Factors Associated for Mild Cognitive Impairment in Older Korean Adults with Type 2 Diabetes Mellitus
Article information
Abstract
Background
The aim of this study was to identify factors associated with mild cognitive impairment (MCI) in older Korean adults with type 2 diabetes mellitus.
Methods
A total of 226 older (age ≥65 years) adults without a history of cerebrovascular disease or dementia participated in this study. Cognitive function was assessed with the Montreal Cognitive Assessment-Korean version (MoCA-K). A MoCA-K score <23 was defined as MCI.
Results
The prevalence of MCI was 32.7%. In a logistic regression analysis, age (≥74 years old vs. 65-68 years old; odds ratio [OR], 3.69; 95% confidence interval [CI], 1.55 to 8.82; P=0.003), educational background (college graduation vs. no school or elementary school graduation; OR, 0.16; 95% CI, 0.05 to 0.46; P=0.001), and systolic blood pressure (≥135 mm Hg vs. ≤120 mm Hg; OR, 3.25; 95% CI, 1.29 to 8.17; P=0.012) were associated with MCI.
Conclusion
More concentrated efforts focused on early detection and appropriate management of MCI may be required in older Korean adults with type 2 diabetes mellitus.
INTRODUCTION
Dementia is a prevalent worldwide problem and one of the most serious problems of late life in an ageing society [1]. People with dementia are impaired in their ability to carry out daily life activities and personal care. Their family members suffer psychological and economic strain [2].
People with mild cognitive impairment (MCI) have an increased risk of developing dementia, with an estimated annual conversion rate to dementia of 12% [3,4]. Type 2 diabetes mellitus is associated with accelerated cognitive decline, development of MCI, and increased risk of dementia, including Alzheimer disease (AD) and vascular dementia [5,6]. Cognitive impairment might result in nonadherence of patients to diabetes treatment, such as diet, medication, and exercise. Among older patients with diabetes, cognitive impairment increases the risk of major cardiovascular events and all-cause death [7,8].
Korea is a rapidly ageing society. In 2005, people >65 years of age represented 9.3% of the total population [9]. Despite the rapidly growing older population and the highest predicted prevalence of diabetes in Korea among The Organisation for Economic Co-operation and Development (OECD) countries by the year 2030 [10,11], few studies of this issue in Korea have been conducted [12,13]. Thus, the aim of this study was to identify factors associated with developing MCI in older Korean adults with type 2 diabetes.
METHODS
Participants
We recruited 226 older adults (≥65 years old) with type 2 diabetes who attended the diabetes outpatient clinic at Inje University Ilsan Paik Hospital between October 1 and December 31, 2011.
Exclusion criteria were blindness, illiteracy, stroke history, definite cognitive impairment, or psychiatric disorders. We also excluded participants with cerebral artery disease or those with previous brain lacunar infarctions detected by computed tomography (CT) or magnetic resonance imaging.
Variables
Demographic characteristics such as sex, age, education status, duration of diabetes, medical history of hypertension, dyslipidemia, smoking, and alcohol drinking were assessed by questionnaire and medical records. We defined heavy alcohol drinking as three or more alcoholic drinks per week, and social alcohol drinking as one to two alcoholic drinks per week. A questionnaire was administered to assess the presence of hypoglycemia. Participants were asked if they had felt symptoms of hypoglycemia in the 3 months prior to study enrollment, based on a list of provided symptoms (sweating, confusion/feeling disoriented, shakiness, clumsy or jerky movements, dizziness, sudden moodiness or behavior changes, hunger, tingling sensations around the mouth, difficulty concentrating, headache, and pale skin color).
All participants were examined and interviewed for past and present evidence of coronary heart disease (CHD). CHD was diagnosed when typical clinical features of angina pectoris or myocardial infarction were proven by selected cardiac tests (electrocardiography during rest and graded treadmill exercise, echocardiography, coronary CT, or coronary angiography).
Weight and height were measured to the nearest 0.1 kg and 0.1 cm, respectively. Blood pressure (BP) was measured after a 10-minute rest in a sitting position with a standard 12.5-cm cuff mercury sphygmomanometer. The recorded BP was the mean of three or more measurements.
Fasting plasma glucose (FPG), serum triglycerides (TG), total cholesterol, low density lipoprotein cholesterol (LDL-C), high density lipoprotein cholesterol (HDL-C), and serum creatinine concentrations were measured with an automated biochemical analyzer (Type AU5421; Olympus, Tokyo, Japan). Glycated hemoglobin (HbA1c) was measured using a high-performance liquid chromatography method and a HLC-723 G8 chromatograph (Tosoh Corp., Tokyo, Japan). Glomerular filtration rate (GFR) was estimated using the six-variable Modification of Diet in Renal Disease equation, as follows:
We defined chronic kidney disease (CKD) as eGFR <60 mL/min/1.73 m2.
The Montreal Cognitive Assessment (MoCA) is a brief cognitive screening tool with high sensitivity and specificity for detecting MCI, and it has been validated in several studies [14,15]. Cognitive function was assessed in all patients using the MoCA-Korean version (MoCA-K). A trained interviewer assessed cognitive function with the MoCA-K. The MoCA-K has a maximum score of 30 points. In the MoCA-K validation study, Lee et al. [16] reported that the MoCA-K has excellent sensitivity of 89%, and good specificity of 84% for screening MCI, using a cutoff score of 23. Thus, in this study, a MoCA-K score <23 was defined as MCI. Park et al. [17] reported that MoCA-K is more sensitive for MCI than the Mini Mental Status Exam-Korean version.
Statistical analysis
Clinical characteristics are summarized for males and females and presented as means or percentages±standard errors. All statistical techniques were performed using the SPSS version 18 (SPSS Inc., Chicago, IL, USA). An analysis of covariance was conducted to examine age- and sex-adjusted characteristics according to the presence of MCI (Table 1). A logistic regression analysis was performed to identify parameters associated with MCI, such as age, sex, educational background, diabetes duration, SBP, LDL-C, and TG (Table 2). Significance was determined by a two-tailed analysis where P<0.05.
Ethics statement
This study was approved by the Institutional Review Board of Ilsan Paik Hospital (IB-1109-042).
RESULTS
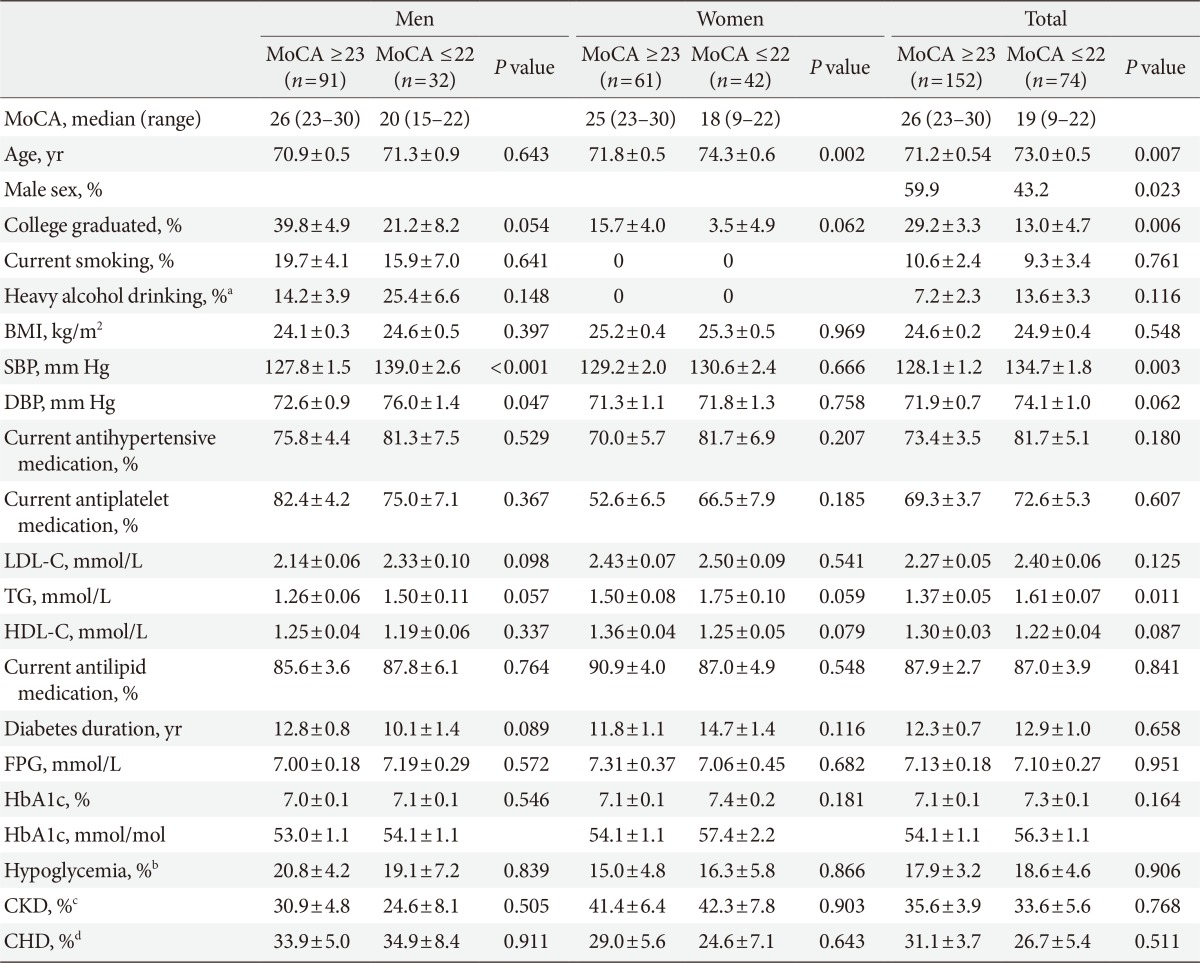
A total of 226 older (≥65 years old) adults (123 males and 103 females) participated in this study. The prevalence of MCI as defined by a MoCA-K score <23 was 32.7% (74/226). Diabetes duration in comprehensive study participants was 12.5±8.4 years. Participants showed relatively well-controlled plasma glucose, BP, and serum lipids (HbA1c, 7.16%±0.91%, 54.7±9.9 mmol/mol; FPG, 7.12±2.26 mmol/L; SBP, 130.0±15.2 mmHg; LDL-C, 2.31±0.57 mmol/L). The proportions of participants taking antihypertensive medication, antilipid medication, or antiplatelet medication were 76.1%, 87.6%, and 70.4%, respectively.
Table 1 shows the age and sex-adjusted clinical characteristics according to the presence of MCI. Participants with MCI were older (73.0±0.5 years vs. 71.2±0.5 years, P=0.007), and were less likely to be male (43.2% vs. 59.9%, P=0.023). In the age and sex-adjusted comparisons, serum TG in patients with MCI was higher (1.61±0.07 mmol/L vs. 1.37±0.05 mmol/L, P=0.011), and SBP in patients with MCI was higher (134.7±1.8 vs. 128.1±1.2, P=0.003). Fewer participants with MCI were college graduates (13.0%±4.7% vs. 29.2%±3.3%, P=0.006). Other variables, including diabetes duration, HbA1c, CKD, and CHD, did not vary between the groups.
Table 2 shows the logistic regression analysis for MCI. age (≥74 years old vs. 65-68 years old; odds ratio [OR], 3.69; 95% confidence interval [CI], 1.55 to 8.82; P=0.003), educational background (college graduation vs. no school or elementary school graduation; OR, 0.16; 95% CI, 0.05 to 0.46; P=0.001), and systolic blood pressure (≥135 mm Hg vs. ≤120 mm Hg; OR, 3.25; 95% CI, 1.29 to 8.17; P=0.012) were associated with MCI.
DISCUSSION
The principal finding of this study was that the prevalence of MCI was high in older Korean adults with type 2 diabetes (32.7%). Although this was not a population-based study, the results strongly suggest that more concentrated efforts focused on early detection and proper management of MCI in Korean older adults with type 2 diabetes are required.
According to a recent Korean study, the prevalence of dementia in persons aged ≥65 years was 9.2% (95% CI, 7.9 to 10.4), and the prevalence of MCI was 23.7% (95% CI, 20.6 to 26.8) [18]. In our study, the prevalence of MCI in patients with type 2 diabetes was 32.7%, which was similar to the 21.9% to 37% reported in other studies [7,12,19]. The prevalence of MCI among studies could vary due to differences in the cognitive impairment test methods used (i.e., MMSE vs. MoCA) or in the clinical characteristics of the study population.
Age, sex, and education are not consistently related to the prevalence of MCI [20]. A Korean study reported that older females in the general population with lower education levels have an increased dementia risk [18]. In our study, older age and lower education level were independently associated with MCI. However, sex was not associated with MCI after adjusting for several clinical variables.
In a recent meta-analysis of 25 prospective studies, patients with diabetes had a 1.5-fold greater risk of MCI and a 1.6-fold greater risk of dementia compared to people without diabetes [21]. Glycemic control and duration of diabetes are important factors related to the development of cognitive impairment in patients with type 2 diabetes [6,22,23,24]. According to a study in menopausal females with diabetes, an increase in the level of HbA1c by 1% is associated with a 1.5-fold greater risk of developing MCI, and a 1.4-fold higher risk of developing dementia [22].
Some possible mechanisms for hyperglycemia-induced cognitive impairment include cerebral macro- and micro-vascular alterations due to hyperglycemia. Stroke is known to be associated with dementia and cognitive impairment [25]. One meta-analysis reported that the pooled relative risk associated with a 1% increase in HbA1c among patients with type 2 diabetes was 1.11 for stroke [26]. The Edinburgh Type 2 Diabetes Study demonstrated that retinopathy is independently associated with cognitive decline in older males with type 2 diabetes, suggesting that cerebral microvascular disease may contribute to the observed cognitive decline [27]. Moreover, a study of 259 autopsies reported that individuals with diabetes mellitus and dementia have more microvascular infarcts compared to those without diabetes mellitus but with dementia [28]. The second possibility is the role of advanced glycation end-products (AGEs) and receptors for advanced glycation end product (RAGE). Increased AGEs in patients with hyperglycemia may contribute to the formation of amyloid plaques, neurofibrillary tangles (NFTs), or activated microglia, which are histopathological, and biochemical hallmarks of AD [29]. Hyperglycemia may also increase the concentration of RAGE, which is a transporter of β-amyloid across the blood-brain barrier into the brain from the systemic circulation [30]. One autopsy study reported a much higher concentration of RAGE protein in AD hippocampi compared with that in controls, suggesting that RAGE may affect AD development by altering β-amyloid metabolism [31]. Another possibility is the association of hyperinsulinemia with hyperglycemia. One study reported that hyperinsulinemia may play a role promoting phosphorylation of tau protein, the main component of NFTs [32].
However, we found no significant correlation between FPG or HbA1c levels and MCI. In addition, no association was observed between diabetes duration and MCI. The difference may partly be explained by the fact that our participants showed relatively well-controlled glycemia (HbA1c, 7.16±0.91%, 54.7±9.9 mmol/mol). We should mention that we analyzed only one HbA1c value that was measured within 3 months of the MoCA-K test. Considering the limitation that we did not consider any previous glycemic control measures, we could not conclude a nonassociation of glycemic control with MCI. However, the results suggest that glycemic control may not be a major determinant for MCI in well-managed older adults.
Many studies have reported conflicting results for the association between dyslipidemia and cognitive impairment. An observational study of 1,037 postmenopausal females with coronary heart disease reported that those in the highest total and LDL-C quartile showed an increased likelihood of cognitive impairment compared with subjects in the lower quartiles. However, no association was observed between HDL-C and TG quartiles and cognitive impairment [33]. One U.S. prospective community-based cohort study of 854 participants ≥65 years with a follow up of 4,189 person-years, reported that higher levels of total cholesterol and LDL are associated with a decreased risk of total MCI in models adjusted for age and sex. However, these associations were attenuated after adjusting for other vascular risk factors. In that study, no association was observed between HDL-C and TG levels and cognitive impairment [34]. In a Japanese longitudinal study that enrolled 261 patients with diabetes (age, ≥65 years), higher serum TG and lower HDL-C at baseline were significantly associated with cognitive decline after 6 years (per 10 mg/dL increase, for TG, adjusted OR, 1.07, for HDL-C, adjusted OR, 0.67) [35]. In the present study, HDL-C and LDL-C did not show associations with MCI. We found that serum TG level was associated with MCI in an age- and sex-adjusted comparison. However, we did not observe a significant association between serum TG level and MCI in the logistic regression analysis with several parameters.
Hypertension is associated with cognitive decline through atherosclerosis, microcirculation disorder, endothelial dysfunction, and white matter lesions [36]. However, a considerable number of studies examining the relationships between hypertension with dementia and cognitive decline have reported inconsistent results. A diabetes study of the Japanese elderly reported that higher SBP at baseline is significantly associated with cognitive decline after 6 years (per 10 mm Hg increase, adjusted OR, 1.42) [35]. In contrast, a U.S. longitudinal cohort study of 824 older Catholic clergy (mean age, 75 years) reported that neither SBP nor DBP was related to AD incidence during a 6-year follow-up [37]. In the present study, SBP was associated with MCI in a logistic regression analysis, in concordance with some previous reports.
Participants in this study showed relatively well-controlled plasma glucose, blood pressure, and serum lipid levels. Most participants were taking antihypertensive medication (76.1%), antilipid medication (87.6%), or antiplatelet medication (70.4%). Moreover, we excluded participants with any evidence of cerebrovascular disease including lacunar infarction on previous imaging studies. The abovementioned clinical characteristics of our participants could explain the lack of an association between several parameters, including diabetic complications, and MCI.
In our study, heavy alcohol drinking was not related with MoCA-K score. However, several studies have reported that alcohol has a U- or J-shaped relationship with cognitive impairment [38,39]. A Finnish study that included 1,464 males and females aged 65 to 79 years with an average follow-up of 23 years showed that alcohol drinking in middle age has a U-shaped relationship with the risk of MCI [38]. Another study from the Finnish Twin Cohort that included 1,486 people with an average follow-up of 22.8 years reported that no alcohol drinkers and heavy drinkers had an increased risk of cognitive impairment in comparison to light drinkers (relative risk, 1.44 [95% CI, 1.02 to 2.10], 1.94 [95% CI, 1.10 to 3.44], respectively); moreover, binge drinking was an independent risk factor for cognitive impairment [39]. The reason for this discrepancy is not clear. We could not exclude the possibility of insufficient statistical power. According to the 2010 Korean National Health and Nutrition Examination Survey (KNHANES), the rate of high-risk drinking (five or more drinks in males and four or more in females, more than twice per week) is 25.5% in males and 7.6% in females [40]. Another large study of the association between MCI and alcohol drinking in Korean is needed.
Some limitations of this study should be mentioned. First, this was a cross-sectional study with a relatively small number of participants who visited one university hospital. Therefore, this study does not comprehensively represent patients with diabetes, and we cannot confirm the nonsignificant association between MCI and several clinical parameters due to insufficient statistical power. We also could not determine a causal relationship. The exclusion criteria of previous lacunar infarction and cerebral artery disease should be considered when interpreting the results.
In conclusion, the prevalence of MCI was high (32.7%) in older Korean adults with type 2 diabetes, considering that our patients with diabetes had good metabolic control without evidence of cerebrovascular disease and more than three-quarters of the participants were taking antihypertensive, antilipid, or antiplatelet medication. The data suggest that more concentrated efforts focused on early detection and proper management of MCI are required in older Korean adults with type 2 diabetes.
Notes
No potential conflict of interest relevant to this article was reported.