Targeting the Peroxisome Proliferator-Activated Receptor-γ to Counter the Inflammatory Milieu in Obesity
Article information
Abstract
Adipose tissue, which was once viewed as a simple organ for storage of triglycerides, is now considered an important endocrine organ. Abnormal adipose tissue mass is associated with defects in endocrine and metabolic functions which are the underlying causes of the metabolic syndrome. Many adipokines, hormones secreted by adipose tissue, regulate cells from the immune system. Interestingly, most of these adipokines are proinflammatory mediators, which increase dramatically in the obese state and are believed to be involved in the pathogenesis of insulin resistance. Drugs that target peroxisome proliferator-activated receptor-γ have been shown to possess anti-inflammatory effects in animal models of diabetes. These findings, and the link between inflammation and the metabolic syndrome, will be reviewed here.
INTRODUCTION
The percentage of the global population that is obese has skyrocketed over the past few decades and it is predicted that this percentage will continue to rise significantly as the developing world adopts more sedentary lifestyles and gains access to high calorie diets. As recently as 10 years ago in the United States, obese adults (defined as body mass index >30) made up less than 12% of the population. Now, more than 20% of the adult population meets the Centers for Disease Control and Prevention criteria for obesity and greater than 40% are considered overweight. The metabolic syndrome is characterized as a clustering of factors associated with an increased risk of cardiovascular disease and stroke and is becoming more common [1]. The essential risk factors that constitute the metabolic syndrome are age, atherogenic dyslipidemia (high triglycerides and low high density lipoprotein cholesterol), hypertension, elevated plasma glucose, a prothrombotic state, and a proinflammatory state. The two major underlying causes of the metabolic syndrome are obesity and type 2 diabetes mellitus (T2DM) [2]. Obesity is conceptually defined as an excess of body lipids of sufficient magnitude to impair health or longevity. T2DM is a chronic metabolic disorder that is caused, in part, by the inability of the body to respond adequately to circulating insulin, a condition termed insulin resistance. The comorbidities of the metabolic syndrome will continue to strain global health care systems requiring development of strategies to combat this epidemic.
The therapeutic approach for the treatment of the metabolic syndrome is to identify and treat each risk factor separately. Current standard of care includes pharmacologic intervention and lifestyle modification such as nutritional consultation and modification of diet, rigorous weight control, and increased regular exercise. Modest weight loss can result in an improvement in metabolic parameters [3]. However, weight loss and exercise often are not sufficient due to poor compliance or perhaps genetic factors [4,5]. Pharmacologic approaches have focused on the use of combination therapy for diabetes treatment in an effort to postpone the inevitable need for insulin therapy [6]. Molecular targets for treatment of the metabolic syndrome include the PPAR subfamily of nuclear receptors. Here we review current thoughts on the role of the γ isoform of the PPAR subfamily in metabolic disease. We also review recent mechanistic studies that pave the pathway to develop novel approaches to pharmacologically target PPAR-γ for the treatment of the metabolic syndrome.
PPARs AND METABOLIC DISORDERS
Nuclear receptors (NRs) are a major target of drug discovery. NRs are ligand-dependent transcription factors that possess the ability to directly interact with DNA and they regulate DNA transcription, in part, by recruitment of chromatin remodeling enzymes to promoters of target genes. These receptors play essential roles in development, cellular homeostasis and metabolism [7]. Moreover, NRs have been implicated in a wide range of diseases and, as such, have been the focus of drug development efforts for the pharmaceutical industry. One of the NR families intimately involved in maintaining metabolic homeostasis is the peroxisome proliferator-activated receptor (PPARs) or NR1C subfamily. The PPAR subfamily consists of three members encoded by different genes and is involved in a myriad of physiological processes that impact lipid homeostasis, inflammatory responses, adipogenesis, insulin sensitivity, reproduction, wound healing, and carcinogenesis [8,9].
PPAR-α was first characterized as mediating the effect of peroxisome proliferators [10]. Subsequently, the other closely related receptors were cloned and named PPAR-γ and PPAR-δ despite neither responding to peroxisome proliferators [11]. Similar to other NRs, PPARs possess a N-terminal ligand-independent transactivation domain (AF-1), a two zinc-finger DNA-binding domain, a hinge domain, and a C-terminal ligand-binding domain containing a ligand responsive activation domain (AF-2). The PPARs have been shown to heterodimerize with the 9-cis-retinoic acid receptor α [12] and regulate transcriptional activity by binding to direct repeat 1 PPAR response elements (5' AGGTCA (N)X AGGTCA 3') in the promoter or enhancer regions of its target genes [13]. The PPAR family members are differentially expressed with PPAR-α being highly abundant in tissues with high potential for fatty acid oxidation such as the skeletal muscle, liver, kidney, heart, and brown adipose tissue. It regulates peroxisome proliferation, lipid catabolism, inflammatory responses, and skin wound healing. PPAR-δ is ubiquitously expressed and is suggested to influence myelination, embryonic implantation, and adipocyte differentiation. In addition, PPAR-δ activity contributes to the restoration of metabolic homeostasis by improving hypertriglyceridemia and insulin resistance, controlling body weight, and suppressing inflammation [14]. PPAR-γ is mainly expressed in adipose tissue where it plays a critical role in adipocyte differentiation and lipogenesis. PPAR-γ is also expressed in monocytic cells and its modulation by synthetic ligands can influence inflammatory processes. We will briefly review the importance of PPAR-γ in inflammation and its connection to the metabolic syndrome observed in obesity.
INFLAMMATION IN ADIPOSE TISSUE
One of the main contributing factors to the development of the metabolic syndrome in diabetics is the excessive expansion and enlargement of adipocytes. This results in the increased secretion of inflammatory factors that can interfere with insulin signaling and glucose disposal. Insulin secreted from pancreatic islet β-cells interacts with its receptor in its major target tissues which are skeletal muscle, liver, and adipose tissue [15]. Insulin is arguably the most important hormone influencing lipogenesis. In order to maximize glucose uptake and clearance, insulin must regulate the availability of free fatty acids in circulation. It does so by opposing the rate of lipolysis in adipose tissue and stimulating the re-esterification of fatty acids into triglycerides for storage. The expression of enzymes involved in fatty acid and triglyceride synthesis, such as acetyl-CoA carboxylase, fatty acid synthase, and diacylglycerol acyltransferase, are induced upon activation of the insulin receptor (IR) [16].
The IR is a heterotetramer consisting of two extracellular α-subunits and two transmembrane β-subunits held together by disulfide bonds [17]. The intracellular portion of the protein contains a tyrosine kinase domain and phosphotyrosine binding sites for signaling molecules. Insulin binding to the extracellular portion of the receptor induces conformational changes in the protein which activate the tyrosine kinase domain in the intracellular region. Activation of the kinase domain results in rapid phosphorylation of intracellular insulin receptor substrates (IRS 1 to 4) that serve as docking sites for downstream signaling molecules [18]. The metabolic effects of insulin are mediated through the PI3k-Akt-PKC pathway which is critical for proper translocation of the insulin-regulated glucose transporter GLUT4 to the plasma membrane [19]. Whereas tyrosine phosphorylation of the docking proteins is indispensable for efficient insulin action, serine/threonine phosphorylation of IRS1 blocks proper downstream signaling and contributes to the desensitization to insulin in T2DM [20]. The chronic low-grade inflammatory state associated with obesity is known to interfere with IR signaling, contributing to the development of insulin resistance. Development of this inflammatory state is initiated, in part, by the release of proinflammatory mediators released from adipocytes that have become excessively large. Stress and inflammatory pathways are subsequently activated by these mediators resulting in the infiltration of a heterogeneous population of cells into adipose tissue consisting of lymphocytes, granulocytes, and monocytes.
Monocytes develop in the bone marrow where they differentiate from common myeloid progenitors. After differentiation, monocytes are released into circulation where they rapidly move to the site of inflammation upon receiving proper migratory signals. The chemokine receptor CCR2, a prominent receptor for the monocyte chemoattractant protein 1 (MCP-1) plays a key role in the migration and extravasation of monocytes. In response to insulin, 3T3-L1 mouse preadipocyte fibroblasts secrete substantial amounts of MCP-1. Injection of insulin into rodents also results in a substantial increase in circulating MCP-1 [21]. Relative to liver, kidney, and lung, fat from obese leptin-deficient ob/ob mice expresses high levels of MCP-1 indicating that adipose tissue is a major producer of this chemokine. Thus, MCP-1 is likely a major factor involved in the recruitment of monocytic cells into adipose tissues in the obese setting. In fact, the percentage of the monocyte population in adipose tissue can reach up to 50% in the ob/ob mouse and up to 40% in obese humans [22].
Once monocytes infiltrate adipose tissue, they undergo a process of maturation into macrophages that consists of cell enlargement and development of higher mitochondrial numbers and larger lysosomal structures with increased content of hydrolytic enzymes [23]. Macrophages are found in a multitude of tissues throughout the body and are essential for normal tissue development and function [24,25]. Macrophages are also essential for metabolic homeostasis. Pancreatic macrophages help maintain appropriate islet cell morphology and proper insulin production. In adipose tissue, resident macrophages produce interleukin (IL)-10, which facilitates insulin action in adipocytes [26]. In order to maintain metabolic homeostasis, the proper function of macrophages is essential.
Macrophages show remarkable plasticity and functional heterogeneity, as they participate in both the initiation and resolution of inflammation. This ability to perform opposite functions is controlled by the type of stimuli encountered. In the presence of type I interferon and microbial triggers, macrophages undergo classical activation, in which they are distinguishable by the generation of reactive oxygen species (ROS), nitric oxide (NO), and proinflammatory cytokines like tumor necrosis factor (TNF)-α, IL-6, and IL-12. In contrast, the type II cytokines IL-4 and IL-12 promote maturation of alternatively activated macrophages, a distinct program associated with reduced ROS generation, a diversion of arginine metabolism through upregulation of arginase I, production of the anti-inflammatory cytokine IL-10, and expression of distinct phagocytic receptors [27]. Each macrophage subtype has independent functions: the M1 subtype plays a pivotal role in the clearance of invading pathogens, whereas M2 macrophages are involved in tissue repair.
In the lean state, resident macrophages in adipose tissue exhibit M2-like characteristics. However, as adipocytes undergo hypertrophy leading to cellular stress and apoptosis, fresh monocytes are recruited to remove the cellular debris and eventually become macrophages that form crown-like structures around the dead adipocytes. Saturated fatty acids released from adipocytes are capable of binding and inducing the toll-like receptor 4 signaling cascade [28] that ultimately results in the activation of the M1 inflammatory program and secretion of the proinflammatory cytokines TNF-α and IL-6, and the generation of NO. These activated macrophages potentiate the inflammatory response and, in turn, attract more leukocytes, forming a positive feedback loop and a state of chronic inflammation. Demonstrating the importance of macrophages in insulin sensitization and metabolic syndrome, CCR2 deficient mice show resistance to diet-induced obesity and improved glucose homeostasis. Therapeutic treatment with a selective CCR2 antagonist mimicked these effects in mice maintained on a high-fat diet [29].
Inflammatory mediators have been extensively studied for their ability to interfere with insulin signaling in adipocytes and muscle cells, negatively altering downstream kinase-dependent signaling and GLUT4 translocation [30,31] and opposing the action of insulin in order to limit nutrient storage. Secretion of TNF-α was the initial factor proposed to link insulin resistance and inflammatory signaling. TNF-α is overexpressed in both muscle and adipose tissues from obese subjects [32,33] and appears to mediate its anti-insulinemic effects by targeting the phosphorylation status of the IR. In obese TNF-α null mice, the IR shows higher levels of tyrosine phosphorylation [34] and these mice respond more efficiently to exogenous glucose and insulin administration. Like TNF-α, IL-6 also inhibits insulin signaling in adipocytes [35]. Furthermore, inhibitory effects of IL-6 on insulin signaling have also been shown in primary hepatocytes [36].
The role of NO in insulin resistance is more complex. NO is synthesized by the nitric oxide synthase (NOS) family of enzymes, of which there are three isoforms differentially expressed in various tissues [37]. The inducible isoform of NOS (iNOS) is activated by inflammatory mediators and there is a wealth of data supporting the notion that overproduction of NO inhibits the action of insulin. In vivo application of the NOS inhibitor L-NAME improved insulin sensitivity in mice fed a high fat diet (HFD) and also reduced the size of adipocytes accompanied with reduced triglyceride accumulation [38]. Furthermore, iNOS deficiency protected mice from HFD-induced insulin resistance and improved brown adipose tissue function. iNOS-/- obese mice displayed normal insulin sensitivity and glucose disposal [39] and iNOS deletion in ob/ob mice enhanced the expression of mitochondrial proteins such as uncoupling proteins UCP1 and UCP3, and the transcription factor PRDM16 [40], reducing body weight and weight of total fat pads, and increasing rectal temperatures.
While PPAR-γ positively regulates the anabolism of lipids in macrophages, activation of PPAR-γ acts as a negative regulator of the inflammatory mediators mentioned above. This inhibitory function appears to be mediated by sumoylation of PPAR-γ at K365 [41]. Pascual et al. [41] demonstrated that sumoylation at K365 enhances the interaction of PPAR-γ with the nuclear corepressor (NCoR). Certain promoters, such as those controlling inflammatory genes, were shown to have NCoR-HDAC3-TBL corepressor complexes present in the basal state. Stimulation with mediators such as lipopolysaccharide triggered removal of these repressor complexes from DNA allowing the initiation of transcription of inflammatory genes. Increased sumoylation of PPAR-γ resulted in retention of repressor complex at these promoters and a reduction in transcription of inflammatory genes.
POLARIZATION OF MACROPHAGES AS A STRATEGY FOR IMPROVING OF METABOLIC DISORDERS
As mentioned above, macrophages are highly adaptable due to their extreme plasticity, which allows them to switch between M1 and M2 phenotypes upon stimulation. Hence, it is tempting to speculate that manipulation of this attribute of macrophages could ameliorate the inflammatory milieu in adipose and muscle tissue of obese individuals and restore normal insulin sensitivity. PPAR-γ could provide such a molecular switch necessary to return macrophage polarization back to the M2 state. PPAR-γ is expressed in macrophages and its genetic disruption in murine monocytes predisposes mice to insulin resistance and glucose intolerance [42]. Additionally, PPAR-γ null macrophages have a reduced rate of fatty acid oxidation and reduced number of mitochondria. PPAR-γ, therefore, is essential to macrophages for the molecular transition to alternative polarization.
The thiazolidinediones (TZDs) are potent PPAR-γ activators (potent full agonists) that are used clinically for the treatment of T2DM. TZDs increase the expression of many proteins within the insulin signaling cascade such as the IR docking proteins, PI3-kinase, glucose transporters 1, and 4. TZD treatment also leads to a reduction of circulatory levels of low density lipoprotein and triglycerides [43-45]. An additional property of TZDs is their anti-inflammatory effects in adipose tissue where TZDs attenuate production of several inflammatory mediators including TNF-α, IL-6, and iNOS [46]. Recently Nguyen et al. [47] showed that a switch to the M2 phenotype in brown and white adipose tissue improves the adaptation to cold temperatures as IL-4-stimulated macrophages release noradrenaline, which facilitates fatty acid mobilization and energy expenditure.
TZDs, THEIR SIDE EFFECTS, AND SPPARγMs
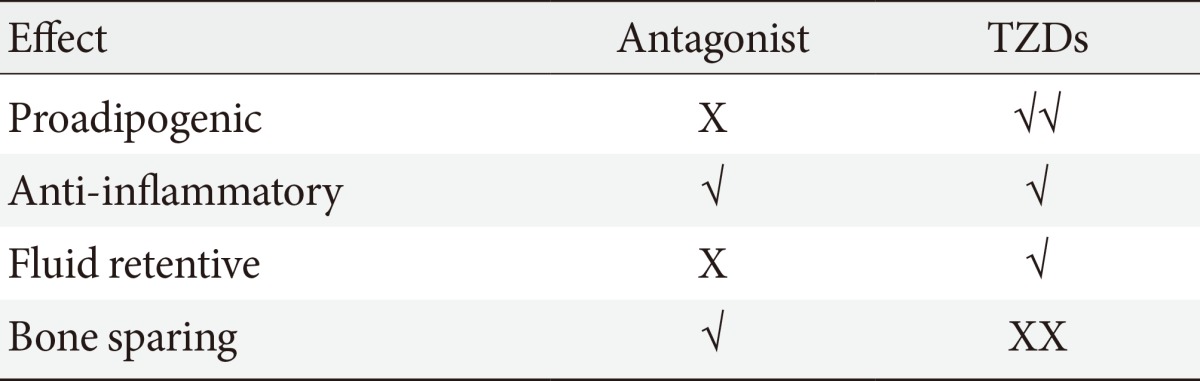
Unfortunately, the beneficial effects of TZDs in terms of improvement of glucose homeostasis, insulin sensitivity, and lipid profile is largely negated by well documented side effects such as fluid retention which is associated with increase for heart failure and the propensity for bone fractures. The increased incidence of bone fractures in patients on TZD treatment likely results from the ability of PPAR-γ to act as a negative regulator of osteoblastogenesis; hence, its hyper activation (superaphysicalogical agonism) carries negative skeletal effects. In cultured murine and human cells, the activation of PPAR-γ and its overexpression induces bone marrow stromal cell differentiation into the adipocyte lineage [48]. The use of the PPAR-γ agonist pioglitazone in ovariectomized rats reduced whole body and femoral bone mineral density [49]. Clinical studies have also provided evidence of the negative effect of TZDs on bone formation [50,51].
Systemic fluid retention is a major factor that has complicated the clinical use of TZDs. PPAR-γ is highly abundant in the collecting duct system of the kidney. Sodium channels present in the collecting duct have been shown to be target genes of PPAR-γ and their expression increases upon stimulation with TZDs resulting in increased Na+ absorption. The reduced Na+ excretion has been accredited with increased systemic water retention or plasma volume expansion (PVE). Increased total body water is partly responsible for the early weight gain observed in animal models treated with TZDs and weight gain can be prevented by deletion of PPAR-γ in the collecting ducts. In addition to weight gain, additional complications of PVE are edema and cardiomegaly. In clinical trials the incidence of edema is greater when TZDs are used in combination with other glucose-lowering agents, such as metformin, sulfonylurea, and insulin [52,53]. In preclinical trials, cardiac hypertrophy has been observed after TZD treatment. The cardiac effects are independent of insulin signaling or PPAR-γ expression in the heart and appear to stem from the cardiac volume overload (increased back pressure) caused by PVE [54].
Because of these side effects the use of TZDs has been restricted or, in some cases, the compounds have been withdrawn from the market altogether. This is unfortunate as this class of compounds have demonstrated clear robust efficacy. Thus, significant efforts had gone into the development of second-generation PPAR-γ activators called selective PPAR-γ modulators or SPPARγMs. In general, partial agonists of PPAR-γ demonstrate the SPPARγMs phenotype in preclinical models. In such models, SPPARγMs demonstrate a clear advantage over TZDs in terms of their side effect profile; however, clinical development of all SPPARγMs have been discontinued.
ALTERNATIVE APPROACHES TO MODULATING PPAR-γ
Similar to other NRs, PPAR-γ activity is regulated at the posttranslational level by modifications at key residues [55,56]. Obesity, in particular, promotes the phosphorylation of PPAR-γ at S273 (pS273), and this posttranslational modification (PTM) is correlated with the dysregulation of a subset of PPAR-γ target genes, many of which are dysregulated in obesity. pS273 sensitive genes include the insulin-sensitizing adipokines such as adiponectin and adipsin [57]. It was shown that partial agonist SPPARMγS block pS273 to the same extent of full agonists TZDs and there was a strong correlation in both obese mice and obese humans between the antidiabetic effects of drug treatment and the reduction of pS273. These results suggest that efficacy may be derived from blockage of this PTM and not from activation of the receptor itself, which partially explains the improved side effect profile of the partial agonist SPPARγMs. Based on this discovery, and in collaboration with Bruce Spiegelman, we set out to develop compounds that were non-agonists (neutral antagonists) of the receptor but retained the ability to block pS273. This effort led to the development of SR1664. We demonstrated that the antagonist SR1664 was equally efficacious as TZDs in correcting elevated plasma glucose and fasting insulin levels in diabetic mice. We also demonstrated that SR1664 was antiadipogenic and did not cause weight gain or PVE in the same mice. Cell culture experiments demonstrated that SR1664 was neutral on bone whereas TZD treatment was detrimental, as expected [58,59]. While SR1664 offers hope for targeting PPAR-γ with significantly improved therapeutic index, the pharmaceutical properties of this compound warrant significant optimization. As such, we have continued the development of compounds with similar functional properties of SR1664, but with significantly improved pharmacokinetics.
It is plausible that such neutral PPAR-γ antagonists that maintain the ability to block pS273 will retain the transcriptional repressive properties of TZDs in the macrophage, thus maintaining the ability to dampen the inflammation that accompanies obesity (Fig. 1). Such a compound would normalize expression of adipokines repressed by pS273, improve IR signaling, yet be devoid of adipogenesis and fluid retention, and will be neutral on bone density. The functional properties sought for an ideal compound are displayed in Table 1.
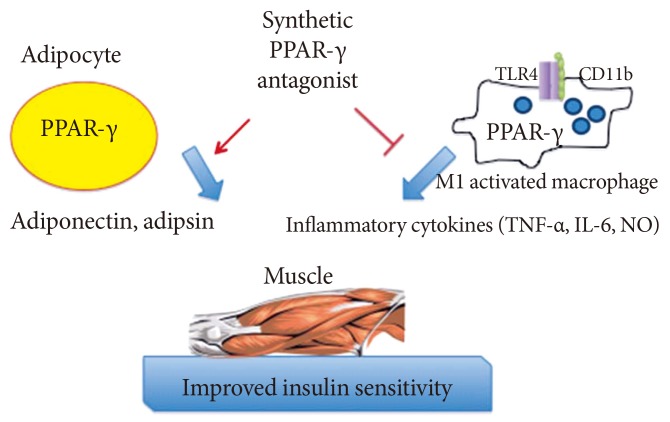
Anticipated regulatory effects of selective peroxisome proliferator-activated receptor-γ (PPAR-γ) antagonists in adipocytes and macrophages: improved expression of insulin-sensitiziting adipokines and lessened inflammatory response. TLR, Toll-like receptor; TNF, tumor necrosis factor; IL-6, interleukin-6; NO, nitric oxide.
CONCLUSIONS
The PPAR subfamily of nuclear receptors plays major roles in the energy homeostasis by promoting either the utilization or the storage of the energy sources glucose and free fatty acids. Several drugs targeting PPAR-γ for the treatment of T2DM have been developed and used clinically. Unfortunately, due to significant concerns over side effects, the utility of these drugs are limited. Significant effort had gone into the development of second-generation compounds but none of these have made it to the market. All of the second-generation compounds were either selective PPAR-γ agonists (partial or full agonists) or dual or pan PPAR modulators activating alpha and delta. Recent studies show that blockage of a specific PTM, and not activation of the receptor, correlates with the efficacy of PPAR-γ modulators. These studies open up the possibility that antagonists of PPAR-γ may offer the same potential to improve insulin sensitivity without activation of pathways responsible for the many side effects of PPAR-γ agonists, such as weight gain, water retention, and bone loss. One such compound, SR1664, that blocks phosphorylation of S273 and is antidiabetic yet does not activate PPAR-γ target genes, has been described. Efforts continue to optimize this chemical scaffold for both potency and pharmaceutical properties. We are hopeful that these efforts will lead to a new generation of safer antidiabetic drugs.
Notes
Patrick Griffin is a founder and SAB member of Ember Therapeutics.