Resistin in Rodents and Humans
Article information
Abstract
Obesity is characterized by excess accumulation of lipids in adipose tissue and other organs, and chronic inflammation associated with insulin resistance and an increased risk of type 2 diabetes. Obesity, type 2 diabetes, and cardiovascular diseases are major health concerns. Resistin was first discovered as an adipose-secreted hormone (adipokine) linked to obesity and insulin resistance in rodents. Adipocyte-derived resistin is increased in obese rodents and strongly related to insulin resistance. However, in contrast to rodents, resistin is expressed and secreted from macrophages in humans and is increased in inflammatory conditions. Some studies have also suggested an association between increased resistin levels and insulin resistance, diabetes and cardiovascular disease. Genetic studies have provided additional evidence for a role of resistin in insulin resistance and inflammation. Resistin appears to mediate the pathogenesis of atherosclerosis by promoting endothelial dysfunction, vascular smooth muscle cell proliferation, arterial inflammation, and formation of foam cells. Indeed, resistin is predictive of atherosclerosis and poor clinical outcomes in patients with coronary artery disease and ischemic stroke. There is also growing evidence that elevated resistin is associated with the development of heart failure. This review will focus on the biology of resistin in rodents and humans, and evidence linking resistin with type 2 diabetes, atherosclerosis, and cardiovascular disease.
INTRODUCTION
The prevalence of obesity has increased worldwide and is associated with increased risk of type 2 diabetes mellitus (T2DM), cardiovascular disease (CVD), sleep apnea, cancer, fatty liver, and other diseases. It has become clear that obesity promotes a state of chronic inflammation in white adipose tissue (WAT), which leads to insulin resistance. Obesity results in excessive lipid accumulation in adipocytes and macrophages in WAT, which plays an active role in the development of insulin resistance through the release of free fatty acids, proinflammatory cytokines, and adipokines [1]. Adipose tissue secretes adipokines that play major roles in metabolism, immunity, and inflammation. Resistin is secreted primarily by adipocytes in rodents and was initially proposed as a link between obesity and insulin resistance [2]. In humans, resistin is mainly secreted by macrophages [3], suggesting that resistin is linked to inflammation. Obesity, T2DM, and CVD have been recently recognized as chronic inflammatory disorders which may be connected to proinflammatory cytokines as well as adipokines such as resistin [4]. In this review, we will discuss the biology of resistin in rodents and humans, and focus on the evolving roles of resistin in atherosclerosis and CVD in humans.
REGULATION OF RESISTIN SYNTHESIS AND SECRETION
Resistin is a cysteine-rich protein which was discovered using a screening strategy for genes down-regulated in mouse adipocytes by thiazolidinedione (TZD) drugs [2]. In rodents, resistin is primarily expressed in mature white adipocytes. Mouse resistin expression is reduced in 3T3-L1 adipocytes by TZD drugs [5,6] and insulin [5,7]. In contrast, high glucose up-regulates resistin expression in adipocytes [5,8]. There were also conflicting results showing insulin can stimulate resistin secretion in mouse adipocytes [9-11]. Resistin expression in mouse adipocytes is suppressed by inflammatory cytokines, e.g., tumor necrosis factor α (TNF-α), [5,12]. Unlike rodents, human resistin is expressed in macrophages and is induced by TNF-α [13,14]. The lack of human resistin expression in adipocytes may be due to loss of a genomic binding site for the nuclear receptor peroxisome proliferator-activated receptor γ that normally controls resistin gene (retn) expression in mouse adipocytes [15]. Dietary intake has a significant effect on the regulation of resistin in rodents. The expression of resistin is decreased in the adipose tissue during fasting, and tends to increase upon refeeding in mice [2,8,16]. Serum resistin is also reduced in fasted mice. Diet-induced obese mice have elevated circulating resistin levels, however, resistin mRNA expression is suppressed in adipose tissue in these mice. Similarly, serum resistin is increased in ob/ob mice while resistin mRNA expression is reduced [16,17].
Resistin circulates in two distinct forms in mice. A disulfide-linked hexamer of resistin is the major form, but a smaller trimeric protein is also detected. The low molecular weight form of trimer is shown to be more bioactive, significantly decreasing hepatic insulin sensitivity during pancreatic clamp study [18]. However, oligomerization is required for the inhibitory action of resistin on glucose uptake in mouse cardiomyocytes [19]. In humans, there are also different molecular isoforms of circulating resistin such as trimeric and oligomeric forms, and the oligomeric form of human resistin is considered to have a more potent effect on the stimulation of proinflammatory cytokines [20-22].
RESISTIN IN GLUCOSE HOMEOSTASIS IN RODENTS
Several lines of evidence support a role of resistin in glucose metabolism in rodents. Circulating levels of resistin were higher in obese mice, and the administration of recombinant resistin in normal mice impaired glucose tolerance and insulin action. Furthermore, neutralization of resistin with antiresistin antibody improved insulin sensitivity in diet-induced obese mice [2]. Both central and peripheral administration of recombinant resistin, or transgenic overexpression of resistin induced hepatic insulin resistance in mice [2,23-25]. Conversely, knockdown or deletion of resistin increased hepatic insulin sensitivity in mice on high-fat diet, and in muscle and WAT in ob/ob mice, leading to a decrease in glucose production and an increase in glucose uptake from peripheral tissue [26,27]. Resistin knockout mice showed low glucose levels after fasting, associated with a decrease in expression of gluconeogenic enzymes in liver [26]. Treatment of 3T3-L1 adipocytes, murine cardiomyocytes, and cultured skeletal muscle cells with murine resistin decreased insulin-stimulated glucose uptake into the cells [2,19,28,29]. All of these findings suggest resistin plays an important role in insulin sensitivity in rodents.
Nevertheless, the resistin receptor or the intracellular signaling pathways of resistin have not yet been clearly identified. Recent studies proposed an isoform of decorin or tyrosine kinase-like orphan receptor 1 as functional resistin receptors which may regulate WAT expansion or modulate glucose homeostasis in rodents [30,31]. Murine resistin has been demonstrated to decrease the phosphorylation of AMP-activated kinase (AMPK) in liver, skeletal muscle, and WAT [24,26,32]. Inhibition of resistin, conversely, led to an increase in the phosphorylation of AMPK which is involved in glucose homeostasis. Resistin interferes with multiple steps involved in the insulin signaling cascade, including phosphorylation of insulin receptor substrates, activation of phosphatidylinositol-3-kinase (PI3K) and protein kinase B/Akt in liver, muscle, and WAT [24,32,33]. Resistin treatment in rodents induces the expression of suppressor of cytokine signaling-3, a known inhibitor of insulin signaling, in liver, muscle, and WAT [17,27,33-35]. Considering adipocyte-derived resistin is linked to insulin resistance in rodents, the potential that rodent data may be applicable to human biology has led to much scientific research.
HUMAN RESISTIN IN OBESITY, INSULIN RESISTANCE AND DIABETES
Resistin is predominantly expressed in peripheral blood mononuclear cells (PBMCs), and its expression increases as the cells differentiate into macrophages in humans [3,14,36]. As in murine resistin, TZD drugs down-regulated the expression of human resistin in macrophages or reduced serum resistin levels [3,14,37]. Resistin expression is also found in the nonadipocyte stromal vascular fraction in WAT, fibrotic liver, and atherosclerotic lesions, indicating that macrophages are the main source of resistin in humans [38-41]. Indeed, obese individuals who are likely to have greater infiltration of macrophages in adipose tissue showed increased expression of resistin in adipose tissue samples or had higher circulating levels of resistin than in lean individuals [36,42]. Studies of obese subjects undergoing weight reduction through diet or bariatric surgery have shown inconsistent results. Serum resistin levels were reduced, or not changed, despite weight loss in obese subjects [43-45]. There were also several reports that serum resistin levels are not associated with the parameters of obesity or insulin resistance [46-48]. However, resistin levels have been associated with visceral, intrathoracic, and pericardial fat in a cross-sectional study [49].
Given the close relationship of murine resistin and insulin resistance, numerous clinical studies have examined a possible relationship of resistin and insulin resistance in obese people with or without diabetes. Since obesity and T2DM are both associated with chronic inflammation in WAT, and resistin is mainly produced by macrophages in humans, it is possible that hyperresistinemia is a contributing factor to these pathophysiological states. However, studies relating human resistin to insulin resistance, or T2DM have shown conflicting results [35,46,47,50-52]. Qatanani et al. [53] generated transgenic mice expressing human resistin via a macrophage promoter and bred them with resistin knockout mice. The humanized resistin mice developed WAT inflammation and insulin resistance, demonstrating that human resistin may link inflammatory responses and glucose homeostasis. This is consistent with prospective case-control studies showing that people with elevated baseline levels of resistin have significantly increased risk of developing T2DM even after adjusting for other risk factors [54,55].
RESISTIN POLYMORPHISMS IN INSULIN RESISTANCE AND ATHEROSCLEROSIS
Epidemiological studies also suggest that resistin plays a role in insulin resistance and T2DM in humans. Several single-nucleotide polymorphisms (SNPs) have been demonstrated to be associated with elevated resistin levels, and up to two-thirds of the variation of serum resistin levels may be attributable to genetic factors [56]. The -638 G>A, -420 C>G, and -358 G>A polymorphisms in the promoter region of human resistin gene (RETN) were associated with resistin levels in Japanese obese individuals [57]. In a Japanese population, the G/G genotype at SNP -420 in RETN was associated with susceptibility to T2DM and also was correlated with monocyte resistin expression and with increased serum resistin levels [58,59]. Moreover, a cross-sectional analysis of 2,078 Japanese subjects showed that plasma resistin was associated with SNP -420 and also correlated with insulin resistance [60]. The -420G and -537A alleles were associated with increased resistin levels but not with T2DM in a Korean population [61]. In the 5-year prospective study from a Chinese subjects, both -420G and +62A alleles were significant predictors of glycemic progression [62]. Although -420 C>G SNP associated with high resistin levels appears to be related to T2DM in many studies of Asian populations, the data in Caucasian populations have been conflicting [55]. An Italian study showed that the presence of -420 C/G SNP in RETN is associated with increased obesity and metabolic syndrome but not with resistin levels [63]. An American cohort of Caucasian nondiabetic subjects has shown an association of -420G allele with resistin level, but no association with insulin resistance [64]. In the Framingham Offspring Study cohort of 2,531 participants, it has been shown that not -420 C>G SNP but SNPs in the 3' region of RETN are associated with resistin levels [65]. However, El-Shal et al. [66] have recently demonstrated that both -420 C>G and +299 G>A SNP were significantly associated with resistin level, obesity, and insulin resistance in Egyptian obese subjects.
The role of human resistin in the pathogenesis of atherosclerosis is also gaining research attention. Tang et al. [67] have shown that -420 C/G SNP in RETN is associated with an increased risk of coronary artery disease (CAD) in a Chinese population. In contrast, a couple of studies of the -420 variant in Europeans and Caucasians found no correlation with carotid and coronary atherosclerosis, respectively [63,64]. The discrepancy in the results among populations might be attributable to ethnic differences.
ROLE OF HUMAN RESISTIN IN INFLAMMATION
Murine and human resistin share about 60% sequence homology. As noted before, resistin levels decrease with TZD treatment in both humans and rodents. Unlike murine resistin, human resistin is highly expressed in macrophages, and robustly induced in response to various proinflammatory stimuli such as lipopolysaccharide (LPS), TNF-α, interleukin (IL)-6, IL-1β, and resistin itself, suggesting a role for resistin in inflammation in humans [13,14,68]. Furthermore, resistin has been shown to up-regulate the expression of proinflammatory cytokines such as TNF-α, IL-6, IL-12, or monocyte chemoattractant protein (MCP)-1 in PBMC, macrophages, and hepatic stellate cells, mediated through the nuclear factor-κB signaling pathway [39,68,69]. In line with these findings, circulating resistin levels correlate with inflammatory and fibrinolytic markers such as C-reactive protein (CRP), TNF-α, IL-6, or plasminogen activator inhibitor (PAI)-1 in subjects with T2DM, coronary atherosclerosis, chronic kidney disease, rheumatoid arthritis (RA), and sepsis [14,46,48,70-73] as well as in general population [48,60,74-78]. Increased resistin levels have been observed in patients with RA and inflammatory bowel disease and shown to be associated with disease activity [22,72,78]. In addition, circulating resistin levels were elevated in patients with severe sepsis and acute pancreatitis. Interestingly, resistin was related with disease severity in patients with severe sepsis as well as acute pancreatitis, and also predicted an unfavorable outcome in nonseptic critically ill patients [73,79,80].
It is noteworthy that numerous inflammatory factors up-regulated by human resistin are known to be involved in the development of insulin resistance. Therefore, it is possible that resistin induced by inflammation may play a role in the development of insulin resistance in humans. To address this issue, Park et al. [81] generated mice lacking murine resistin but transgenic for a bacterial artificial chromosome containing human resistin (BAC-Retn), whose expression was similar to levels in humans. LPS increased serum resistin levels in this model, and resulted in mild hypoglycemia compared with mice lacking murine and human resistin. In addition, the BAC-Retn mice developed hepatic insulin resistance under chronic endotoxemia, accompanied by inflammation in liver and skeletal muscle, supporting the role of resistin in the pathophysiology of inflammation-induced insulin resistance in humans [81]. A recent study suggested that human resistin competes with LPS for binding to Toll-like receptor 4 (TLR4) which could mediate some of the proinflammatory effects of resistin [82], however, it is unknown whether TLR4 is the predominant resistin receptor in humans.
HUMAN RESISTIN IN ATHEROSCLEROSIS
Given the evidence that resistin is found in human atherosclerotic lesions and affects vascular endothelial function [40,41,83], it is possible resistin is involved in the development of atherosclerosis and CVD. Treatment of human resistin increased proliferation and migration of human endothelial cells (ECs) and vascular smooth muscle cells (VSMCs). Cell proliferation and migration induced by resistin in both EC and VSMC appears to be mediated through the extracellular signal-regulated kinase, PI3K, or p38 mitogen-activated protein kinase signaling pathways [84-86]. Resistin was also shown to attenuate the ability of insulin signaling, inhibit endothelial nitric-oxide synthase, and increase oxidative stress in human aortic and coronary artery EC [86,87]. In addition, resistin up-regulated the expression of adhesion molecules such as intercellular adhesion molecule-1, vascular cell adhesion molecule-1, P-selectin, and fractalkine, and also increased proinflammatory factors such as MCP-1, PAI-1, endothelin-1, matrix metalloproteinases, and vascular endothelial growth factor receptors, and, thereby, promoted monocyte adhesion in vascular EC [17,41,83,84,88,89]. Furthermore, resistin was expressed in human atheroma samples, and increased lipid accumulation through the up-regulation of CD36 expression and an increase in cholesteryl ester deposition in human macrophages, suggesting a role of resistin as a modulator for macrophage to foam cell transformation [40,41,90,91]. In addition, resistin has been shown to induce a prothrombotic phenotype by up-regulating tissue factor expression in human coronary artery EC [92,93]. Taken together, these findings indicate that resistin may contribute to EC dysfunction, VSMC proliferation, arterial inflammation, and accumulation of cholesterol in macrophages, suggesting that resistin plays an important role in the pathogenesis and progression of atherosclerosis in humans.
CLINICAL IMPLICATIONS OF RESISTIN IN CVD
There is the growing evidence that resistin is involved in the pathogenesis of CVD. Reilly et al. [48] showed that elevated resistin levels were predictive of coronary atherosclerosis, independent of CRP, in asymptomatic individuals. Resistin was associated with the presence and severity of CAD in patients undergoing coronary angiography and those with stable CAD [94,95]. Moreover, resistin predicted restenosis after coronary artery stenting and was an independent predictor of major adverse cardiovascular events in patients with CAD [96-99]. In Korean patients with acute myocardial infarction (MI), resistin was an independent predictor of all cause mortality during the mean follow-up period of 12 months [100]. A European study found that high plasma resistin levels were associated with an increased risk of MI but not with risk of ischemic stroke in healthy subjects, even after adjustment for established cardiovascular risk factors including CRP [101]. In regard to the risk of ischemic stroke, a cross-sectional study from Japan and two nested case-control studies from Europe and United States showed that resistin was significantly associated with ischemic stroke among healthy individuals [102-104]. Higher resistin levels were also associated with poorer prognosis in patients with CAD or atherothrombotic stroke [99,105-107]. More recently, an analysis of 2,313 diabetic patients of European ancestry from two cross-sectional and two prospective studies showed that high serum resistin was a risk factor for CVD and all cause mortality in patients with T2DM [108]. Interestingly, resistin was also shown to be associated with vascular inflammation measured using 18F-fluorodeoxyglucose-positron emission tomography among healthy individuals [109]. Despite some studies showing no association between resistin and CVD [110-112], a number of animal and clinical studies are strongly supporting the idea that resistin is a potential mediator of atherosclerosis and CAD in humans.
Several animal studies have investigated the effects of resistin on cardiomyocytes. Murine resistin directly reduced both basal and insulin-stimulated glucose uptake in mouse cardiomyocytes [19]. Murine resistin was shown to be up-regulated by mechanical stretch and the overexpression of resistin depressed cardiac contractility, promoting cardiac hypertrophy in rat cardiomyocytes [113,114], suggesting resistin may affect cardiac function in animal models. Regarding the effects of human resistin on cardiomyocytes, however, very few studies have been reported. Human resistin inhibited insulin-stimulated glucose uptake in isolated mouse cardiomyocytes [19]. Preconditioning with recombinant human resistin prior to ischemia significantly impaired contractile recovery during reperfusion and stimulated cardiac TNF-α secretion in isolated rat hearts, suggesting human resistin has direct cardiac actions [115]. Emerging evidence suggests that elevated resistin is associated with the development of heart failure (HF). Takeishi et al. [116] have found that resistin is correlated with the severity of HF and predicts adverse cardiac events in patients with established HF. In addition, two large cohort studies have shown that high resistin is independently associated with new-onset HF, even after adjusting for known risk factors [117-119]. However, the causal role of resistin in HF is unclear and needs further investigation.
CONCLUSIONS
Resistin was initially described as a link between obesity and insulin resistance in rodents. In rodents, resistin is almost exclusively expressed in white adipocytes, whereas human resistin is predominantly expressed in macrophages. The phenotypes of humanized resistin transgenic mice suggest similar roles of murine and human resistin in insulin resistance. Studies in humanized resistin transgenic mice and epidemiological data also suggest that human resistin is a potential mediator between inflammation, and insulin resistance and atherosclerosis (Fig. 1). The identification of the resistin receptor and specific signaling mechanisms will help address crucial questions concerning the biology of resistin in health and disease. Insights into the pathophysiological role of resistin will facilitate the development of novel diagnostic and treatment tools for diabetes, and inflammatory and CVDs.
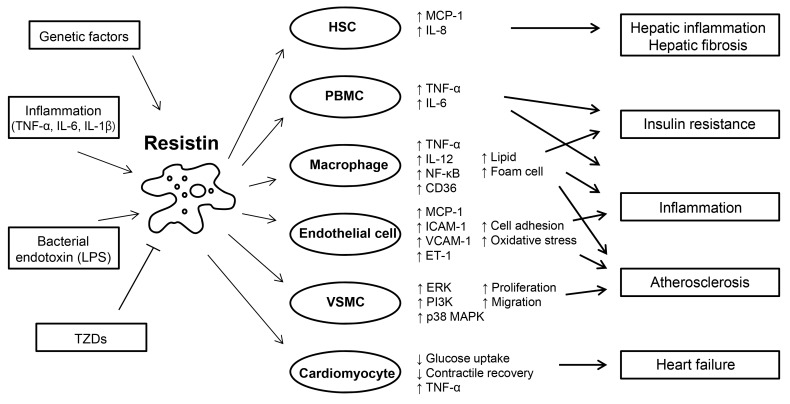
Roles of resistin in inflammation, glucose homeostasis, and cardiovascular diseases. Resistin is induced in response to various stimuli, including proinflammatory cytokines, lipopolysaccharide (LPS), and genetically determined factors. Resistin targets several types of cells, promoting inflammation, insulin resistance, and atherosclerosis. Resistin is up-regulated and secreted from PBMC and macrophages, and in turn, acts on these cells, leading to the development of insulin resistance and enhancing inflammatory processes through activation of nuclear factor (NF)-κB. Resistin contributes to the pathogenesis of atherosclerosis by disrupting vascular endothelial cellular function, and increasing proliferation of smooth muscle cells, and foam cell transformation. Resistin directly affects the function of cardiomyocytes predisposing to myocardial injury. Adapted from Schwartz et al. Trends Endocrinol Metab 2011;22:259-65, with permission from Elsevier [55]. HSC, hepatic stellate cell; MCP, monocyte chemoattractant protein; IL, interleukin; TNF, tumor necrosis factor; PBMC, peripheral blood mononuclear cell; ICAM, intercellular adhesion molecule; VCAM, vascular cell adhesion molecule; ET, endothelin; VSMC, vascular smooth muscle cell; ERK, extracellular signal-regulated kinase; PI3K, phosphatidylinositol-3-kinase; MAPK, mitogen-activated protein kinase; TZD, thiazolidinedione.
ACKNOWLEDGMENTS
This work was supported by grants P01-DK-049210 and P30-DK-19525 from the National Institutes of Health, USA.
Notes
No potential conflict of interest relevant to this article was reported.