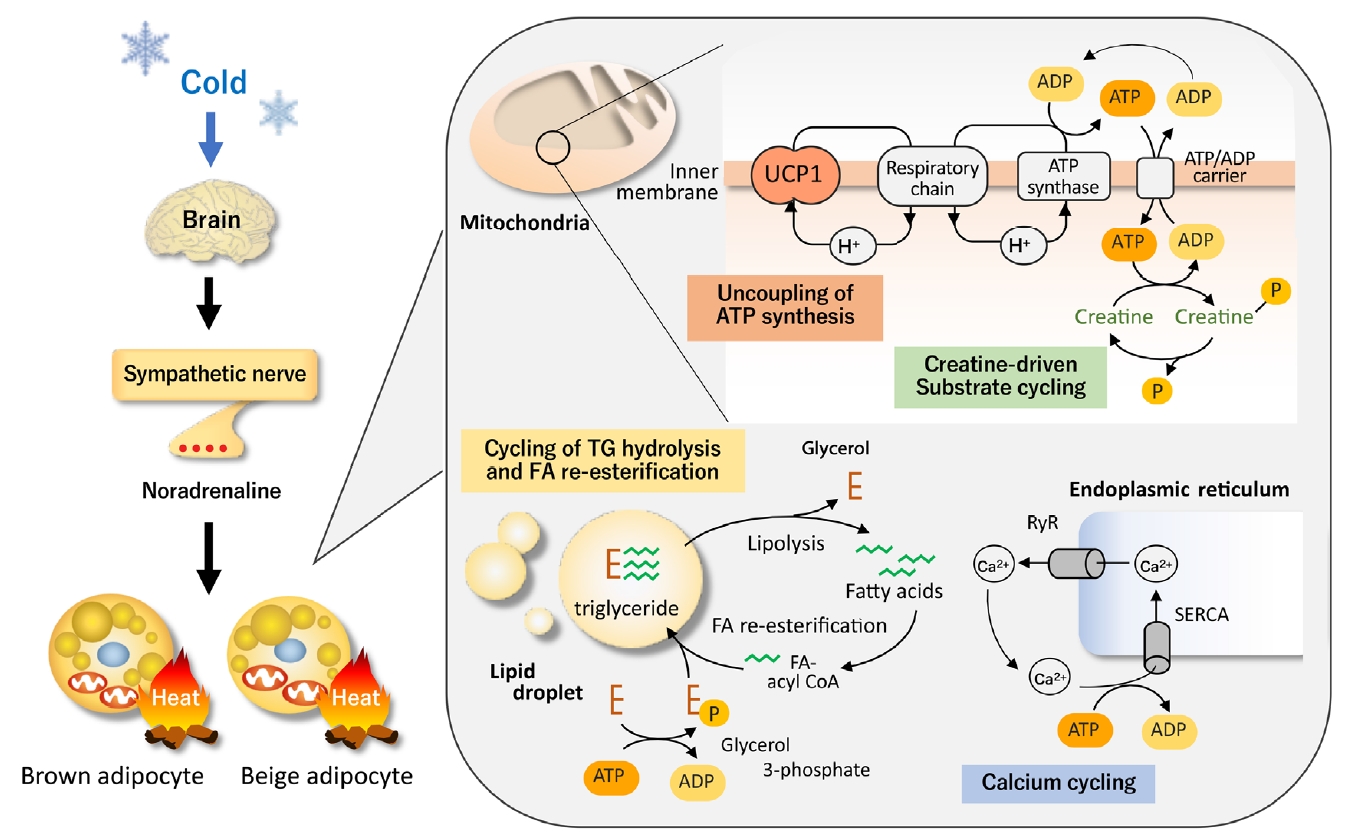
Uncoupling protein 1 (UCP1)-dependent and independent thermogenesis in brown and beige adipocytes. UCP1, a protein located in the mitochondrial inner membrane, has the ability to leak proton gradient across the mitochondrial membrane, and thereby uncouples oxidative phosphorylation to generate heat. Thus, UCP1 thermogenesis is not dependent on adenosine triphosphate (ATP). On the other hand, thermogenesis by the futile cycles of creatine and creatine phosphate, release and re-uptake of calcium, and triglyceride (TG) hydrolysis and fatty acid (FA) re-esterification is dependent on the breakdown of ATP to adenosine diphosphate (ADP). These thermogenic mechanisms are activated by cold exposure primarily through the sympathetic nerve activation. RyR, ryanodine receptor; SERCA, sarco-endoplasmic reticulum ATPase.
The critical role of UCP1 in CIT is typically indicated in mice with genetic deletion of UCP1 (UCP1-knockout [KO]), making them cold-sensitive and unable to maintain their body temperature after acute cold exposure [12]. However, it is also true that UCP1-KO mice can survive during mild and gradual cold exposure, suggesting some thermogenic mechanisms other than UCP1 [13,14]. In general, when animals are exposed to cold temperatures for a long time, they adapt to their surroundings by increasing the number of brown adipocytes and the amount of UCP1 through the proliferation of interstitial preadipocytes and mature adipocytes [15,16]. In addition to BAT hyperplasia, prolonged cold exposure increases an apparent induction of beige adipocyte in WAT. Beige adipocyte expresses considerable amounts of UCP1 and has thermogenic activity comparable with that of brown adipocyte [17,18]. However, recent studies have indicated the existence of UCP1-independent thermogenic mechanisms, particularly in beige adipocytes (Fig. 1).
Beige adipocytes contain large amounts of mitochondria and a high capacity of oxidative phosphorylation for adenosine triphosphate (ATP) synthesis. Thus, ATP-dependent futile cycles can compensate for the lack of UCP1 thermogenesis. Calcium cycling is one of the ATP-dependent and UCP1-independent futile mechanisms. Calcium transport has been known to contribute to NST in muscles through sarco-endoplasmic reticulum ATPase (SERCA) and ryanodine receptors (RyR) [19]. Ikeda et al. [20] demonstrated that this ATP-dependent calcium cycling also occurs through SERCA2b and RyR2 in beige adipocytes. Notably, the SERCA2b-mediated Ca2+ cycling mechanism is necessary for beige adipocyte thermogenesis, but dispensable in brown adipocytes.
Another example of UCP1-independent thermogenic mechanisms is creatine cycling through ATP-dependent phosphorylation and dephosphorylation [21]. Cycling of FA and TG through hydrolysis of TG and re-esterification of released FA is also possible as an ATP-dependent thermogenic mechanism in beige adipocytes [22]. Thus, all of these UCP1-independent thermogenic mechanisms would compensate for the lack of UCP1 and enable cold tolerance in UCP1-KO mice.
In adult humans, BAT is usually assessed by cold-activated glucose utilization using 18F-fluorodeoxyglucose (FDG)-positron emission tomography (PET) [2-6]; that is, when FDGPET is performed after either cold exposure or β3-AR agonist administration, it detects symmetrical FDG uptake in adipose tissue at the supraclavicular and thoracic spine regions. Histological examinations revealed the presence of UCP1-positive adipocytes surrounded by numerous UCP1-negative white adipocytes in these regions. Gene expression analysis has demonstrated that the fat pat at these regions is largely composed of beige adipocyte more than classical brown adipocytes [23,24], suggesting both UCP1-dependent and -independent thermogenic mechanisms in adult humans; however, their individual roles remain to be elucidated.
THERMOGENESIS-ASSOCIATED METABOLIC CHANGES IN BAT
As noted above, the primary energy source of BAT thermogenesis is FA. FA is also indispensable for BAT thermogenesis as an activator of UCP1 [25-28]. Thus, BAT thermogenesis seems intimately associated with intracellular TG hydrolysis and FA oxidation. On the other hand, there have been reports that incorporation of FA from circulation and its synthesis in BAT are also enhanced after cold exposure and/or sympathetic stimulation [29-31]. As such, lipid metabolism is drastically changed in parallel with activation of BAT thermogenesis; however, their causal relation to UCP1-dependent thermogenesis is not fully understood.
Recently, we conducted metabolomic and GeneChip array analyses of BAT after acute cold exposure in wild-type (WT) and UCP1-KO mice [32]. Our results suggest that the FA uptake, oxidation, and intracellular lipolysis simultaneously occur after cold exposure in WT and UCP1-KO mice, indicating these changes are UCP1-independently induced probably through the sympathetic nerve activation. On the other hand, FA-derived acetyl-coenzyme A (CoA) is oxidized through the tricarboxylic acid (TCA) cycle and oxidative phosphorylation in WT mice, while it is converted to citric acid and utilized for active de novo FA synthesis in UCP1-KO mice (Fig. 2). We also found that succinate, an intermediate of the TCA cycle, was accumulated after cold exposure in BAT of WT, but not in UCP1-KO mice. This is consistent with the reports that succinate is incorporated into brown adipocytes upon cold exposure and activates thermogenesis by producing reactive oxygen species [33], which activate thermogenesis through UCP1 sulfenylation [34].
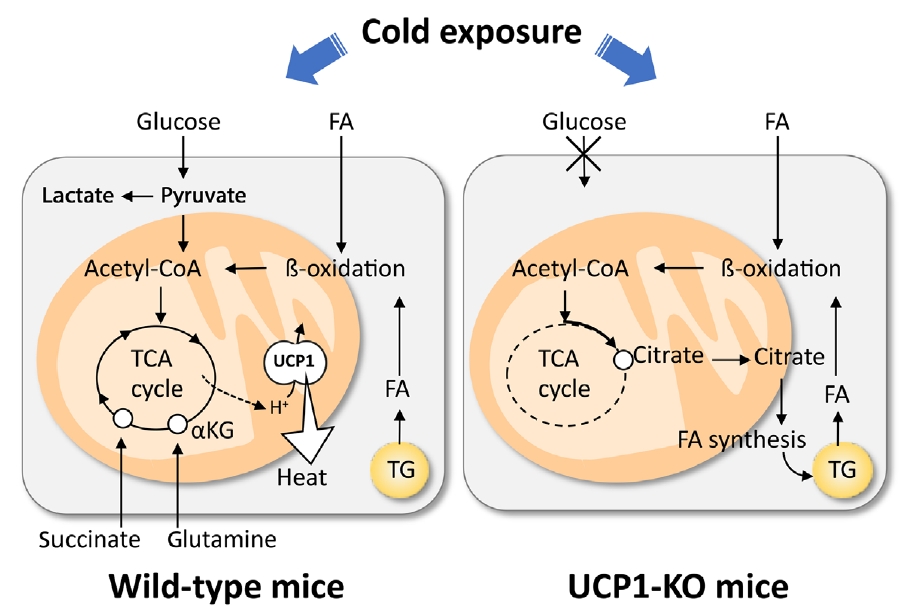
Cod-induced uncoupling protein 1 (UCP1)-dependent and -independent metabolic changes in brown adipose tissue (BAT). Cold exposure triggers triglyceride (TG) hydrolysis, fatty acid (FA) uptake, oxidation of FA via β-oxidation and tricarboxylic acid (TCA) cycle, and UCP1 activation. These are intimately associated with anaerobic glycolysis, and uptake of succinate and glutamine. In UCP1-KO brown adipocytes, cold exposure triggers TG hydrolysis, FA uptake, and β-oxidation of FA, similarly in wild-type brown adipocytes. Resulting acetyl-coenzyme A (CoA) is not oxidized by TCA cycle but used to re-synthesis of FA and TG. αKG, α-ketoglutaric acid.
In addition to lipid, glucose metabolism is also drastically changed in parallel with the activation of BAT thermogenesis. Cold exposure has been known to increase 2-deoxy-D-glucose (2-DG) uptake into BAT but only slightly into WAT [35,36]. The stimulatory effects of cold exposure can be mimicked by electrical stimulation of sympathetic nerves into BAT and β-adrenergic agonist administration, but are abolished by either sympathetic nerve surgery, β-adrenergic blockade, or genetic deletion of UCP1 [37,38]. Thus, the β-adrenergically stimulated 2-DG uptake into BAT is dependent on UCP1-mediated thermogenesis. Moreover, our metabolomic analysis revealed that glycolytic metabolites in BAT robustly increased by cold exposure in WT mice, but not in UCP1-KO mice [32]. In addition, lactic acid levels elevated only in WT mice, suggesting that a large proportion of glucose incorporated in BAT is metabolized via anaerobic glycolysis. It is most likely that glucose is used to replenish ATP through aerobic glycolysis to compensate for the decreased ATP production in mitochondria caused by UCP1 uncoupling. Thus, cold-induced increase in glucose utilization in BAT is secondary to UCP1 thermogenesis. This is a basis to use FDG-PET for the assessment of human BAT. It was also reported that inhibition of glycolysis impairs BAT thermogenesis [39,40]. This may contradict with the above-mentioned idea but may easily be explained by assuming that increased glycolysis is favorable and indispensable for a sufficient supply of oxaloacetate to enable rapid oxidation of FA and acetyl-CoA by the TCA cycle.
Some studies demonstrated an apparent association of amino acid metabolism and thermogenesis in BAT [41-43]. For example, acute cold exposure in rats increased BAT influx of amino acids, including glutamine and branched-chain amino acids (BCAAs), accounting for approximately one-third of the energy supplied by glucose to the tissue [44]. Indeed, glutamine level increased upon cold exposure in WT mice, but not in UCP1-KO mice [32]. Adipose tissue is known to have active glutamine metabolism [43] and contains a high level of glutaminase, an enzyme responsible for the conversion of glutamine to glutamate, reaching approximately 33% of the entire enzyme activity in rat lymphocytes or kidney [45]. Cold-induced uptake of glutamine is possibly related to the UCP1-thermogenesis induced tissue hypoxia [46], which is reported to enhance tissue uptake of glutamine used for citrate production from α-ketoglutaric acid [47] or de novo FA synthesis [48]. BCAA is also actively used as a substrate for thermogenesis and promotes systemic BCAA clearance. Recently, Yoneshiro et al. [49] demonstrated that BAT highly expresses the enzymes for BCAA catabolism and actively imports BCAA into mitochondria via the specific transporter SLC25A44.
BAT AS A REGULATOR OF SYSTEMIC METABOLISM
BAT thermogenesis is associated with metabolic changes in BAT itself and those in a wide range of systemic metabolism. The most representative is the regulatory role of whole-body energy expenditure (EE) and body fatness. Activation of BAT thermogenesis by cold exposure or βAR stimulation leads to an increase in whole-body EE and a concomitant decrease in WAT fat content. In diet-induced or genetically obese animals, whole-body EE and BAT activity are reduced, but BAT transplantation increases whole-body EE and reverses obesity [50,51]. The significant role of BAT in body fat regulation appears controversial because UCP1-KO mice are cold-sensitive but not obese [52,53]. This paradoxical finding was re-examined by Kontani et al. [54], who demonstrated that UCP1-KO mice get obese when they are kept on a high-fat diet for more than half a year. Feldmann et al. [55] also reported that UCP1-KO are susceptible to diet-induced obesity when they are maintained at thermoneutral temperatures (29°C), but not at conventional animal room temperatures (18°C to 22°C). Thus, the preventive effect of UCP1 against body fat accumulation is actualized only when animals need not to increase CIT. Therefore, in the absence of UCP1, some alternative calorically more costly mechanisms are used for thermogenesis to maintain body temperature, which also contribute to body fat regulation. The ATP-dependent futile cycles may be a likely mechanism. In fact, the calcium cycling mechanism activated by sarcolipin is upregulated in the skeletal muscle of UCP1-KO mice, and UCP1 expression is increased in sarcolipin-KO mice [14].
As noted in section 2, the main energy source of BAT thermogenesis is FA derived from intracellular TG and incorporated from circulation. BAT activation by cold exposure drastically accelerates clearance of plasma TG-rich lipoproteins (TRL) due to increased uptake into BAT. This depends on lipoprotein lipase and a lipid transporter CD36, and corrects hyperlipidemia in Apoa5-KO mice [56]. Similar effects were also found by pharmacologic activation of BAT with β3AR agonist CL316,243, which improves dyslipidemia, and more importantly, protects hyperlipidemic model mice from atherosclerosis [57]. Activation of BAT thermogenesis increases high-density lipoprotein (HDL) levels, promoting HDL cholesterol turnover [58], which may also contribute to the atheroprotective effects of BAT. All these facts suggest a direct contribution of BAT to the regulation of blood lipoprotein metabolism.
The significant contribution of BAT to blood metabolite levels was also documented in BCAA; that is, BCAA uptake into activated BAT produces an apparent reduction of plasma BCAA levels [49], which may improve insulin sensitivity in other peripheral tissues as noted in section 5.
Possible roles of BAT in whole-body glucose homeostasis have been suggested over the years. It has been reported that cold exposure potentiates the stimulatory effect of insulin and increases glucose uptake in peripheral tissues, including skeletal muscle, WAT, and BAT, resulting in improved glucose tolerance [59]. Direct evidence for the beneficial effects of BAT on glucose homeostasis has been accumulated in studies of BAT transplantation. Gunawardana and Piston [60] first demonstrated that subcutaneous transplants of embryonic BAT can correct type 1 diabetes mellitus in streptozotocin-treated mice with severely impaired glucose tolerance. In support of their observation, transplantation of BAT was shown to result in improved glucose tolerance, increased insulin sensitivity, lower body weight, decreased fat mass, and a complete reversal of high-fat diet-induced insulin resistance [61]. Inversely, surgical removal of the interscapular BAT in obese diabetic mice impairs insulin sensitivity [62]. Moreover, Nishio et al. [63] reported that mice with transplanted brown adipocytes, derived from human pluripotent stem cells (hPSC), show augmented glucose tolerance, while those with hPSC-derived white adipocytes show deteriorated glucose tolerance. Notably, co-transplantation of brown adipocytes normalizes glucose intolerance induced by white adipocyte transplantation. There have also been reports that induction of beige adipocytes by transgenic techniques improves glucose tolerance in WT and even in UCP1-KO mice [20], indicating that UCP1 is not necessarily indispensable for the beneficial effects of brown and beige adipocytes on glucose homeostasis.
BAT-DERIVED ENDOCRINE FACTORS REGULATING SYSTEMIC METABOLISM
As discussed above, even small amounts of BAT influence the broad range of systemic metabolisms, suggesting the role of BAT as a regulator of metabolism beyond thermogenesis. Mechanistically, some of the effects may be attributed to the relatively high metabolic activity of BAT itself, as in the case of the lowering effect on TRL and BCAA; however, those on glucose metabolism and insulin sensitivity may not be explained by this activity. In this connection, it is interesting that BAT secretes various molecules into extracellular fluid, collectively called as BATkines, which may mediate the effects of BAT on other tissues (Fig. 3) [11].
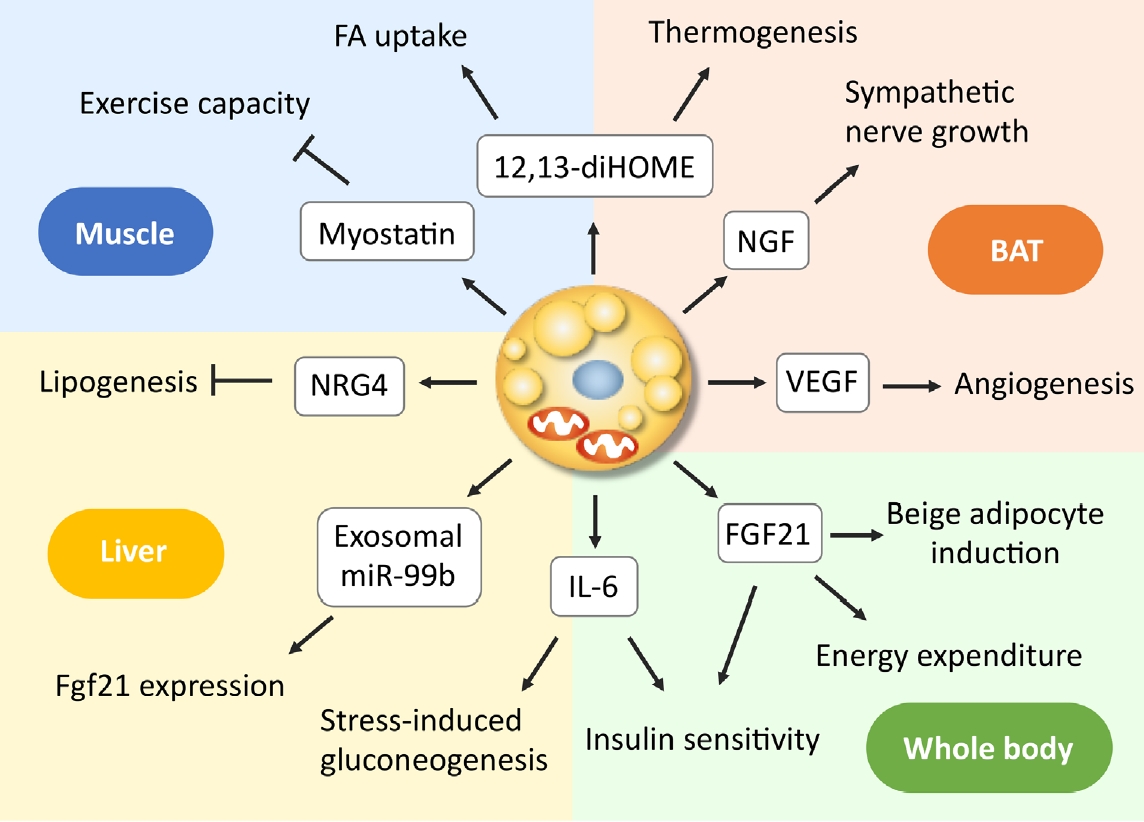
Endocrine actions of brown adipose tissue (BAT)-derived factors. Brown/beige adipocytes secrete various molecules, some of which such as nerve growth factor (NGF) and vascular endothelial growth factor (VEGF) function as paracrine factors, while others as endocrine factors, acting on the liver and skeletal muscle, thereby regulating systemic metabolism. 12,13-diHOME, 12,13-dihydroxyoctadecaenoic acid; FA, fatty acid; FGF21, fibroblast growth factor 21; IL-6, interleukin-6; miR, microRNA; NRG4, neuregulin 4.
It is known that BAT and WAT synthesize and secrete various adipokines, including adiponectin. In addition, activated BAT secretes some growth factors, such as vascular endothelial growth factor and nerve growth factors, which promote angiogenesis and sympathetic nerve growth associated with tissue hyperplasia [64-66]. In addition to these paracrine factors, BAT secretes a wide variety of molecules into circulating blood, including polypeptides, FA metabolites, and microRNA (miRNA).
Interleukin 6 (IL-6) is one of the polypeptides secreted from BAT. In the study of BAT transplantation mentioned in section 3, Stanford et al. [61] found that the improved metabolic profile was lost when the BAT used for transplantation was obtained from IL-6-KO mice, demonstrating that BAT-derived IL-6 is required for the profound effects of BAT transplantation on glucose homeostasis and insulin sensitivity. Recently, Qing et al. [67] reported that acute stress induces IL-6 secretion from BAT, which is the required instructive signal for mediating hyperglycemia through hepatic gluconeogenesis. Thus, IL-6 may be a likely BATkine to regulate systemic glucose metabolism. Interestingly, the secretion of IL-6 from BAT is mediated by β3AR but independently of UCP1 thermogenesis.
Another candidate of BATkines is fibroblast growth factor 21 (FGF21). FGF21 is an important regulator of insulin sensitivity, systemic metabolism, and EE [68]. Although FGF21 is released abundantly from the liver, it is also secreted from BAT in response to cold exposure or β3AR stimulation and contributes to increased circulating levels in mice and humans [69-71]. Some studies have demonstrated that UCP1 is dispensable for the action of FGF21 on body weight and glucose metabolism [72,73]. In contrast, FGF21 induces beige adipocyte biogenesis [74], suggesting that the FGF21 action is to stimulate some UCP1-independent mechanisms in beige adipocytes. In fact, Keipert et al. [75] reported that FGF-signaling controls paradoxical obesity resistance of UCP1-KO mice. Ruan et al. [76] also reported that brown adipocyte-specific FGF21 KO impaired the observed effects of adenosine A2A receptor agonism in attenuating hypertensive cardiac remodeling, suggesting an endocrine role of BAT in controlling hypertensive cardiac remodeling though the release of FGF21.
miRNAs are small noncoding RNAs with 20 to 22 nucleotides, that function as negative regulators of protein synthesis at the translational level and are involved in many cellular processes. miRNAs produced in adipocytes have a role in the differentiation and function of adipocyte itself and are also secreted through exosomes and taken-up into other cells. Thomou et al. [77] reported that mice with adipose-tissue-specific KO of the miRNA-processing enzyme exhibit a substantial decrease in the levels of circulating exosomal miRNAs, including miRNA-99b, and that the transplantation of BAT into the KO mice restores the circulating miRNA level, improves glucose tolerance, and suppresses hepatic FGF21 expression. These results suggest that exosomal miRNA-99b is secreted from BAT into blood circulation, acts on the liver, and suppresses hepatic FGF21 expression. It was also reported that circulating levels of miR-92a-3p and miRNA-122 are inversely correlated with BAT activity in humans [78,79]. Thus, it is likely that miRNA is a group of BATkines, although its precise role as a BATkine is largely elusive to date.
Lipid molecules also serve as signals between BAT and other tissues. When FA derived from cold-induced lipolysis in WAT is oxidized in liver and in BAT, the acylcarnitine formed from carnitine and acyl-CoA by carnitine acyltransferases are secreted into circulation, and incorporated into BAT to be utilized as energy fuel for thermogenesis [80]. Oxylipins, molecules derived from the oxidation of polyunsaturated FAs, are attracted interest because of their action as intercellular signaling molecules and involvement in the regulation of many cell and tissue responses. 12,13-Dihydroxyoctadecaenoic acid (12,13-diHOME) is secreted from BAT upon cold exposure and activates BAT in paracrine manner to enhance thermogenesis, resulting in decreased levels of serum TGs [81]. 12,13-diHOME secreted from BAT is also enhanced by exercise and increases FA uptake in muscle [82].
In addition, many molecules such as neureglin 4 (NRG4) [83] and myostatin [84] were identified as BATkines, but a comprehensive overview is beyond the scope of this article and found in other literatures [11,66,85,86].
Besides these BATkines, afferent nerve may also be involved in the signaling from BAT. We found that chronic β3AR agonist treatment potentiates anorexigenic effects of leptin at the hypothalamus level, in parallel with UCP1 induction in WAT, in WT mice, but not in UCP1-KO mice [87]. Yamada et al. [88] also showed that ectopic expression of very low levels of UCP1 in epididymal WAT improves glucose tolerance and reverses both insulin and leptin resistance in obese mouse models. Interestingly, local-nerve dissection in the epididymis or pharmacological afferent blockade blunts the leptin action, suggesting that afferent-nerve signals from intra-abdominal fat tissue regulate food intake by modulating hypothalamic leptin sensitivity. Histological and physiological evidence has indicated that BAT has sensory system innervation overlapping with the sympathetic efferent system [89]. Thus, it may be possible, that the BAT-derived sensory nervous system, in addition to and in combination with BATkines, plays a role in the regulation of systemic metabolism through the central nervous system.
BAT IN HUMAN PHYSIOLOGY AND DISEASE
Although our understanding on BAT discussed above is based largely on the studies in small rodents, the rediscovery of metabolically active BAT using FDG-PET in adult humans [2-5] has dramatically accelerated the translational studies on BAT in health and diseases.
A role of BAT to NST in humans has been demonstrated for CIT and diet-induced thermogenesis (DIT); that is, whole-body EE after mild cold exposure or meal intake is greater in individuals with higher BAT activities than those with lower activities [7,90-95]. This is also supported by the observations that a single nucleotide substitution in the UCP1 gene at -3826A to G, which lowers UCP1 mRNA expression [96], attenuates both CIT and DIT [97,98]. Muzik et al. [99] reported that CIT estimated from whole-body EE is much smaller than EE by BAT directly estimated by 15O2-PET, contradicting a significant role of BAT detected by FDG-PET in whole-body EE and CIT. This discrepant result may be caused by FDG-PET which underestimated the total mass of BAT [100]. Consistent with the role of BAT in short-term regulation of EE, BAT is thought as a long-term regulator of energy and body fats in humans. Both clinical and experimental studies have revealed an inverse relationship between the activity/prevalence of BAT and adiposity-related parameters, such as body mass index, body fat content, and visceral fat accumulation [101-104]. Moreover, cold-induced reactivation and recruitment of BAT in individuals with low BAT activities results in a significant increase in CIT and a concomitant reduction of body fat content [105-107].
Accumulated evidence has suggested a regulatory role of BAT in glucose homeostasis and insulin sensitivity in humans. We found in healthy adults that blood glucose and glycosylated hemoglobin (HbA1c) levels are lower in individuals with higher BAT activities [104]. Regression analysis revealed that BAT, in addition to age, sex, and body fatness, is a significantly independent determinant of glucose and HbA1c. These results seem compatible with some retrospective analyses of patient data showing blood glucose as a determinant of BAT prevalence [4,102,103]. Chondronikola et al. [108] reported that mild cold exposure for 5 hours increased whole-body glucose disposal, plasma glucose oxidation, and insulin sensitivity in men with significant amounts of BAT, but not in those without detectable BAT. Improved insulin sensitivity in parallel with BAT recruitment after cold acclimation was also shown in healthy men [109,110] and in patients with type 2 diabetes mellitus [111].
In connection with the beneficial effects of BAT on insulin sensitivity, BAT also actively metabolizes BCAA to lower their plasma levels in humans [49]. Accordingly, impaired BAT activity reduces BCAA clearance, thereby resulting in increased circulating BCAA levels, which are often seen in obesity and aging [112]. The accumulation of intracellular BCAA results in mammalian target of rapamycin activation and subsequent inhibition of insulin signaling [113].
Very recent studies also revealed significant beneficial effects of BAT on the cardiovascular system in humans, in agreement with the atheroprotective effects of BAT in mice [57]. Becher et al. [114] categorized 53,475 patients by presence or absence of BAT, and found improved profiles of blood glucose, TG, and HDL-cholesterol in individuals with BAT, as already reported in healthy humans. Notably, BAT independently correlated with lower rates of hypertension, type 2 diabetes mellitus, coronary artery disease, and congestive heart failure. These results are well consistent with a 5-year follow-up study reported by Raiko et al. [115] showing that BAT activity correlated with lower carotid intima-media thickness and higher carotid elasticity.
CONCLUSIONS
As discussed in the previous sections, the preclinical and clinical studies, together with experimental studies in mice, have indicated that BAT is important as a regulator of whole-body energy balance, systemic metabolism, and cardiovascular functions. Hence, BAT is now recognized as an intriguing therapeutic target of obesity and metabolic disorders such as diabetes mellitus, dyslipidemia, and related cardiovascular diseases. In fact, several drugs and food ingredients targeting BAT have been tested for pharmacological and nutritional therapy of obesity and metabolic syndrome [116,117].
Despite these advances in experimental and clinical researches of BAT, some important questions remain to be solved: for example, in mouse models, beige fat induction is greatly influenced by their genetic background [118,119], but in humans little is known about possible effects of genetic variants on brown and beige adipocytes, which undoubtedly participate in the remarkable individual difference in the amount and activity of BAT [120]. Another problem is related to the method used in assessing human BAT. To date, FDG-PET is a standard tool used to measure human BAT [121]; however, this option has serious limitations, including the enormous cost of devices, radiation exposure, and acute cold exposure, which make repeated measurements difficult and an impediment in basic and clinical studies. There is therefore an urgent need to establish less invasive and simpler methods for quantitative assessment of human BAT. This would promote dedicated human studies, including longitudinal observations and the development of practical, easy, and effective regimens that can activate and recruit BAT.
Notes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
FUNDING
This study was supported by Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan, 17K08118 to Okamatsu-Ogura Yuko, and 16K15485, 18K11013 to Masayuki Saito.
Acknowledgements
None