Sulforaphane Ameliorates Diabetes-Induced Renal Fibrosis through Epigenetic Up-Regulation of BMP-7
Article information
Abstract
Background
The dietary agent sulforaphane (SFN) has been reported to reduce diabetes-induced renal fibrosis, as well as inhibit histone deacetylase (HDAC) activity. Bone morphologic protein 7 (BMP-7) has been shown to reduce renal fibrosis induced by transforming growth factor-beta1. The aim of this study was to investigate the epigenetic effect of SFN on BMP-7 expression in diabetes-induced renal fibrosis.
Methods
Streptozotocin (STZ)-induced diabetic mice and age-matched controls were subcutaneously injected with SFN or vehicle for 4 months to measure the in vivo effects of SFN on the kidneys. The human renal proximal tubular (HK11) cell line was used to mimic diabetic conditions in vitro. HK11 cells were transfected to over-express HDAC2 and treated with high glucose/palmitate (HG/Pal) to explore the epigenetic modulation of BMP-7 in SFN-mediated protection against HG/Pal-induced renal fibrosis.
Results
SFN significantly attenuated diabetes-induced renal fibrosis in vivo. Among all of the HDACs we detected, HDAC2 activity was markedly elevated in the STZ-induced diabetic kidneys and HG/Pal-treated HK11 cells. SFN inhibited the diabetes-induced increase in HDAC2 activity which was associated with histone acetylation and transcriptional activation of the BMP-7 promoter. HDAC2 over-expression reduced BMP-7 expression and abolished the SFN-mediated protection against HG/Pal-induced fibrosis in vitro.
Conclusion
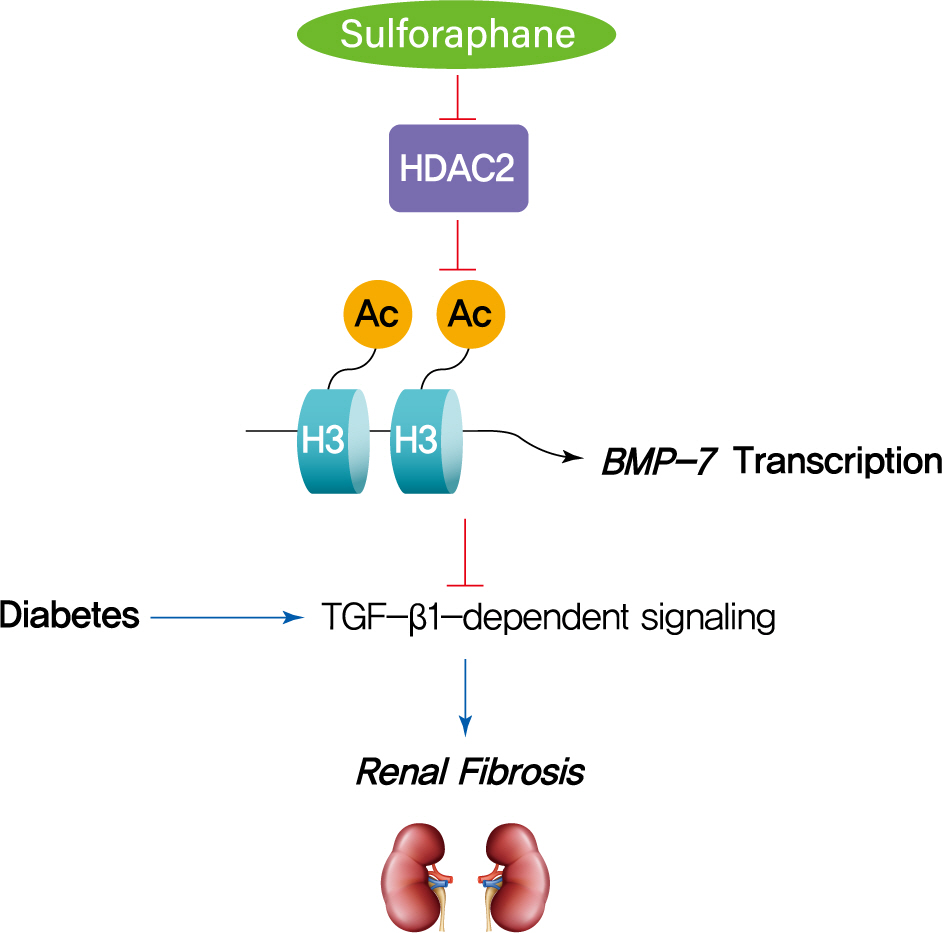
Our study demonstrates that the HDAC inhibitor SFN protects against diabetes-induced renal fibrosis through epigenetic up-regulation of BMP-7.
INTRODUCTION
Acetylation is a highly conserved post-translational modification that occurs on lysine residues. Histone acetylation promotes relaxed chromatin formation, thereby facilitating gene transcription. In contrast, histone deacetylation leads to chromatin compaction and transcription repression. Therefore, histone acetyltransferases (HATs) and histone deacetylases (HDACs) play a key role in the dynamic regulation of gene transcription. Increasing evidence has demonstrated that a decrease in histone acetylation is associated with a higher risk of diabetic vascular complications, such as diabetic nephropathy (DN) [1], diabetic retinopathy [2], and diabetic cardiomyopathy [3]. HDAC inhibitors have been shown to reduce fibrosis in some organs, such as the kidney [4,5], heart [6], and lung [7], but the mechanisms are not well understood.
Sulforaphane (SFN), a natural isothiocyanate extracted from broccoli sprouts, serves as a cancer chemo-preventive agent by inhibiting HDAC activity [8-10]. Moreover, there is evidence that SFN can attenuate diabetes-induced renal fibrosis, inflammation, and albuminuria [11,12]. However, the epigenetic effects of SFN on the diabetic kidney remain unclear.
Renal fibrosis is believed to be a pathological basis for DN. Transforming growth factor beta1 (TGF-β1) is an important cytokine that induces renal fibrosis, and TGF-β1-induced tubular epithelial-to-mesenchymal transition (EMT) is the key process in renal fibrogenesis associated with DN. Bone morphologic protein 7 (BMP-7), which belongs to the TGF-β superfamily, is expressed in the normal kidney parenchyma, especially in the epithelium of the proximal tubules [13]. BMP-7 has been shown to antagonize EMT and renal fibrosis induced by TGF-β1 via inducing the epithelial adhesion molecule Ecadherin in various renal diseases [14]. Exogenous administration of BMP-7 reverses renal fibrosis, as well as restores renal function and structure in animals with unilateral ureteral obstruction [15,16], DN [17], nephrotoxic serum nephritis [14], lupus nephritis [18], and acute kidney injury [19]. Therefore, BMP-7 has become a new therapeutic target for treating DN.
Epigenetic regulation of BMP-7 has been recently investigated. An in vitro study showed that the HDAC inhibitor trichostatin A (TSA) suppressed TGF-β1-induced EMT which was associated with the up-regulation of BMP-7. Chromatin immunoprecipitation (ChIP) assays also confirmed the role of histone acetylation in the up-regulation of BMP-7 [20]. Furthermore, TSA was shown to increase BMP-7 expression and subsequently inhibit TGF-β-dependent signaling pathways and renal fibrosis in an in vivo study [21]. Down-regulation of HDAC5 in an ischemia/reperfusion mouse model was reported to be involved in BMP-7 up-regulation, likely mediated by histone acetylation [22]. Given these findings, we hypothesized that the HDAC inhibitor SFN could prevent diabetes-induced renal fibrosis through epigenetic up-regulation of BMP-7.
METHODS
Animals
The FVB/N (FVB) mice were purchased from the animal center of Jilin University. All experimental procedures were approved by the Animal Ethics Review Committee of Jilin University (approved No., 2019-231), and were in compliance with the regulations set by the Institutional Committee for the Care and Use of Laboratory Animals of the Experimental Animal Center of Jilin University, China.
Streptozotocin (STZ) (Sigma-Aldrich, St. Louis, MO, USA) or sodium citrate was injected intraperitoneally into 8-weekold FVB mice for 5 consecutive days. STZ was administered at a dose of 50 mg/kg dissolved in 0.1 M sodium citrate with a pH of 4.5. Blood glucose levels were tested after 5 days of injection. Hyperglycemic mice (blood glucose levels ≥250 mg/dL) were defined as having diabetes. All mice were randomly allocated into the following four groups: control, SFN, diabetes mellitus (DM), and DM/SFN, with at least six mice in each group.
SFN (Sigma-Aldrich) or the vehicle (phosphate buffered saline [PBS] with 1% dimethyl sulfoxide [DMSO]) was injected subcutaneously for 5 days every week for 4 consecutive months. SFN was dissolved in DMSO and diluted with PBS to a final dose of 0.5 mg/kg [11,23].
Mouse urinary albumin to creatinine ratio
Mouse urine was collected after 4 months of SFN treatment. Urinary albumin and creatinine levels were measured according to the test kit manuals (Bethyl Laboratories Inc., Montgomery, TX; and BioAssay Systems, Hayward, CA, respectively). The urinary albumin to creatinine ratio (UACR) was calculated as the direct ratio of urinary albumin/urinary creatinine and expressed in terms of μg/mg.
Renal histopathological examination
Kidney tissues were fixed in 10% buffered formalin, then dehydrated through a 70%, 90%, 96%, and 100% alcohol gradient. Specimens were cleared in xylene, embedded in paraffin, and sectioned at 5 μm thickness. Sections were stained with both Periodic acid Schiff (PAS) and Masson’s trichrome as described previously [24], then assessed using a Nikon Eclipse E600 microscopy system (Nikon, Tokyo, Japan) by a histologist in a blinded fashion. Morphometric analyses were performed using Image-Pro Plus 6.0 software (Media Cybernetics, Bethesda, MD, USA). At least 30 glomeruli per section were selected for image analysis. The mesangial matrix and Masson’s trichrome-positive area in each glomerulus to the full glomerular area were assessed for renal fibrosis.
Real-time polymerase chain reaction
Kidney tissues that were stored at –80°C were pulverized in liquid nitrogen and homogenized in Trizol (Invitrogen, Carlsbad, CA, USA) to extract total RNA. RNA purity and concentration were quantified using a Nanodrop ND-1000 spectrophotometer (Thermo Fisher, Waltham, MA, USA). The cDNA product was synthesized and amplified from the RNA according to the manufacturer’s polymerase chain reaction (PCR) protocol (Promega, Madison, WI, USA) using primers for BMP-7 and actin (Life Technologies, Grand Island, NY, USA).
Western blot analysis
Western blot analysis was conducted as previously described [11]. The primary antibodies used in the experiments were as follows: fibronectin (FN; 1:500), collagen I (1:2,000), collagen IV (1:2,000), α-smooth muscle actin (α-SMA; 1:1,000), H3K9/14Ac (1:1,000), HDAC2 (1:1,000), TGF-β1 (1:1,000), phosphoSmad1/5/8 (1:1,000), phospho-Smad2/3 (1:1,000), BMP-7 (1:500), and β-actin (1:3,000). All antibodies were purchased from Santa Cruz Biotechnology (Dallas, TX, USA), except BMP-7 and phospho-Smad1/5/8 (Cell Signaling Technology, Danvers, MA, USA) and phospho-Smad2/3 (Abcam, Cambridge, UK).
Chromatin immunoprecipitation
A commercially available kit (Epigentek, Farmingdale, NY, USA) was used for the ChIP assay according to the manufacturer’s instructions. Antibodies included H3K9/14Ac (Cell Signaling Technology) and immunoglobulin G (negative control [NC], included in the tissue ChIP kit). The immunoprecipitated DNA samples, as well as input DNA samples, were analyzed using real-time PCR with a promoter-specific primer for BMP-7: 5´-TCACTCACTGCCATTCTG-3´and 5´-GTGGATTGCTGCTCTTTG-3´.
Cell culture
Human renal proximal tubular (HK11) cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM)-F12 supplemented with 5% fetal bovine serum (FBS) [25]. To mimic diabetes, HK11 cells were exposed to D-glucose at a concentration of 27.5 mM (high glucose [HG]) with 2% bovine serum albumin and 1% FBS for 48 hours. Palmitate (Pal; 300 μM) was added during the last 6 hours of glucose incubation [25]. SFN dissolved in DMSO (3 μM final concentration) was added for 48 hours [11], and DMSO without SFN was used as the vehicle control.
Plasmid constructs and over-expression of HDAC2
The primers used to amplify the full coding region sequence of HDAC2 with the total cDNA as the template were as follows: 5´-CTGTCTAGAATGGCGTACAGTCAAGGA-3´ (forward) and 5´-CTGGGATCCTCAGGGGTTGCTGAGCTG-3´ (reverse). The restriction endonuclease sites are underlined. The PCR product was digested with XbaI and BamHI and cloned into the vector VR1012. The recombinant vector VR1012-HDAC2 was transfected into HK11 cells using the Viafect transfection reagent (Promega) to over-express HDAC2, as previously described [26]. Cells transfected with empty vectors were used as NCs.
Immunoprecipitation and HDAC activity assay
A commercially available HDAC assay kit (Upstate) was used to measure HDAC activity according to the manufacturer’s instructions. Briefly, kidney tissue and HK11 cell lysates were prepared in radioimmunoprecipitation assay (RIPA) lysis buffer containing phosphatase inhibitors and protease inhibitors. The protein concentration was measured using the bicinchoninic acid assay (BCA; Pierce, Rockford, IL, USA). Lysates were incubated overnight at 4°C with antibodies against HDAC1-5 and HDAC8 (Santa Cruz Biotechnology). Protein A/G PLUSAgarose (Santa Cruz Biotechnology) was added and incubation continued at 4°C for 2 hours. Precipitates were washed with RIPA buffer and HDAC assay buffer. HDAC activity was measured at a 405 nm wavelength using a spectrophotometer.
Statistical analysis
In vivo and in vitro data were collected from at least six mice or four separate cell cultures for each group and are presented as mean±standard deviation. Comparisons were performed using one-way Analysis of variance (ANOVA) for different groups, followed by Tukey’s post hoc test. Unpaired Student’s t-tests were used to compare the means of two groups. Statistical analysis was performed with Origin 7.5 Laboratory data analysis and graphing software (OriginLab Corporation, Northampton, MA, USA). Statistical significance was considered as P<0.05.
RESULTS
Effects of SFN on diabetes-induced systemic changes and renal disfunction
In the present study, both non-diabetic and diabetic mice were treated with SFN or vehicle for a period of 4 months. As a primary index of renal function, UACR was calculated post-SFN treatment. As shown in Fig. 1A, the UACR was significantly higher in the diabetic mice compared to the control mice. Compared with the mice in the DM group, SFN treatment for four months attenuated the diabetes-induced elevation in UACR. Similar to the UACR, kidney weight to tibia length was higher in the DM group, which was decreased with SFN treatment (Fig. 1B). Blood glucose levels were higher in the diabetic mice compared to the control mice (Fig. 1C), but SFN treatment did not affect blood glucose levels. As shown in Fig. 1D, body weight gain was comparatively slower in the diabetic mice compared to the control mice. SFN treatment increased body weight in the diabetic group, which indicates that the catabolic status of the diabetic mice was alleviated by SFN.
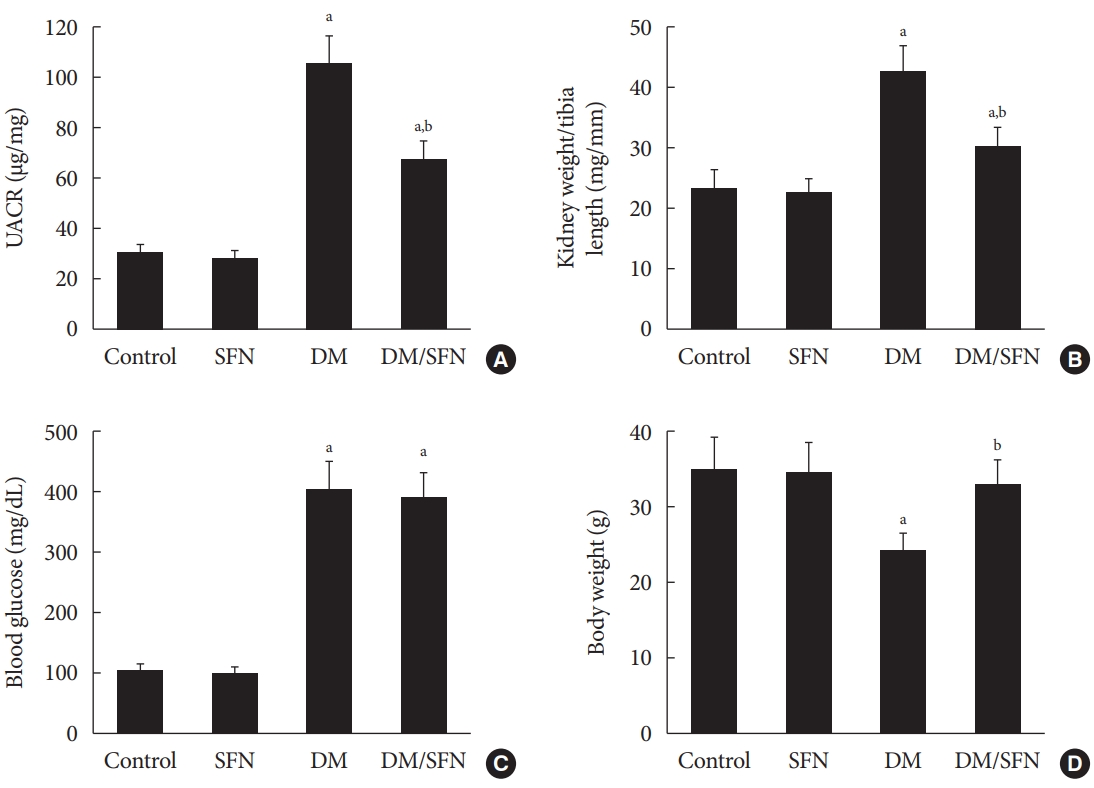
Effects of sulforaphane (SFN) on diabetes-induced systemic changes and renal disfunction. (A) Urinary albumin to creatinine ratio (UACR), (B) kidney weight/tibia length, (C) blood glucose, and (D) body weight were measured in all mice. Data are presented as mean±standard deviation (n=at least 6 per group). DM, diabetes mellitus. aP<0.05 vs. control, bP<0.05 vs. DM group.
SFN alleviates diabetes-induced glycogen and collagen accumulation in the kidney
Extracellular matrix (ECM) is a non-cellular three-dimensional macromolecular network composed of collagens, FN, laminins, and several other glycoproteins [27]. ECM and collagen deposition are important hallmarks of glomerulosclerosis and renal fibrosis [24]. PAS and Masson’s trichrome staining were used to quantify the glycogen and collagen deposition, respectively. Glomerular enlargement (Fig. 2A and B), mesangial matrix expansion (Fig. 2A and C), and enlarged trichromepositive areas (Fig. 2B and D) were found in kidney tissues collected from the diabetic mice. SFN treatment significantly reduced these lesions.

Sulforaphane (SFN) alleviates diabetes-induced glycogen and collagen accumulation in the kidney. Kidney pathology was examined with Periodic acid Schiff (PAS; A, ×400) and Masson’s trichrome staining (B, ×400) in all mice. Mesangial matrix expansion (C) was quantified from PAS staining and fibrotic accumulation (D) was quantified from Masson’s trichrome staining. Glycogen- and collagen-positive areas are displayed in microscopic images of PAS and Masson’s trichrome staining, respectively. Data are presented as mean±standard deviation (n=at least 6 per group). DM, diabetes mellitus. aP<0.05 vs. control, bP<0.05 vs. DM group.
SFN reduces diabetes-induced kidney fibrosis
Diabetes-induced renal fibrosis was further determined by measuring protein expression of collagen I, collagen IV, and FN, which are the main components of the ECM. In agreement with Fig. 2B, these proteins were significantly up-regulated in kidney tissues collected from the diabetic mice (Fig. 3A-C), whereas SFN treatment decreased the expression of collagen I (Fig. 3A), collagen IV (Fig. 3B), and FN (Fig. 3C). α-SMA, which is a typical marker of myofibroblasts and fibrosis, was measured using Western blot analysis. α-SMA protein expression was higher in the diabetic kidney tissues compared to the control kidney tissues. SFN treatment significantly reduced the protein expression of α-SMA in the diabetic kidney tissues (Fig. 3D).
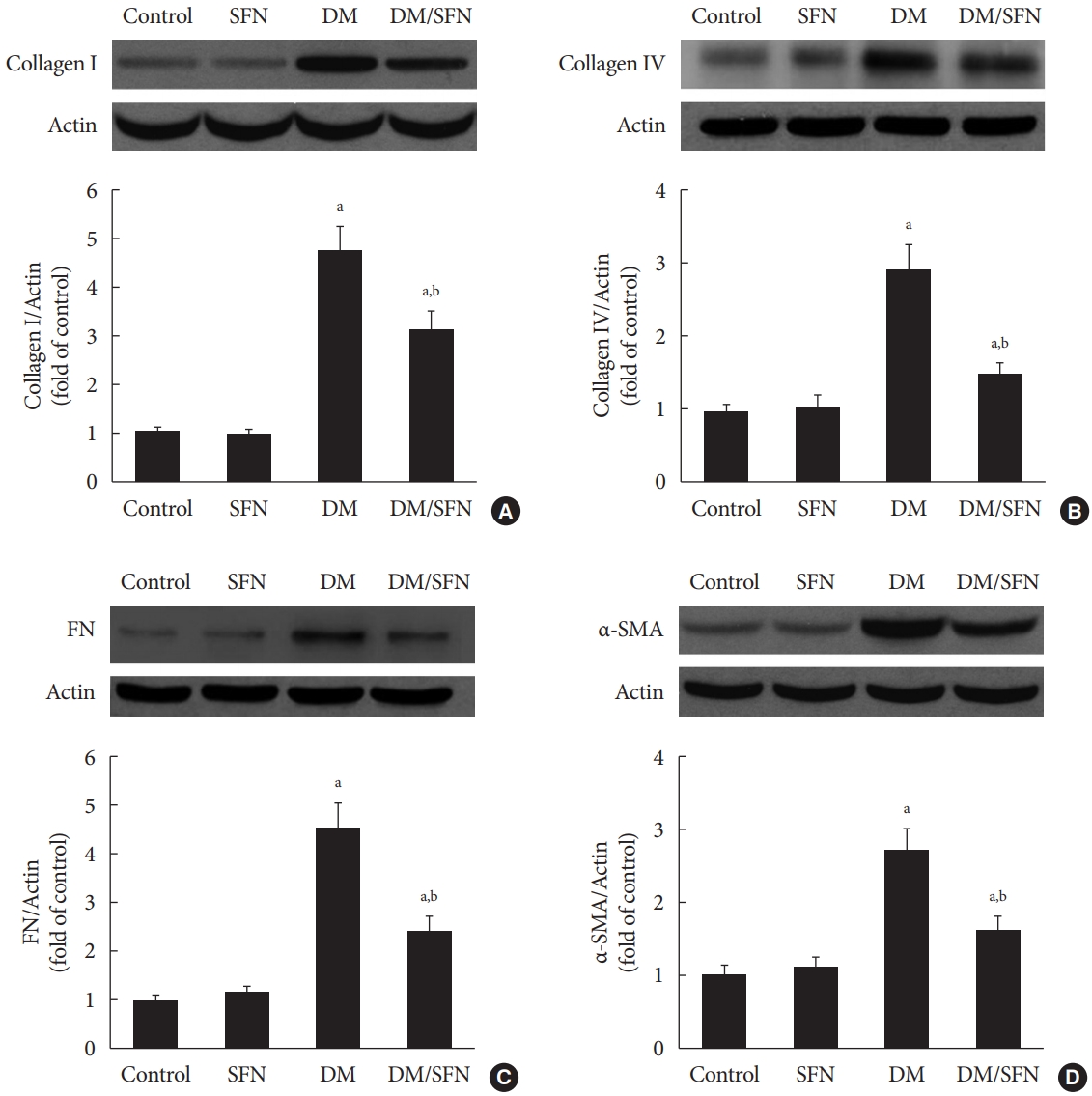
Sulforaphane (SFN) attenuated diabetes-induced renal fibrosis. Protein expression of collagen I (A), collagen IV (B), fibronectin (FN) (C), and α-smooth muscle actin (α-SMA) (D) were detected using Western blot analysis. Data are presented as mean±standard deviation (n=at least 6 per group). DM, diabetes mellitus. aP<0.05 vs. control, bP<0.05 vs. DM group.
SFN-induced histone acetylation is involved in BMP-7 up-regulation
To determine the effect of SFN on the expression of BMP-7, we measured BMP-7 mRNA levels using real-time PCR and protein levels using Western blot analysis. BMP-7 expression was significantly reduced in the diabetic mice (Fig. 4A and B). SFN treatment restored the BMP-7 expression both at the mRNA and protein levels (Fig. 4A and B). Next, we determined if the up-regulation of BMP-7 by SFN was associated with histone acetylation. Total H3K9/14Ac expression in the kidney tissues of each group was evaluated using Western blot analysis. The H3K9/14Ac levels in the BMP-7 promoter were measured using ChIP assays. As shown in Fig. 4C and D, diabetes decreased H3K9/14Ac expression and the H3K9/14Ac levels in the BMP-7 promoter. SFN treatment significantly reversed the diabetes-induced decrease in H3K9/14Ac expression and the H3K9/14Ac levels in the BMP-7 promoter, which was in agreement with BMP-7 up-regulation. These results suggest that SFN-induced histone acetylation is involved in the up-regulation of BMP-7. Given that SFN is an HDAC inhibitor, we hypothesized that SFN restores BMP-7 expression by inhibiting diabetes-increased HDAC activity.
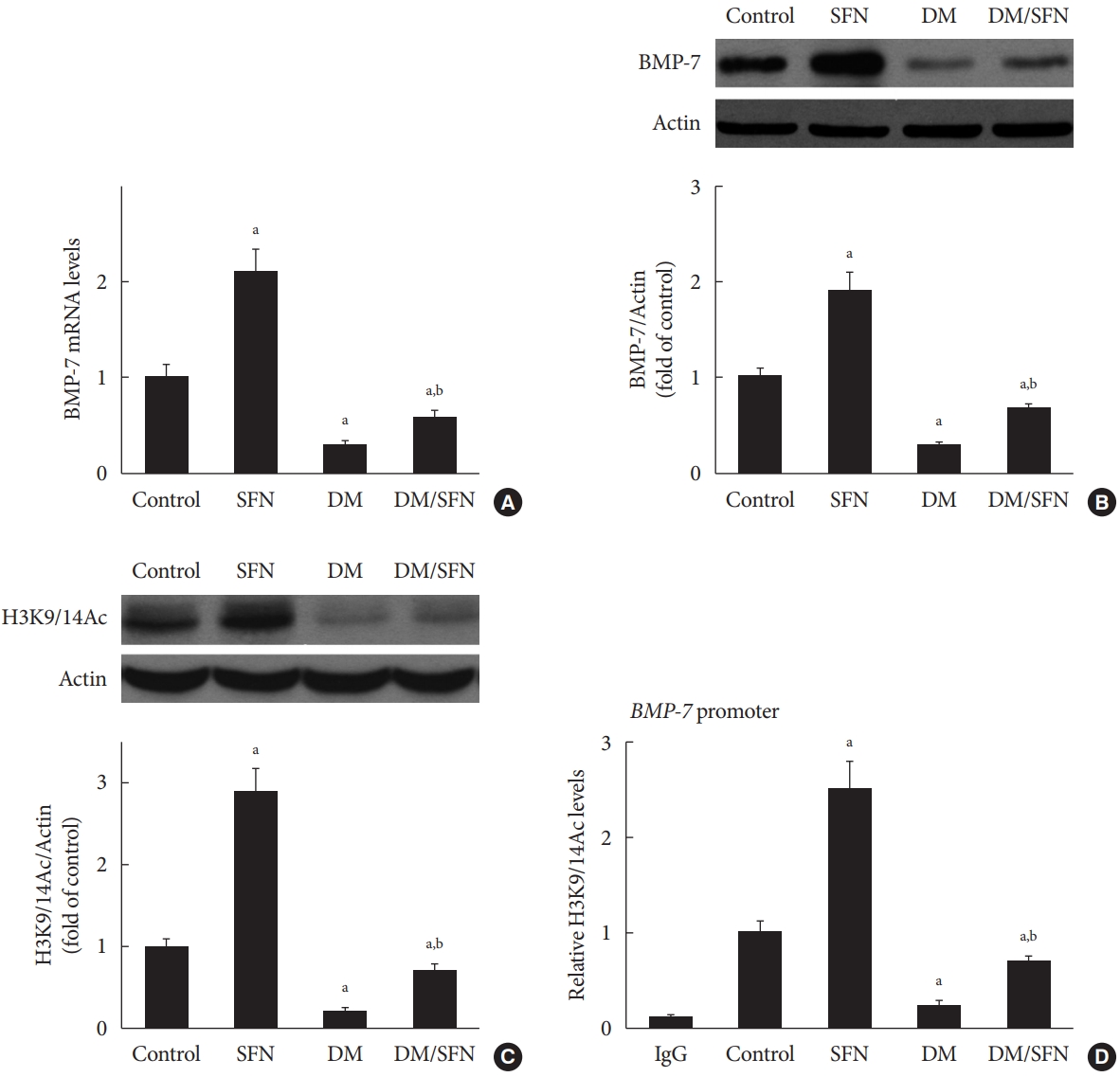
Sulforaphane (SFN)-induced histone acetylation is involved in the up-regulation of bone morphologic protein 7 (BMP-7). BMP-7 mRNA levels (A) and protein expression (B) were detected using real-time polymerase chain reaction and Western blot analysis, respectively. Expression of H3K9/14Ac was assessed using Western blot analysis (C). H3K9/14Ac levels in the BMP-7 promoter were measured using the chromatin immunoprecipitation (ChIP) assay (D). Data are presented as mean±standard deviation (n=at least 6 per group). DM, diabetes mellitus; IgG, immunoglobulin G. aP<0.05 vs. control, bP<0.05 vs. DM group.
HDAC2 is a crucial regulator of diabetes-induced renal fibrosis
Since each HDAC isoform appears to have a different function, it was necessary to determine which isoform was involved in the diabetes-induced renal fibrosis in our model. Emerging evidence has shown that class I and class IIa HDACs play an important role in diabetes-induced renal damage [20,28]. Therefore, we measured the activity of HDAC1, 2, 3, and 8 (class I HDACs) and HDAC4 and 5 (class IIa HDACs). We found that only HDAC2 activity was increased in the STZ-induced diabetic kidneys (Fig. 5A) and HG/Pal-treated HK11 cells (Fig. 5B). However, diabetes and HG/Pal had no effect on HDAC2 mRNA levels and protein expression (data not shown). To further confirm the crucial role of HDAC2 in DN, HDAC2-overexpressing HK11 cells were used to test whether HDAC2 overexpression aggravates HG/Pal-induced renal fibrosis. Western blot analysis showed that transfection with an HDAC2 overexpression plasmid increased HDAC2 protein expression (Fig. 5C). Interestingly, over-expression of HDAC2 decreased BMP-7 protein expression (Fig. 5C) and aggravated HG/Pal-induced renal fibrosis, as exhibited by increased α-SMA and FN expression in vitro (Fig. 5D).
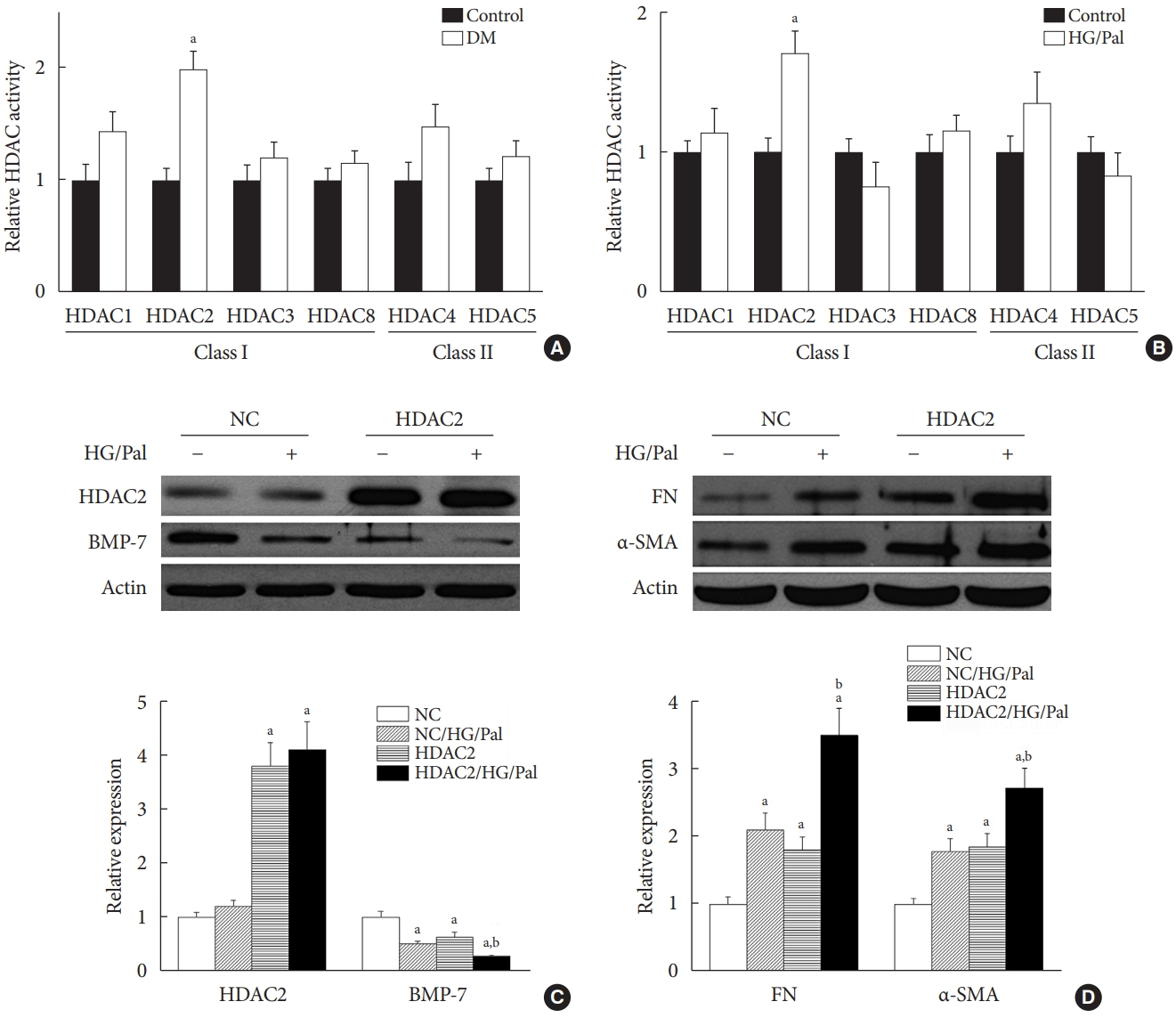
Histone deacetylase 2 (HDAC2) is a crucial regulator of diabetes-induced renal fibrosis. (A) Effect of streptozotocin-induced diabetes on HDAC activity (n=at least 6 per group). (B) Effect of hyperglycemia/hyperlipidemia on HDAC activity in human renal proximal tubular (HK11) cells. (C, D) HK11 cells were transfected with an HDAC2 over-expression plasmid. The transfection efficiency was determined using Western blot analysis (C). Effects of HDAC2 over-expression on bone morphologic protein 7 (BMP-7) expression (C) and hyperglycemia/hyperlipidemia-induced renal fibrosis (D) were determined using Western blot analysis. Data are presented as mean±standard deviation of four experiments. DM, diabetes mellitus; HG/Pal, high glucose/palmitate; NC, negative control transfected with empty vector; FN, fibronectin; α-SMA, α-smooth muscle actin. aP<0.05 vs. control or NC correspondingly, bP<0.05 vs. NC/HG/Pal.
SFN activates BMP-7-Smad1/5/8 and suppresses TGF-β1-Smad2/3 pathways by inhibiting HDAC2 activity
The results in Fig. 5 provide evidence of the involvement of HDAC2 in DN. Furthermore, SFN has been shown to potently inhibit HDAC2 activity and mildly inhibit the activity of class IIa HDACs [29]. Therefore, we measured HDAC2 activity in the kidneys of the four groups. We found that SFN prevented the diabetes-mediated increase in HDAC2 activity in the kidneys of the diabetic mice (Fig. 6A).

Sulforaphane (SFN) activates bone morphologic protein 7 (BMP-7)-Smad1/5/8 and suppresses transforming growth factor beta1 (TGF-β1)-Smad2/3 pathways by inhibiting histone deacetylase 2 (HDAC2) activity. (A) Effect of SFN on HDAC2 activity. (B) Effect of SFN on the expression of phospho-Smad2/3 and TGF-β1. (C) Effect of SFN on the expression of phospho-Smad1/5/8. Data are presented as mean±standard deviation (n=at least 6 per group). (D, E, F, G) Effects of HDAC2 over-expression on SFN-mediated prevention of hyperglycemia/hyperlipidemia-induced fibrosis and on SFN-induced BMP-7 expression. Data are presented as mean±standard deviation of four experiments. DM, diabetes mellitus; HG/Pal, high glucose/palmitate; FN, fibronectin; NC, negative control transfected with empty vector. aP<0.05 vs. control, bP<0.05 vs. DM group, cP<0.05 vs. NC/control or HDAC2/control correspondingly, dP<0.05 vs. NC/HG/Pal.
Both BMP-7 and TGF-β belong to the TGF-β superfamily, but they activate phosphorylation of distinct Smad proteins. BMP-7 activates Smad1/5/8 and TGF-β activates Smad2/3 [30]. Renal fibrosis has been reported to be attenuated by BMP-7 via activating Smad1/5/8 to inhibit TGF-β/Smad pathways [30]. We next measured the expression of phosphoSmad1/5/8, phospho-Smad2/3, and TGF-β1 in the kidneys. Fig. 6B shows that diabetes increased the expression of TGF-β1 and phospho-Smad2/3, which were decreased by SFN treatment. Phospho-Smad1/5/8 was decreased in the diabetic kidneys (Fig. 6C), but SFN restored phospho-Smad1/5/8 expression (Fig. 6C), which was paralleled with changes in expression of its upstream protein, BMP-7 (Fig. 4B).
The above results indicate that SFN reduces diabetes-induced renal fibrosis (Figs 2 and 3), inhibits HDAC2 activity (Fig. 6A), and stimulates BMP-7 expression, histone acetylation, and transcriptional activation of the BMP-7 promoter (Fig. 4). However, whether there exists a causal relationship between BMP-7 epigenetic regulation and renal fibrosis remains unclear. To determine the role of BMP-7 epigenetic regulation in renal fibrosis, HDAC2-over-expressing HK11 cells were again used. Our results showed that in the NC group, SFN significantly reduced HG/Pal-induced FN expression (Fig. 6D and E) and inhibited the activation of TGF-β target proteins, Smad2/3 (Fig. 6D and F), which was associated with BMP-7 up-regulation (Fig. 6D and G). However, over-expression of HDAC2 significantly decreased SFN-induced BMP-7 expression (Fig. 6D and G) and abolished the SFN-mediated prevention of HG/Pal-induced fibrosis (Fig. 6D-F).
DISCUSSION
Increasing evidence has shown the vital role of histone acetylation in gene transcription [31,32]. Histone acetylation is regulated by a balance between HATs and HDACs. HATs, which add acetyl groups to histones, promote an open chromatin formation, thereby facilitating the binding of basal transcription factors and RNA polymerase II, with subsequent gene transcription [33]. In contrast, HDACs remove acetyl groups from histones, leading to chromatin compaction and transcription repression. It has become increasingly recognized that epigenetic disturbances are found in fibrotic organs, and HDAC inhibitors prevent fibrosis in the kidney [4,5], heart [6], and lung [7]. However, the underlying mechanisms have yet to be elucidated.
The HDAC inhibitor SFN has been shown to prevent DN, but the epigenetic effect of SFN as an HDAC inhibitor on diabetes-induced renal fibrosis has not been explored. In this study, we found that SFN significantly attenuated diabetes-induced renal fibrosis. Among all the HDACs we assayed, HDAC2 activity was elevated in the STZ-induced diabetic kidneys and HG/Pal-treated HK11 cells. Furthermore, over-expression of HDAC2 in vitro decreased BMP-7 expression and aggravated the HG/Pal-induced profibrotic response. These results suggest the pivotal role of HDAC2 in diabetes-induced renal fibrosis. Interestingly, SFN inhibited the diabetes-induced increase in HDAC2 activity, which was associated with histone acetylation and transcriptional activation of the BMP-7 promoter. In vitro, HDAC2 over-expression reduced BMP-7 expression and abolished the SFN-mediated prevention of HG/Pal-induced fibrosis. These results suggest that SFN-mediated inhibition of HDAC2 ameliorates diabetes-induced renal fibrosis by up-regulating BMP-7.
During the last decade, BMP-7 has been identified as an essential renal protective factor that prevents kidney damage in response to various stimuli [21]. As a member of the TGF-β superfamily, BMP-7 has been found to inhibit TGF-β-dependent signaling pathways [34]. The inhibitory effects occur secondary to the activation of the SMAD1/5/8 proteins, which, in turn, reduce the transcriptional activity of TGF-β-dependent transcription factors to stimulate pro-fibrotic gene expression [30,35]. Notably, exogenous administration of BMP-7 has been observed to inhibit renal fibrosis induced by a variety of stimuli [14-19]. Earlier studies have shown that TSA, an HDAC inhibitor, could significantly enhance BMP-7 expression, which antagonized TGF-β1-induced EMT and up-regulation of collagen I in human renal epithelial cells [20]. ChIP assays further confirmed that histone acetylation is involved in the up-regulation of BMP-7 [20]. Similar results were also found in obstructed kidneys in vivo, where TSA up-regulated BMP-7 expression together with BMP-7-mediated inhibition of TGF-β-dependent signaling pathways and renal fibrosis [21]. Thus, we investigated if SFN could up-regulate BMP-7 expression. Our in vivo studies showed that SFN inhibited diabetes-increased HDAC2 activity, which was associated with histone acetylation and transcriptional activation of the BMP-7 promoter. However, over-expression of HDAC2 in vitro significantly decreased the SFN-induced BMP-7 expression and abolished the SFN-mediated prevention of HG/Pal-induced fibrosis. These results suggest that SFN up-regulates BMP-7 expression by inhibiting HDAC2.
Previous studies revealed that chronic renal damage in response to a variety of stimuli, including DN, is related to a reduction in BMP-7 expression [18,21,36,37]. In accordance with the above studies, our findings showed that STZ-induced diabetes reduced BMP-7 expression and increased HDAC2 activity in kidney tissues. Notably, this process was associated with histone deacetylation and transcriptional repression of the BMP-7 promoter. Importantly, over-expression of HDAC2 in vitro decreased BMP-7 protein expression and aggravated HG/Pal-induced renal fibrosis. These results indicate the role of epigenetic modulation of BMP-7 in diabetes-induced renal fibrosis.
The present study indicates that the protective effects of SFN against DN are associated with BMP-7 up-regulation. However, it was reported that SFN can prevent DN in a nuclear factor erythroid-2-related factor 2 (Nrf2)-dependent manner [11,38, 39]. Specifically, SFN prevented DN by increasing Nrf2 expression in wild-type mice. However, SFN did not provide renal protection in Nrf2 knock-out mice. The findings of these two studies may not be contradictory. As a transcription factor, Nrf2 may bind to the BMP-7 gene promoter to activate its expression. That is, BMP-7 expression induced by SFN may be Nrf2-dependent. However, further studies are needed to confirm this hypothesis.
In summary, this study, to the best of our knowledge, is the first to demonstrate that SFN up-regulates BMP-7 expression by inhibiting HDAC2 activity, which may contribute to the anti-fibrotic effect of SFN in DN. Our findings suggest that HDAC inhibitors could be used as therapeutic agents to treat DN.
Notes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTIONS
Conception or design: L.K., Y.Z.
Acquisition, analysis, or interpretation of data: L.K, H.W., C.L., H.C.,Y.C., L.L.
Drafting the work or revising: L.K., Y.Z.
Final approval of the manuscript: Y.Z.
FUNDING
This work was supported by the Foundation of the Eastern Department of the First Hospital of Jilin University (No. B01) and the Natural Science Foundation of China (No. 31520103916).
Acknowledgements
We express our gratitude to the doctors who participated in this study.