Hemoglobin A1c May Be an Inadequate Diagnostic Tool for Diabetes Mellitus in Anemic Subjects
Article information
Abstract
Background
Recently, a hemoglobin A1c (HbA1c) level of 6.5% has been determined to be a criterion for diabetes mellitus (DM), and it is a widely used marker for the diagnosis of DM. However, HbA1c may be influenced by a number of factors. Anemia is one of the most prevalent diseases with an influence on HbA1c; however, its effect on HbA1c varies based on the variable pathophysiology of anemia. The aim of this study was to determine the effect of anemia on HbA1c levels.
Methods
Anemic subjects (n=112) and age- and sex-matched controls (n=217) who were drug naive and suspected of having DM were enrolled. The subjects underwent an oral glucose tolerance test and HbA1c simultaneously. We compared mean HbA1c and its sensitivity and specificity for diagnosing DM between each subgroup.
Results
Clinical characteristics were found to be similar between each subgroup. Also, when glucose levels were within the normal range, the difference in mean HbA1c was not significant (P=0.580). However, when plasma glucose levels were above the diagnostic cutoff for prediabetes and DM, the mean HbA1c of the anemic subgroup was modestly higher than in the nonanemic group. The specificity of HbA1c for diagnosis of DM was significantly lower in the anemic subgroup (P<0.05).
Conclusion
These results suggest that the diagnostic significance of HbA1c might be limited in anemic patients.
INTRODUCTION
The prevalence of diabetes mellitus (DM) has been on the rise over the past few decades and the disease is associated with a number of complications. DM affects many organ systems and is responsible for patient morbidity and mortality, so early diagnosis of DM is important for the prevention of complications [1].
The International Expert Committee and American Diabetes Association (ADA) has recommended using hemoglobin A1c (HbA1c) to diagnose DM, since the test is more convenient compared to previous methods that required patients to fast for more than 8 hours and has achieved worldwide standardization [2]. In addition to convenience, HbA1c has good reproducibility and reflects the chronic hyperglycemic state of diabetic patients better than fasting blood glucose. However, HbA1c is affected by a number of factors. Age, ethnicity, genetic, hematologic, and many disease-related factors can influence HbA1c levels [3-6]. In terms of various disease entities that can affect measurement, previous studies have suggested that anemia, hemoglobinopathy, chronic liver, or renal disease, and rheumatoid arthritis can influence HbA1c, and this influence is variable depending on the pathogenesis of disease [5]. Anemia is the most prevalent disease with an influence on HbA1c, and its prevalence rate is about 7% for the total population and about 12% for women in Korea [7]. The pathogenesis of anemia varies depending on the cause and the influence on HbA1c is also variable. Iron deficiency and vitamin deficiency anemia, which are associated with decreased erythropoiesis and erythrocyte turnover rate, have been found to increase HbA1c levels [8-11]. In contrast, hemolytic anemia, acute hemorrhage, and hemoglobinopathies, which are associated with increased erythropoiesis and decreased erythrocyte life span, have been found to decrease HbA1c levels [5]. Anemia due to chronic renal failure has factors that both increase (decreased erythropoiesis, increased carbamylated hemoglobin, increased contact with external glucose-like dialysate) and decrease (decreased erythrocyte life span) HbA1c levels. The influence of anemia due to chronic disease on HbA1c level is not clear, but it has been reported that rheumatoid arthritis can decrease HbA1c due to the decreased erythrocyte life span [5,12,13].
Therefore, if a physician uses only HbA1c to diagnose DM in patients with anemia, a false diagnosis or management can occur. However, the influence of anemia to HbA1c is difficult to study as has variable influence according to the specific cause of anemia. In Korea, there have been studies on the adequacy of HbA1c for diagnosing DM; however, only a few studies have addressed the influence of anemia on HbA1c. The aim of this study was to determine the effect of clinically common anemia on HbA1c level and to examine the feasibility of diagnosing DM using HbA1c as a marker in anemic patients in Korea.
METHODS
Subjects
We retrospectively reviewed the medical records of 2,119 patients who were ≥20 years old and underwent oral glucose tolerance testing (OGTT) between January 2006 and June 2008 in the Department of Endocrinology and Metabolism at the Kyung Hee University Medical Center. Among the total subjects, 169 patients were anemic and 112 patients were finally analyzed after exclusion. In addition, 217 age- and sex-matched control subjects were selected and analyzed.
Anemia was defined as a hemoglobin level of less than 13 g/dL in men and less than 12 g/dL in women, according to criteria from the World Health Organization [14]. Subjects were excluded if they were diagnosed with DM previously, had a history of transfusion in the past 3 months, or a lag of more than 1 month between OGTT and HbA1c test. Subjects who had chronic renal failure, elevated bilirubin level of more than 1.2 mg/dL, hematologic neoplasms, and acute anemia due to surgical operation or hemorrhage were also excluded. There were no patients with hemoglobinopathies or hemolytic anemia.
Methods
The following data were collected from medical records: age, gender, body mass index, hemoglobin, mean corpuscular volume, mean concentration of hemoglobin, total bilirubin, lipid profile, white blood cell and platelet counts, blood urea nitrogen (BUN), serum creatinine, and HbA1c.
All subjects underwent a 75 g OGTT and were stratified into normal, prediabetic, and DM subgroups. HbA1c was compared between the anemic and nonanemic groups at each glucose tolerance test. Subjects were also classified according to glucose tolerance status by fasting plasma glucose (FPG) and postprandial 2-hour glucose (PP2) and the mean HbA1c of both groups was also compared. Furthermore, we compared the sensitivity and specificity of HbA1c for the diagnosis of DM between each subgroup.
The OGTT was conducted after fasting more than 8 hours and venous blood was drawn at fasting and 2 hours after glucose loading. After centrifuging the blood samples, plasma glucose levels were measured by the hexokinase method. According to criteria suggested by the ADA, DM was defined as a plasma glucose level equal to or greater than 126 mg/dL in the fasting state or equal to or greater than 200 mg/dL 2 hours after glucose load. Impaired fasting glucose (IFG) was defined as a FPG level between 100 and 125 mg/dL, and impaired glucose tolerance (IGT) was defined as a 2-hour plasma glucose level between 140 and 199 mg/dL [15].
Complete blood count parameters were measured by an ADVIA 2120 analyzer (Siemens Healthcare Diagnostics, Surrey, UK) and blood chemistry parameters were measured using a TBA-200FR analyzer (Toshiba, Tokyo, Japan). HbA1c concentrations were determined using high performance liquid chromatography on the HLC-723G8 (Tosoh Biosciences Inc., San Francisco, CA, USA) instrument, which received National Glycohemoglobin Standardization Program certification.
Statistical analysis
Data were analyzed using SPSS software version 12.0 (SPSS Inc., Chicago, IL, USA) and all variables are reported as mean±standard deviation. Clinical characteristics and mean glucose/HbA1c levels were compared using Student t-test, and variables which were not normally distributed were analyzed by the Mann-Whitney U test. Differences of mean hemoglobin level according to glucose tolerance status were analyzed by the one-way analysis of variance test. Difference in sensitivities and specificities were compared using the two-sample proportion z test. The sensitivity and specificity of HbA1c for diagnosing DM were measured using a receiver operating characteristic (ROC) curve by STATA 12.0 program (Stata Corp., College station, TX, USA).
RESULTS
Clinical characteristics
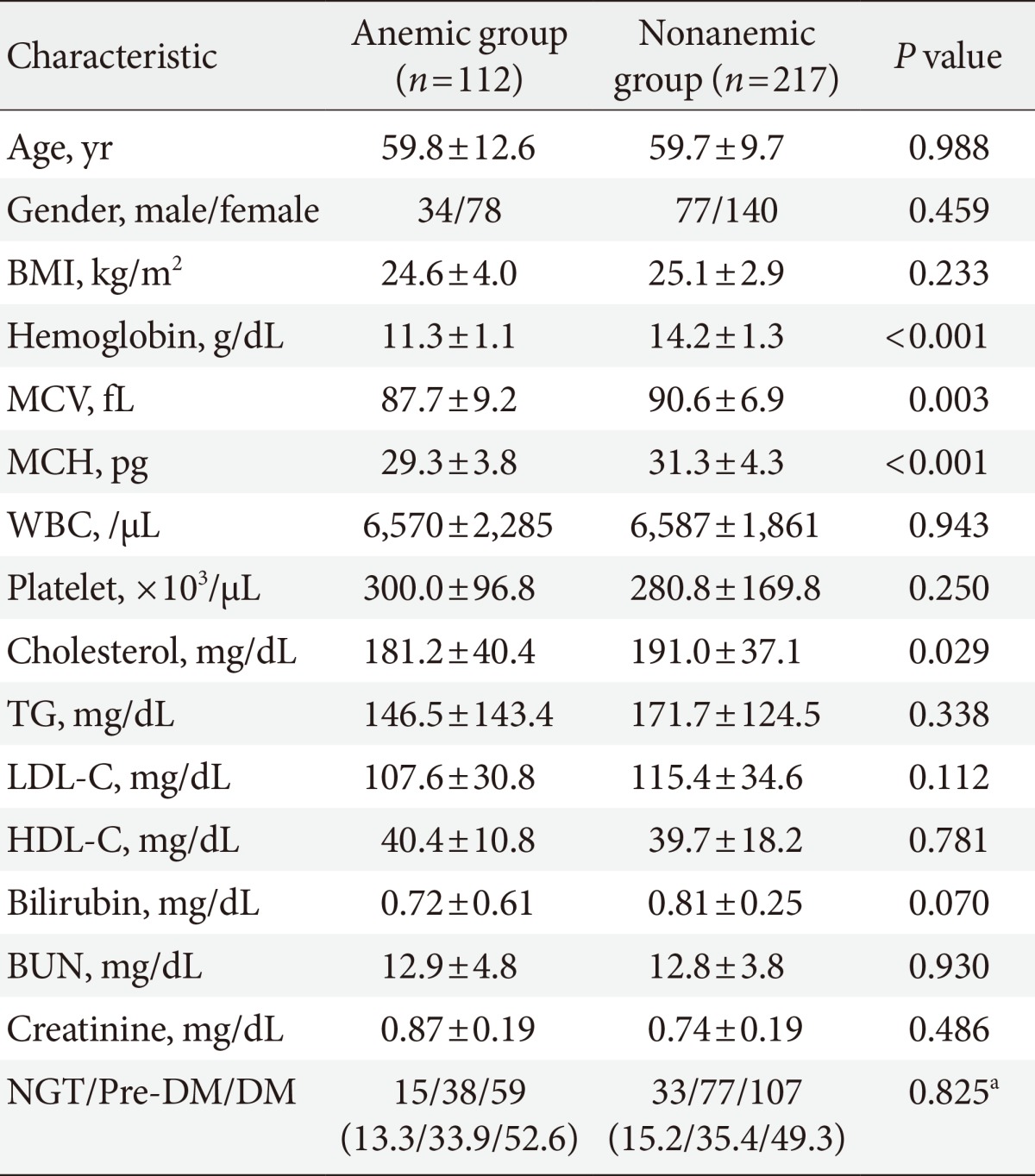
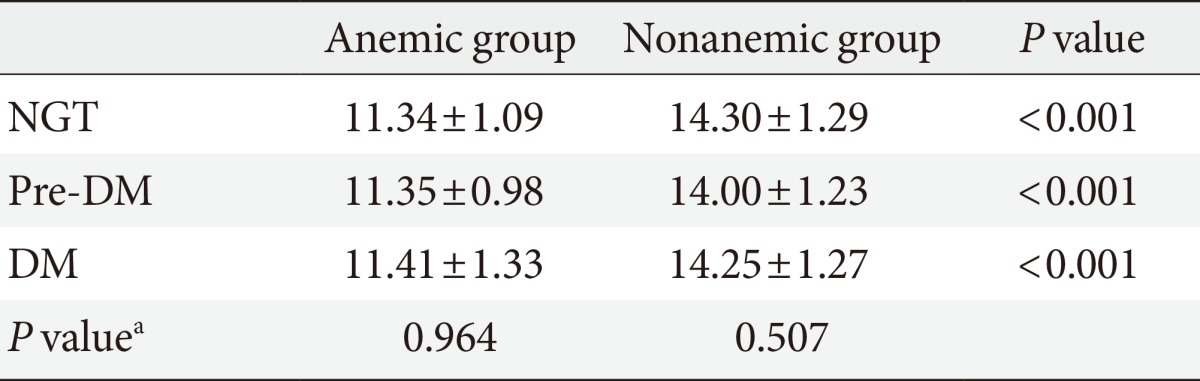
Clinical characteristics and glucose levels of the subjects are shown in Table 1. The age and sex ratios of both groups were not significantly different. The mean hemoglobin was 11.3±1.1 g/dL (range, 8.2 to 12.9 g/dL) in the anemic group and 14.2±1.3 g/dL (range, 12.2 to 20.1 g/dL) in the nonanemic group (P<0.05) and the mean corpuscular volume was 87.7±9.2 fL in the anemic group and 90.6±6.9 fL in the nonanemic group (P<0.05). There were no significant differences observed in any other hematologic parameters, such as white blood cell and platelet counts or blood chemistry parameters, including BUN, serum creatinine, and total bilirubin. Lipid profiles, including total cholesterol, triglyceride, low density lipoprotein cholesterol, and high density lipoprotein cholesterol were similar between the two groups with the exception of total cholesterol, which was higher in the nonanemic group than in the anemic group (P<0.05). There was no significant difference in the proportion of each glucose tolerance status between the anemic and nonanemic groups. Mean hemoglobin levels were not different according to glucose tolerance status in either the anemic or nonanemic group (Table 2).
Comparison of mean HbA1c levels in various glucose tolerance statuses between anemic and nonanemic subjects
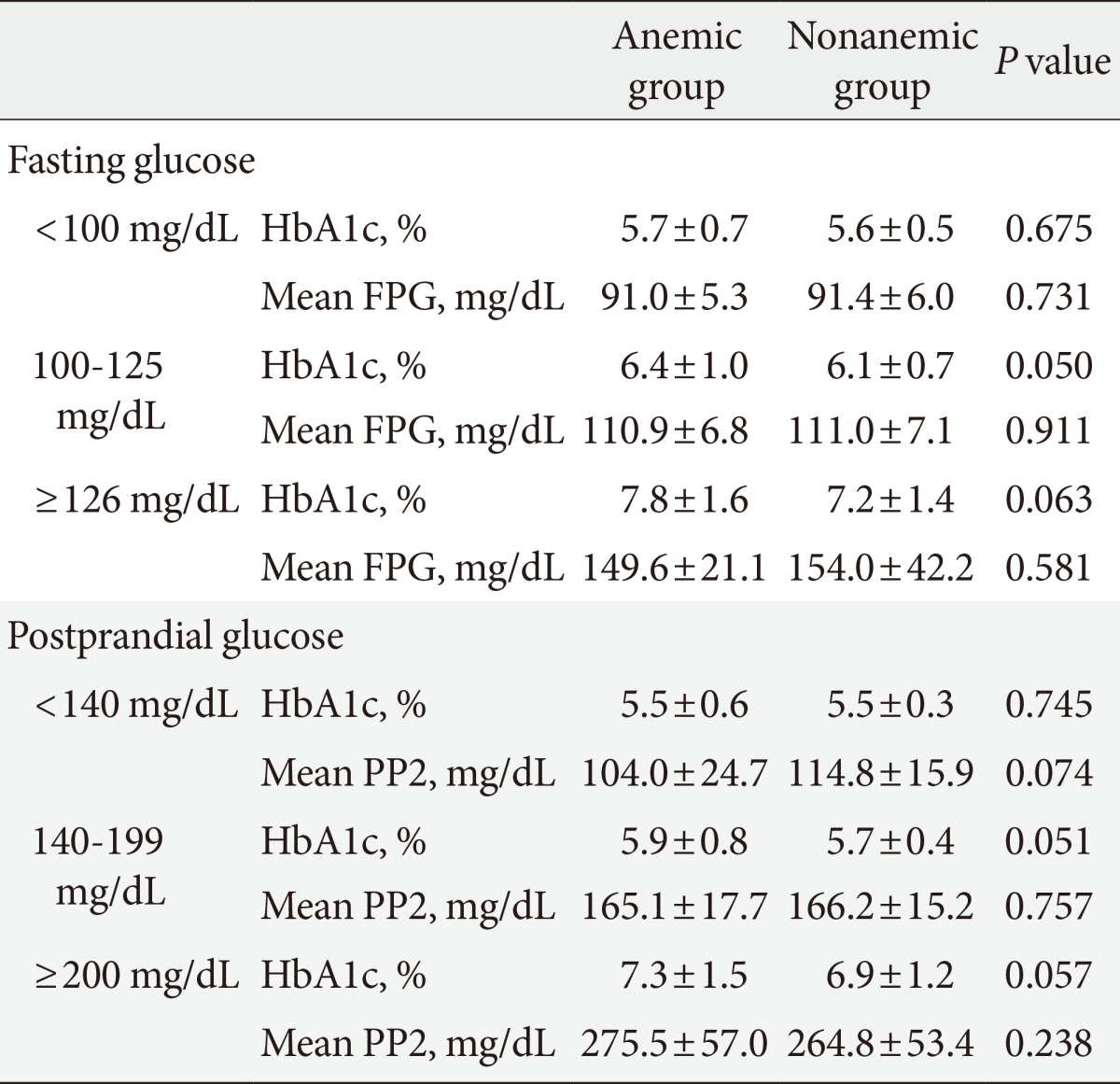
We compared mean HbA1c and mean glucose levels between anemic and nonanemic groups according to FPG and PP2 (Table 3). The mean HbA1c of subjects with an FPG of less than 100 mg/dL was 5.7%±0.7% in the anemic group and 5.6%±0.5% in the nonanemic group, and no significant difference was observed between the two groups. However, in the IFG group, mean HbA1c was 6.4%±1.0% in the anemic group and 6.1%±0.7% in the nonanemic group, a difference with borderline significance (P=0.050). Similarly, in diabetics, mean HbA1c was 7.8%±1.6% in the anemic group and 7.2%±1.4% in the nonanemic group, a difference with borderline significance (P=0.059). Mean glucose levels were not significantly different between groups. A similar tendency was also observed in postprandial glucose. In subjects with a level of less than 140 mg/dL, mean HbA1c was not different between the anemic and nonanemic groups (5.5%±0.6% vs. 5.5%±0.3%; P=0.745). However, in subjects with IGT, there were modestly higher HbA1c levels in the anemic group (5.9%±0.8% vs. 5.7%±0.4%; P=0.051). Likewise, in subjects with a PP2 of more than 200 mg/dL, the mean HbA1c was also higher in the anemic group with borderline significance (7.3%±1.5% vs. 6.9%±1.2%; P=0.057), although there were no significant differences in mean PP2 levels between groups.
Sensitivity and specificity of HbA1c for the diagnosis of diabetes mellitus in anemic subjects
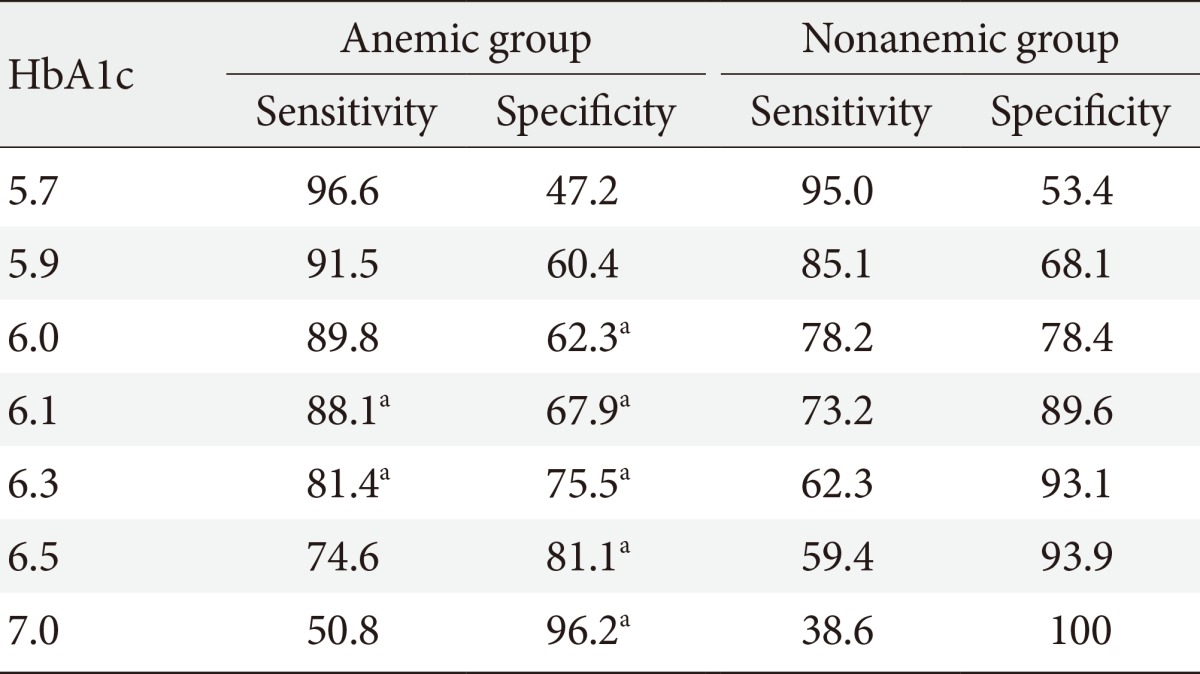
The sensitivity and specificity of HbA1c for the diagnosis of DM were determined using ROC curve analysis. In the nonanemic group, the HbA1c optimal cutoff value of 6.1% had an area under the curve (AUC) of 0.884 with 73.2% sensitivity and 89.6% specificity (Table 4). In the anemic group, the HbA1c optimal cutoff value of 6.2% had an AUC of 0.859 with 86.4% sensitivity and 73.6% specificity. At HbA1c 6.5%, which is currently the diagnostic cutoff, the sensitivity of DM was 59.4% and the specificity was 93.1% in the nonanemic group. In contrast, in the anemic group, the diagnostic sensitivity of DM was 74.6% and the specificity was 81.1%. The specificity of the anemic group was significantly lower than that of the nonanemic group (P<0.05).
DISCUSSION
HbA1c has been proposed as a new diagnostic criterion for DM [16] due to its convenience and high reproducibility and its role in the diagnosis of DM is expected to grow. This study was conducted to determine the effect of anemia on HbA1c, since anemia is highly prevalent among the diseases that can affect HbA1c levels and only a few studies have been performed to investigate the relationship between anemia and HbA1c in Korea. Moreover, although there have been many previous studies conducted in the United States and Europe on the influence of anemia on HbA1c, there is a vacuum in the literature concerning the sensitivity and specificity of using HbA1c in anemic subjects for the diagnosis of DM [8,17-19]. We limited our survey to common forms of anemia and other rare causes of anemia such as aplastic, hemolytic anemia, and other inherited disorders, and acute anemia due to hemorrhage or surgery was excluded.
Danescu et al. [17] reported a case of a β-thalassemia minor patient with an HbA1c level of 1.6% despite an elevated blood glucose concentration and explained that the cause was decreased hemoglobin life span. In a Turkish study, the mean HbA1c level was 7.4% among nondiabetic patients with iron deficiency anemia (IDA) and 5.2% among the controls, with mean HbA1c level decreasing in patients with IDA from 7.4% to 6.2% after iron supplementation [8]. On the contrary, Rai and Pattabiraman [18] were unable to find a difference in the mean concentrations of HbA1c between nondiabetic patients with IDA and controls. The analysis of the National Health and Nutrition Examination Survey (NHANES) population data for 8 years revealed that iron deficiency was associated with shifts in HbA1c distribution from less than 5.5% to more than 5.5% in nondiabetic women. However, another NHANES analysis did not detect a significant difference in mean HbA1c level according to IDA status [20,21].
In the present study, the mean HbA1c level of the anemic group in which the result of OGTT was prediabetes or DM was higher than in the nonanemic group with borderline significance, although how decreased erythropoiesis and altered red blood cell life span affect glycosylation of hemoglobin in those with elevated blood glucose levels remains unanswered.
ROC curve analysis indicated that the sensitivity and specificity of the nonanemic group were not significantly different from previous studies. However, in the anemic group, the specificity was 81.1% at an HbA1c level of 6.5%, the diagnostic cutoff for DM, and was significantly lower than that in the nonanemic group. The diagnostic value of HbA1c was initially suggested to be higher than the optimal cutoff value in order to increase specificity, therefore, the low specificity of HbA1c in the anemic group in this study is meaningful. The results of this study imply that a diagnosis of DM in an anemic patient based primarily on HbA1c levels may have decreased diagnostic significance.
Some limitations of this study should be noted. First, selection bias could have occurred in the selection of subjects in the nonanemic group. However, we considered several factors that can affect HbA1c levels and tried to minimize this bias. Second, because the specific causes of anemia in subjects were not clarified, the influence of specific causes of anemia on HbA1c level could not be determined. However, we excluded relatively rare and specific disease-related anemias, so it could be assumed that most patients had iron or vitamin deficiency anemias or anemia due to chronic disease, and those are the most prevalent forms and easily seen in clinical settings. Third, in the comparison of mean HbA1c, the number of subjects was not large enough to draw statistically significant conclusions. To overcome these limitations, further studies with a greater number of subjects are needed to determine the relationship between anemia and HbA1c.
In conclusion, the data suggest that the mean HbA1c level of the anemic group in which the OGTT result was prediabetes or DM was higher than in the nonanemic group with borderline significance. Moreover, HbA1c had decreased specificity for diagnosing DM in those with anemia and an abnormal glucose tolerance status. Therefore, HbA1c may be a limited tool for diagnosing DM in patients with anemia and clinicians should be cautious in those cases.
ACKNOWLEDGMENTS
This study was supported by a grant from the Korea Healthcare Technology R&D Project, Ministry of Health and Welfare, Republic of Korea (A102065).
Notes
No potential conflict of interest relevant to this article was reported.