Independent Impact of Diabetes on the Severity of Coronavirus Disease 2019 in 5,307 Patients in South Korea: A Nationwide Cohort Study
Article information
Abstract
Background
Inconsistent results have been observed regarding the independent effect of diabetes on the severity of coronavirus disease 2019 (COVID-19). We conducted a nationwide population-based cohort study to evaluate the relationship between diabetes and COVID-19 severity in South Korea.
Methods
Patients with laboratory-confirmed COVID-19 aged ≥30 years were enrolled and medical claims data were obtained from the Korean Health Insurance Review and Assessment Service. Hospitalization, oxygen treatment, ventilator application, and mortality were assessed as severity outcomes. Multivariate logistic regression analyses were performed after adjusting for age, sex, and comorbidities.
Results
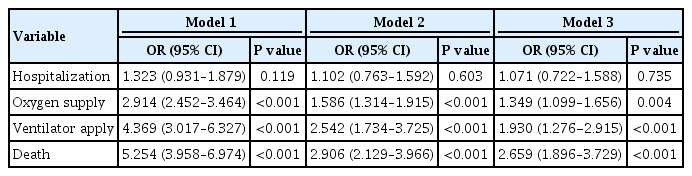
Of 5,307 COVID-19 patients, the mean age was 56.0±14.4 years, 2,043 (38.5%) were male, and 770 (14.5%) had diabetes. The number of patients who were hospitalized, who received oxygen, who required ventilator support, and who died was 4,986 (94.0%), 884 (16.7%), 121 (2.3%), and 211 (4.0%), respectively. The proportion of patients with diabetes in the abovementioned outcome groups was 14.7%, 28.1%, 41.3%, 44.6%, showing an increasing trend according to outcome severity. In multivariate analyses, diabetes was associated with worse outcomes, with an adjusted odds ratio (aOR) of 1.349 (95% confidence interval [CI], 1.099 to 1.656; P=0.004) for oxygen treatment, an aOR of 1.930 (95% CI, 1.276 to 2.915; P<0.001) for ventilator use, and an aOR of 2.659 (95% CI, 1.896 to 3.729; P<0.001) for mortality.
Conclusion
Diabetes was associated with worse clinical outcomes in Korean patients with COVID-19, independent of other comorbidities. Therefore, patients with diabetes and COVID-19 should be treated with caution.
INTRODUCTION
Coronavirus disease 2019 (COVID-19), which is caused by a novel virus severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has had major effects worldwide [1]. Since the first case was reported in Wuhan, China in December 2019, COVID-19 has spread globally, and as of on June 4, 2020 the total number of COVID-19 cases reached 6,416,828 with 382,867 fatalities according to the World Health Organization (WHO) [2].
According to previous studies, risk factors for the severity of COVID-19 are old age, male sex, high body mass index, and chronic disease [3-5]. Since diabetes is a major risk factor for other diseases caused by coronaviruses, such as severe acute respiratory syndrome (SARS) and the Middle East respiratory syndrome (MERS), special attention has been given to diabetes [6,7]. Increased expression of angiotensin converting enzyme 2 (ACE2), the receptor of SARS-CoV-2, impairs innate and adaptive immunity, and the presence of comorbidities other than diabetes has been suggested to be a potential mechanism of diabetes to affect the poor clinical outcomes of patients with COVID-19 [8-11].
To date, several studies from different countries evaluated the impact of diabetes on COVID-19 outcomes [3,5,12-20]. Without considering other comorbidities, most studies showed a significant association between critical illness or death and diabetes. However, in some of those studies, no such association was found after adjusting for other risk factors [3,5,14,18]. Among the studies showing significant associations, the hazard ratios varied substantially [16,17]. Most of these studies were not conducted across different geographic areas and only included hospitalized patients who had relatively severe conditions. To better evaluate the impact of diabetes on the severity of COVID-19, studies with larger and nationwide data are necessary.
South Korea is regarded as a country that has successfully coped with COVID-19 through accurate and aggressive testing; more than one million tests were performed, revealing a 1.2% positive rate and 2.4% mortality rate (as of June 4, 2020), which implies that most cases were detected [21]. A retrospective study of COVID-19 in South Korea has assessed the clinical outcomes of patients with diabetes; however, it was conducted at a single center and included a relatively small number patients (n=117) [19]. Therefore, we conducted a nationwide population-based cohort study to evaluate the impact of diabetes on severity outcomes among patients with COVID-19 in South Korea.
METHODS
Study subjects
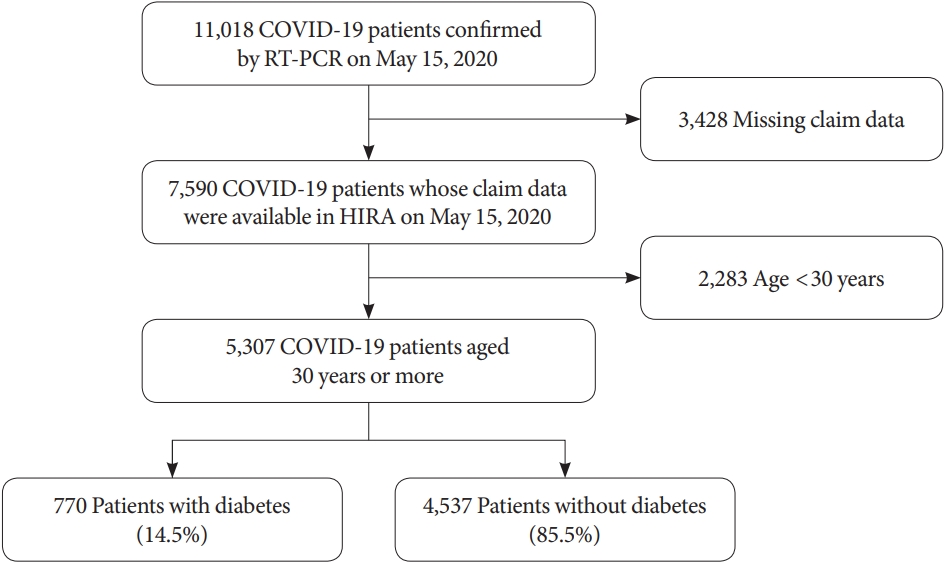
In South Korea, the national health coverage system covers approximately 98% of the total national population, and the Korean Health Insurance Review and Assessment Service (HIRA) is the government agency that reviews medical claims. For research purposes, the South Korean government has shared de-identified nationwide HIRA data on patients with COVID-19 with the public and researchers [22]. This contains data on all patients who were thought to be infected with COVID-19 after January 2020, including data on examinations, prescription drugs, and diagnostic codes. The Korean Classification of Diseases seventh revision (KCD-7), a modified version of the International Classification of Disease (ICD-10), was used as the diagnostic code. All patients with COVID-19 aged ≥30 years were enrolled in this retrospective study, regardless of hospitalization. Among 11,018 patients with COVID-19 confirmed prior to May 15, 2020, 7,590 patients were eligible after excluding subjects with missing claims data, and 5,307 patients aged 30 years or more were finally included in this cohort (Fig. 1) [23].
COVID-19 tests
In South Korea, COVID-19 screening tests are performed with a nasopharyngeal swab or sputum sample using a diagnostic kit and real-time polymerase chain reaction. The South Korean government guidelines on indications for the screening test are as follows: (1) a person exhibiting a fever (≥37.5°C) or respiratory symptoms (such as cough and shortness of breath) within 14 days of contact with a patient with confirmed COVID-19 during their symptom-exhibiting period; (2) a person suspected of COVID-19 according to a physician’s opinion, for reasons such as pneumonia of unknown etiology; (3) a person with an overseas travel history exhibiting a fever (≥37.5°C) or respiratory symptoms (such as cough and shortness of breath) within 14 days of entry into South Korea; or (4) a person exhibiting fever (≥37.5°C) or respiratory symptoms (such as cough and shortness of breath) within 14 days who has an epidemiological link to a domestic COVID-19 cluster [24]. As of May 15, 2020, of the 726,747 tests performed, 11,018 test results were positive for COVID-19.
Disease definitions
Patients with diabetes were defined as those who had a prescription of antidiabetic drugs in the year prior to diagnosis of COVID-19. The following diagnostic codes were used to define other comorbidities: hypertension (HTN), I10-13 and I15; dyslipidemia, E78; chronic kidney disease (CKD), N18-19; chronic obstructive pulmonary disease (COPD), J41-J44; ischemic heart disease (IHD), I20-25; stroke, I121-122; congestive heart failure (CHF), I150; atrial fibrillation (AF), I48; end-stage renal disease (ESRD), rare and incurable disease claim codes V001, V003, V005. Cardiovascular disease (CVD) was defined as a composite of IHD, stroke, and CHF. Hospitalization, oxygen treatment, ventilator use, and mortality were evaluated as outcomes.
Statistical analysis
Continuous variables are presented as mean±standard deviation, and categorical variables are presented as number and proportion. Baseline characteristics were compared between the diabetes and non-diabetes groups using the t-test and chi-square test. To evaluate the association between diabetes and the severity of outcomes, multivariate logistic regression analyses was performed using several models: (1) Model 1, unadjusted; (2) Model 2, age+sex; (3) Model 3: age+sex+HTN+dyslipidemia+CKD+COPD+CVD+AF+ESRD+cancer. In addition, we performed subgroup analyses stratified by sex, age (65 years), and CVD status. For all tests, a P value of <0.05 was considered statistically significant. Statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA).
Ethical statement
This study was approved by the Institutional Review Board (IRB) of Kangbuk Samsung Hospital (IRB No. KBSMC 2020-04-040). Patient data were de-identified and based on the Bioethics and Safety Act in South Korea, the need for informed consent from subjects was waived.
RESULTS
Baseline characteristics and clinical outcomes
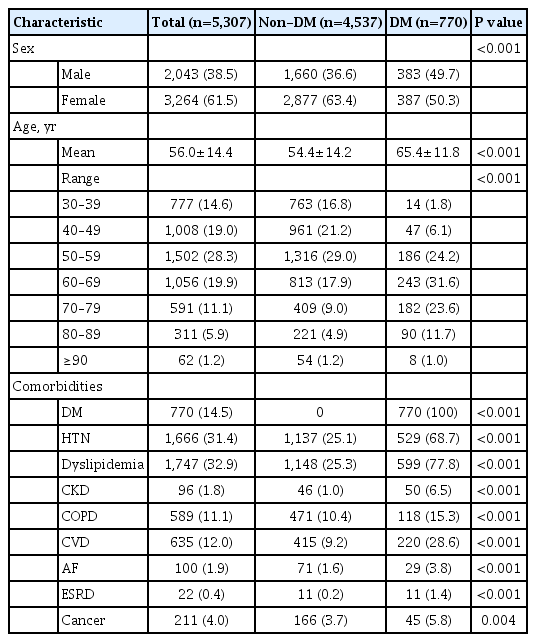
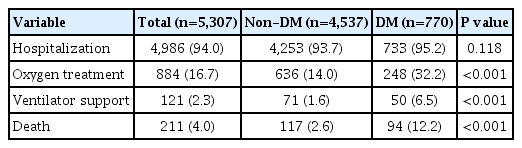
The mean patient age was 56.0±14.4 years and 2,043 (38.5%) were male. A total of 770 (14.5%) patients had diabetes. The diabetes group included more older (65.4±11.8 years vs. 54.4± 14.2 years) and male (49.7% vs. 36.6%) patients than the non-diabetes group (Table 1). All comorbidities, including HTN, dyslipidemia, CKD, COPD, CVD, AF, ESRD, and cancer, were more prevalent in the diabetes group than in the non-diabetes group. In total, 4,986 (94.0%) patients were hospitalized, 884 (16.7%) patients underwent oxygen treatment, 121 (2.3%) patients required ventilator support, and 211 (4.0%) patients died (Table 2). With the exception of hospitalization (P=0.118), all severe outcomes were significantly higher in patients with diabetes than in those without diabetes (all P<0.001).
Clinical outcomes according to age group and comorbidities
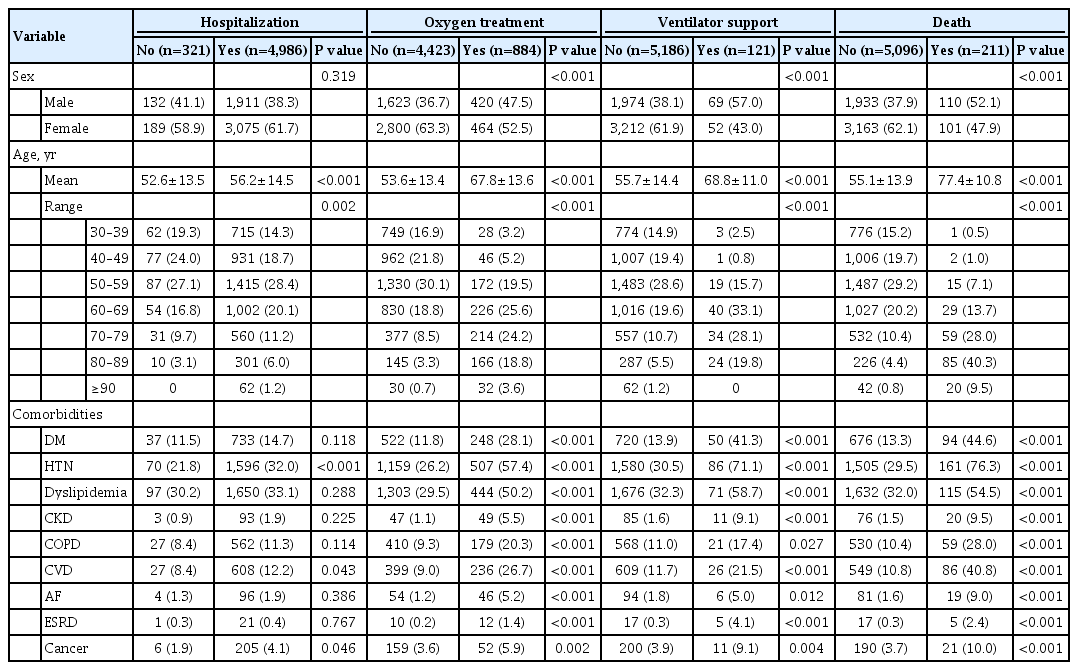
Patients with poor outcomes were significantly older than those with good outcomes (all P<0.001), and age showed a tendency toward increasing as outcomes worsened from simple hospitalization to death (56.2±14.5, 67.8±13.6, 68.8±11.0, and 77.4±10.8 years, respectively) (Table 3). There were more male subjects in groups with poor outcomes than in groups with good outcomes.
The number of patients with HTN, CVD, and cancer was higher in the hospitalized group, but there was no correlation with other comorbidities. With regard to other severe outcomes (oxygen, ventilator support, and death), all comorbidities were associated with worse clinical outcomes. The proportion of patients with diabetes showed a positive correlation with severity of outcomes, accounting for 14.7%, 28.1%, 41.3%, and 44.6% in the hospitalization, oxygen, ventilator support, and death groups, respectively.
Risk of poor clinical outcomes according to diabetes status
The results of logistic regression analyses for severe outcomes according to diabetes status are shown in Table 4. In both univariate and multivariate analyses, the presence of diabetes did not increase risk for hospitalization. In contrast, the presence of diabetes significantly increased the risks for all other poor clinical outcomes, even after adjustment for covariates and comorbidities, although the odds ratios (ORs) were attenuated after full adjustment, with an adjusted odds ratio (aOR) of 1.349 (95% confidence interval [CI], 1.099 to 1.656; P=0.004) for oxygen treatment, an aOR of 1.930 (95% CI, 1.913 to 1.946; P<0.001) for ventilator support, and an aOR of 2.659 (95% CI, 1.896 to 3.729; P<0.001) for mortality. In subgroup analyses stratified by sex, age (65 years), and CVD status, death was significantly associated with diabetes in all subgroups, and there was no significant difference in the association between mortality and diabetes in male and female patients, those aged <65 and ≥65 years, or non-CVD and CVD groups (Table 5).
DISCUSSION
We performed a nationwide population-based cohort study evaluating the impact of diabetes on the severity of COVID-19. Among 5,307 patients with COVID-19 aged ≥30 years, 14.5% had diabetes, and a gradual increasing tendency was shown in the proportion of diabetes with increasing severity of outcomes. Despite a non-significant association with hospitalization, diabetes was significantly associated with the need for oxygen treatment, ventilator support, and mortality even after adjusting for other comorbidities, with ORs of 1.349, 1.930, and 2.659, respectively.
COVID-19 is caused by SARS-CoV-2, which is a single-strand enveloped RNA virus and the seventh known human coronavirus [25]. Other human coronaviruses include SARS coronavirus (SARS-CoV) and MERS coronavirus (MERS-CoV), which caused SARS in 2002 and MERS in 2012, respectively [26]. Similar to SARS-CoV and MERS-CoV, the primary source of SARS-CoV-2 is thought to be bats; it is believed to have moved to an intermediate host (probably a pangolin) before transmission to humans [27]. In all pandemic diseases caused by coronaviruses, diabetes was associated with worse outcomes [6,7]. Several potential mechanisms by which diabetes affects poor outcomes in patients with COVID-19 have been suggested [8,28]. These include (1) increased expression of ACE2 (the receptor of SARS-CoV-2) in diabetes, which has been confirmed in animal studies [11]; (2) poor glycemic control [28]; (3) decreased viral clearance due to impaired innate and adaptive immunity [10]; (4) vulnerability to cytokine storm syndrome [9]; and (5) the presence of other comorbidities, such as CVD.
Hence, international diabetes associations have suggested strict glycemic control to defend against COVID-19, and the American Diabetes Association has created a platform for health professionals to share and discuss their COVID-19 experience [29]. The United States Centers for Disease Control and Prevention classified patients with diabetes as a high-risk group for severe COVID-19 [30]. The Korean Diabetes Association (KDA) has proposed prioritizing COVID-19 screening in elderly (aged ≥70 years) patients with diabetes, based on data that showed a high proportion of patients with diabetes among patients with COVID-19.
Recently, several previous studies have assessed the association between diabetes and poor prognosis in patients with COVID-19. Most results of univariate analyses have shown a positive association between diabetes and severe outcomes [3,5,12-17,19,20]. However, after adjusting for comorbidities, this association disappeared in many studies (Supplementary Table 1). In a large prospective cohort study of 2,729 hospitalized patients with COVID-19 in the United States, the multivariate aOR of diabetes for mortality was 1.23 (95% CI, 0.99 to 1.50), which was not significant [3]. In another United States study conducted in 257 critically-ill patients with COVID-19, no significant association between diabetes and mortality was shown, with an adjusted hazard ratio (aHR) for mortality of 1.31 (95% CI, 0.81 to 2.10) [5]. Furthermore, another United States study involving 200 hospitalized patients with COVID-19 also failed to show a significant association between diabetes and mortality, with an aHR of 1.16 (95% CI, 0.55 to 2.44) [18]. In a large prospective cohort study in the United Kingdom, the multivariate aHR for death was 1.06 (95% CI, 0.99 to 1.14) among 15,194 hospitalized patients with COVID-19; however, no significant association was found [20]. In China, one study including 306 age- and sex-matched hospitalized patients with COVID-19 showed no significant aHR for mortality (1.58; 95% CI, 0.84 to 2.99) [14].
In contrast, in another multivariate study in China, diabetes was significantly associated with a composite outcome of intensive care unit (ICU) admission, ventilator use, and mortality among 1,590 hospitalized patients with COVID-19 (aHR, 1.59; 95% CI, 1.03 to 2.45) [16], while another Chinese study of 453 hospitalized patients with COVID-19 showed a relatively high aHR of 4.63 (95% CI, 1.02 to 21.0) for mortality in patients with diabetes compared to normoglycemic patients [17]. A study conducted in Italy of 59 hospitalized patients with COVID-19 also showed a relatively strong association between diabetes and a composite outcome of ICU admission, ventilator use, and death, with an aHR of 5.81 (95% CI, 1.74 to 19.61) [13]. Moreover, a study of 117 hospitalized patients with COVID-19 in South Korea showed a significant association between diabetes and severe outcomes (composite of acute respiratory distress syndrome, septic shock, and ICU care), with an aOR of 10.77 (95% CI, 3.00 to 38.73) [19].
Despite these discrepancies, most large cohort studies in various countries have shown no significant association between diabetes and severe outcomes (Supplementary Table 1). However, the present study suggested a significant independent association between diabetes and the severity of COVID-19. This difference may be due to the lower mortality rate of COVID-19 in South Korea (3.98% in this study) than in other countries and the fact that more patients with relatively non-severe COVID-19 were included in this cohort. Given the global, large-scale outbreak of COVID-19, the high mortality rates seen in several studies may be because the patients involved were hospitalized at advanced stages of COVID-19, and limited medical resources may have prevented adequate treatment. In this case, it can be assumed that many deaths occur regardless of comorbidities. On the other hand, studies with low mortality, including this study, are conducted in relatively stable situations, and we believe that they better reflect the effects of comorbidities on disease severity. As shown in Supplementary Table 1, the mortality rate in studies that showed no significant findings was as high as 15.4% to 39% [3,5,14,18,20]. However, other studies reporting a positive association have shown a substantially lower mortality rate of 3.1% to 10.2% [13,16,17,19], which supports our hypothesis to some extent.
To the best of our knowledge, this South Korean study is the first study conducted in a national population evaluating the impact of diabetes on the severity of COVID-19 outcomes. Moreover, South Korea is regarded as one of the most successful countries with regard to managing the COVID-19 pandemic, and national data can be interpreted as originating from a relatively well-controlled and stable situation. Since a substantial number of COVID-19 tests were performed in South Korea, and aggregate national data were included, we believe that the results of the present study are more reflective of the real-life situation.
Several limitations should be considered. Due to the nature of claims data, laboratory results were absent, and disease definitions were based solely on the diagnostic codes or prescription data. Since we defined patients with diabetes as those who had a prescription for anti-diabetes drugs, patients with diabetes who were not on medication may have been assigned to the non-diabetes group in this study. However, we expect the number of these patients to be small. In addition, since there was a time lag between actual medical practice and medical claims, there is a possibility that non-collected claims data were missed. However, we believe that missing claims data will occur randomly and are not likely to significantly affect our findings. In addition, due to policy in South Korea, most patients with confirmed COVID-19 are hospitalized for treatment regardless of severity, and the proportion of hospitalization was high. Therefore, this should be taken into account when interpreting the results, especially regarding the outcome of hospitalization.
In conclusion, in this nationwide population-based cohort study of patients with COVID-19 in South Korea, diabetes was associated with worse clinical outcomes in patients with COVID-19 independent of other comorbidities. After full adjustment for comorbidities, the OR for mortality in patients with diabetes was 2.66, which was higher than reported in previous observational studies conducted in other countries with higher mortality rates. Therefore, the importance of caution regarding patients with diabetes, who are vulnerable to COVID-19, cannot be overemphasized, and gathering further experience and knowledge globally will improve our understanding of how comorbidities affect COVID-19 severity and will facilitate the development of more effective treatment strategies in vulnerable patients.
SUPPLEMENTARY MATERIALS
Supplementary materials related to this article can be found online at https://doi.org/10.4093/dmj.2020.0141.
Current studies of multivariate analyses for severity outcomes in diabetes patients
Notes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTIONS
Conception or design: S.R.K., W.Y.L, K.H.Y.
Acquisition, analysis, or interpretation of data: S.J.M., E.J.R., J.H.J., K.D.H.
Drafting the work or revising: S.J.M., E.J.R., W.Y.L., K.H.Y.
Final approval of the manuscript: S.J.M., E.J.R., J.H.J., K.D.H., S.R.K., W.Y.L., K.H.Y.
Acknowledgements
The present study was supported and funded by the Korea Diabetes Association.