Reducing Carbohydrate from Individual Sources Has Differential Effects on Glycosylated Hemoglobin in Type 2 Diabetes Mellitus Patients on Moderate Low-Carbohydrate Diets
Article information
Abstract
Background
We evaluated decreases in glycosylated hemoglobin (HbA1c) achieved by reducing carbohydrate from various sources in type 2 diabetes mellitus patients.
Methods
We followed up 138 male and 107 female outpatients on a moderate low-carbohydrate diet without diabetic medication for 6 months. Changes in carbohydrate sources (Δcarbohydrate) were assessed from 3-day dietary records at baseline and 6 months, and associations with changes in HbA1c (ΔHbA1c) were examined with Spearman's correlation coefficients (rs) and multiple regression analysis.
Results
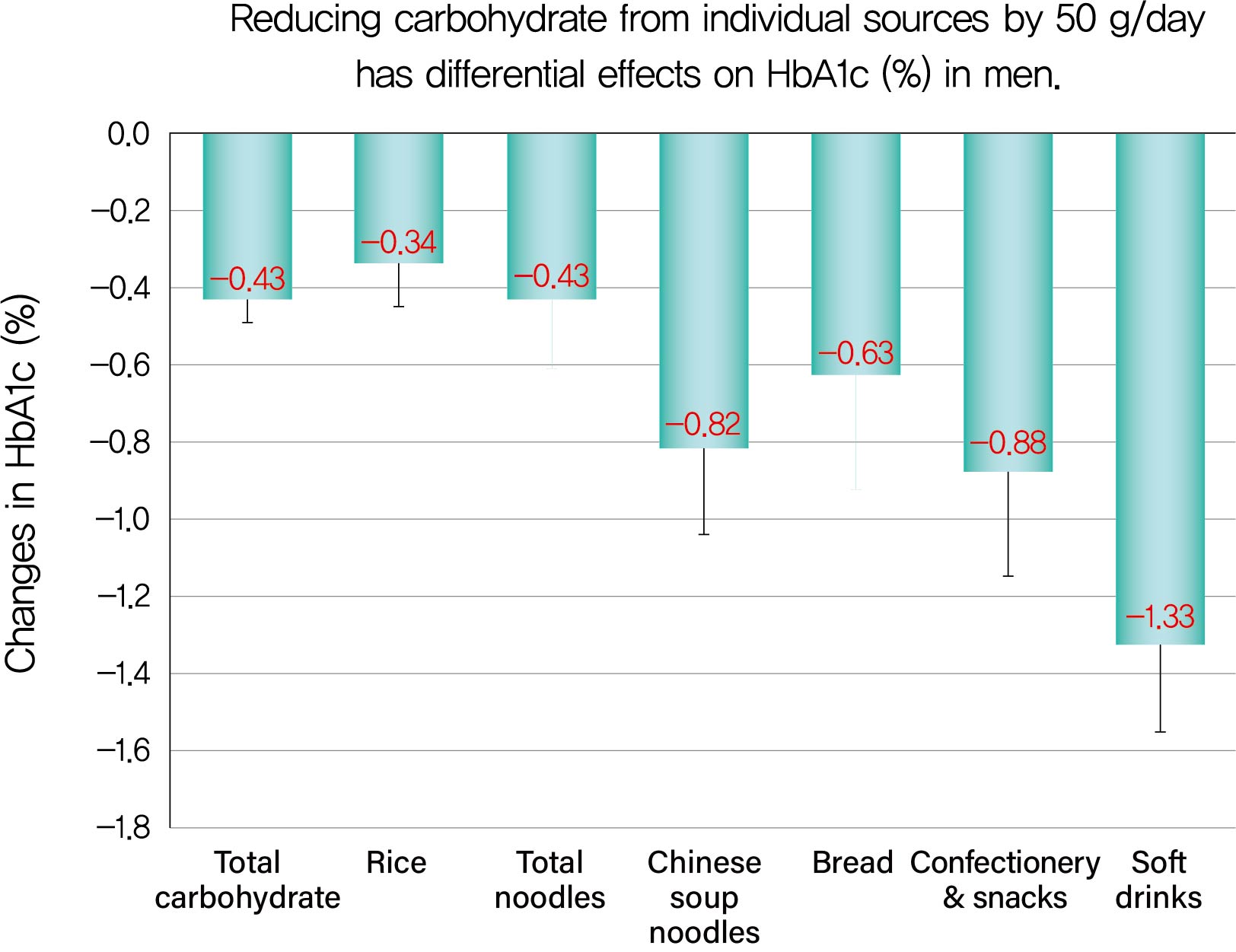
ΔHbA1c was −1.5%±1.6% in men and −0.9%±1.3% in women, while Δtotal carbohydrate was −115.3±103.7 g/day in men and −63.6±71.1 g/day in women. Positive associations with ΔHbA1c were found for Δtotal carbohydrate (rs=0.584), Δcarbohydrate from soft drinks (0.368), confectionery (0.361), rice (0.325), bread (0.221), Chinese soup noodles (0.199) in men, and Δtotal carbohydrate (0.547) and Δcarbohydrate from rice (0.376) and confectionery (0.195) in women. Reducing carbohydrate sources by 50 g achieved decreases in HbA1c of 0.43% for total carbohydrate, 1.33% for soft drinks, 0.88% for confectionery, 0.63% for bread, 0.82% for Chinese soup noodles and 0.34% for rice in men and 0.45% for total carbohydrate, 0.67% for confectionery and 0.34% for rice in women, although mean reductions in carbohydrate from these sources were much smaller than that from rice.
Conclusion
Decreases in HbA1c achieved by reducing carbohydrate from soft drinks, confectionery, bread and Chinese soup noodles were 2- to 4-fold greater than that for rice. Our results will enable patients to decrease HbA1c efficiently (UMIN000009866).
INTRODUCTION
Meal tolerance tests have proven that blood glucose and serum insulin concentrations are largely dependent on carbohydrate intake [12]. In contrast, dietary protein and fat intakes have little effect on concentrations. Incremental rises in blood glucose after ingesting various carbohydrate sources differ greatly: 50 g of carbohydrate from potatoes or rice raised blood glucose by 2/3 and 1/2, respectively, compared with the same amount of glucose [3]. Nevertheless, little quantitative information on the direct impact of carbohydrate sources on glycosylated hemoglobin (HbA1c) in patients with type 2 diabetes mellitus (T2DM) has been reported, except for fructose-containing sugars and fruits [456]. This is probably because it is ethically difficult to impose a diet consisting largely of carbohydrate-rich foods on patients for a few months. Meanwhile, a large number of interventional studies have revealed that low-carbohydrate diets had beneficial effects on glycemic control in patients with T2DM compared to low-fat diets [78]. Most studies, however, have paid attention to only total carbohydrate intake and ignored effects of carbohydrate sources on HbA1c.
In our previous study, we set goals in terms of effects of carbohydrate delta-reduction (Δcabohydrate g/day) on decreases in HbA1c levels (ΔHbA1c) in patients with T2DM treated with a moderate carbohydrate diet [9]. It demonstrated that Δ%carbo-hydrate (energy percent of carbohydrate reduction) was not associated with ΔHbA1c, and reducing total carbohydrate by 106 g decreased HbA1c by 1.0%. However, some patients who followed our moderate low-carbohydrate diet achieved a greater reduction in HbA1c levels than we expected. We speculated that this was because we did not take the effects of carbohydrate sources into account. Furthermore, our recent observational study in patients with T2DM not taking antidiabetic agents revealed that carbohydrate from soft drinks and noodles, whose amounts were less than that of rice, had a greater impact on HbA1c compared with carbohydrate from rice in men but not in women [10].
In the clinical setting, patients with T2DM need specific instructions on what carbohydrate sources they should preferentially reduce to achieve HbA1c targets efficiently. When patients reduce their intake of carbohydrate from various sources by 50 g equivalent to 1 bowl for rice, a 500 mL bottle for soft drinks, 2 slices for white bread, a large piece of cake for confectionery and 1 Chinese-style bowl for Chinese soup noodles, how much will HbA1c levels decrease due to each reduction? Also, will decreases in HbA1c levels due to reducing various carbohydrate sources differ from those achieved by reducing rice?
In consideration of the above, we designed this study to investigate and compare the effects of reducing various carbohydrate sources on decreases in HbA1c by sex in patients with T2DM treated with a 6-month moderate low-carbohydrate diet. We excluded patients taking any type of antidiabetic medication, which would lead to an incorrect estimate of changes in HbA1c.
METHODS
Patients
We recruited all new Japanese outpatients with T2DM and HbA1c levels of 6.5% or above at Haimoto Clinic from March 2013 to June 2018. Both newly and previously diagnosed patients were enrolled. Exclusion criteria included: taking any type of oral hypoglycemic agent, insulin or steroid hormone from 3 months before baseline that would impact HbA1c levels; following strict carbohydrate restriction at baseline based on commercial diet therapies such as the Atkins diet; serum creatinine levels greater than 2.0 mg/dL (176.8 µmol/L), ketoacidosis, soft drink ketosis, cancer, or decompensatory liver cirrhosis.
Of 159 eligible Japanese male outpatients, two declined to participate, nine were voluntarily lost to follow-up, one moved, four did not report dietary information, three suffered from cancer, and two took antidiabetic medications during the study period. Thus, 138 male patients were investigated. Of 117 eligible Japanese female outpatients, three declined to participate, five were voluntarily lost to follow-up, one suffered from cancer, and one took antidiabetic medications during the study period. Thus, 107 female patients were investigated.
After obtaining written informed consent, patients were followed up for 6 months. The main protocol for the present study was approved by the Ethical Committee of Aichi Syukutoku University and it was registered in University Hospital Medical Network (UMIN000009866) before its start.
Moderate low-carbohydrate diet and glycemic control
The main principles of our moderate low-carbohydrate diet are as follows: first, to calculate carbohydrate intake from 3-day dietary records at baseline; second, to reduce carbohydrate intake according to patients' baseline carbohydrate intake and HbA1c levels [1112]. Based on the results of our previous studies [913], patients were divided into three groups according to their baseline HbA1c: ≤7.4%, 7.5% to 8.9%, and ≥9.0%. Patients with HbA1c levels ≤7.4% were instructed to reduce carbohydrate by about 70 g, those with levels 7.5% to 8.9% were instructed to reduce carbohydrate by about 120 g and those with levels ≥9.0% to reduce carbohydrate by about 170 g. We instructed them to reduce major carbohydrate sources with reference to baseline 3-day dietary records. Patients were recommended to eat an amount of fat corresponding to the decrease in energy due to the reduced carbohydrate intake. A dietician (Shio Watanabe) gave instructions to all participants twice during the first month and once a month thereafter.
We provided dietary instruction tailored to each patient for maintaining optimal carbohydrate reduction. From the 2nd month, we asked patients to recall dietary details for 1 or 2 days and, straightaway, roughly calculated daily total carbohydrate and carbohydrate from various sources every month. We asked patients with difficulty in remembering their dietary details to make 3-day written dietary records or take snapshots of all they had eaten for a few days. Based on the findings from interviewing patients every month, we adjusted total carbohydrate intake in units by 25 to 50 g/day. To achieve this, we determined several carbohydrate sources that should be reduced and presented patients with written instructions in this regard. In accordance with their individual likes and dislikes, patients selected carbohydrate sources for reduction by themselves. We relaxed carbohydrate restrictions if patients had over-reduced intakes or felt stress about the reductions. By this procedure, patients were able to achieve stable reductions in carbohydrate intake for the initial 3 months and maintain the reductions for the following 3 months as described previously [91214].
The target HbA1c levels were based on the guidelines of the American Diabetes Association [15]. Patients were requested to maintain their usual level of physical activity throughout the study.
Sources of carbohydrate from various foods
Foods rich in carbohydrate content were divided into 13 groups (rice, noodles, bread, other wheat-based foods, potatoes, sugar and sweetenings, vegetables, fruits, dairy, confectionery, soft drinks, alcoholic drinks, and seasonings) according to the Japanese food composition table. “Noodles” were divided into four food groups: udon (thick white noodles), Chinese soup noodles and spaghetti made from wheat and soba made from buckwheat. “Other wheat-based foods” included gyoza (Chinese-style dumplings), shaomai (steamed dumplings), and okonomiyaki (Japanese-style pizza). “Potatoes” included sweet potatoes, taro, and yams. “Sugar and sweetenings” included non-artificial sweeteners for drinks tea and cooking. “Vegetables” included carbohydrate-rich ones, i.e., carrots, Indian lotus, pumpkin, tomatoes and burdock. “Fruits” were divided into five food groups, of which citrus fruits included mikan (mandarin oranges), oranges, and grapefruits. “Dairy” included milk, cheese, butter, and processed dairy foods such as ice cream. “Confectionery” included cakes, manjyu (buns with bean-jam filling), candy, chocolate, cookies, and snack foods (chips made from rice, wheat or potato with a sweet or salty taste). “Soft drinks” included beverages containing sugar, glucose, and fructose. “Alcoholic drinks” included brewed liquors: beer, sake (rice wine), chuhai (white distilled liquor mixed with soda water and sweetenings), and wine, but did not include distilled liquors because they contain little carbohydrate.
Dietary records and clinical assessment
Intakes of total carbohydrate and carbohydrate sources were assessed at baseline and 6 months based on 3-day dietary records. Patients were requested to record dietary intakes on 3 non-consecutive days: 2 weekdays and a holiday. The dietary records were obtained within 2 weeks from the first visit to Haimoto Clinic and we measured the HbA1c level during this period. Patients were not instructed to make any dietary changes during that time. Supplementary information was obtained in an interview with a dietitian. Dietary intakes were computed from the dietary records using the Healthy Maker Pro 501 software (Mushroomsoft, Okayama, Japan).
We measured the body mass index (BMI) and HbA1c level of each patient every month. Venous blood samples were obtained after an overnight (12-hour) fast at baseline and 6 months for the determination of fasting plasma glucose, fasting serum insulin, triglycerides, low-density lipoprotein cholesterol (LDL-C) and high-density lipoprotein cholesterol (HDL-C). The homeostasis model assessment of insulin resistance (HOMA-IR) parameter was computed as follows: [fasting plasma glucose (mg/dL)]×[fasting serum insulin (µIU/mL)]/405 [16].
Patients were asked about leisure-time physical activity levels in a questionnaire at baseline. The metabolic equivalents (Mets)-hour per week was calculated each week based on the intensity and duration of various leisure-time physical activities. We investigated changes in leisure-time physical activities during the study period in 83 patients (47 men, 36 women) who were registered after July 2016.
Laboratory methods
HbA1c levels were measured by high-performance liquid chromatography (Arkley Co., Kyoto, Japan) and presented as National Glycohemoglobin Standardization Program (NGSP) values (%). Plasma glucose concentrations were determined using enzymatic methods (Shino-Test Co., Kanagawa, Japan). Serum insulin levels were measured using the standard double antibody radioimmunoassay method (Fujirebio Inc., Tokyo, Japan). Enzymatic methods were used to measure serum triglyceride concentrations (Daiichi Pure Chemicals Co., Tokyo, Japan). Direct methods were used to assay serum LDL-C and HDL-C levels (Daiichi Pure Chemicals Co., Tokyo, Japan).
Statistical analysis
The change in each biomarker (Δ) was defined as the level after 6 months minus the level at baseline. In the analysis, we included carbohydrate sources that were consumed by at least 30% of patients at baseline.
Increases in the selected baseline characteristics (age, BMI, HbA1c, leisure-time physical activities, total energy intake, and total carbohydrate intake) with increasing or decreasing trends in ΔHbA1c were tested using linear regression models including an ordinal score for two to four groups. The Wilcoxon test was used to assess the changes in HbA1c levels and other cardiovascular risk factors, leisure-time physical activities, total energy, macronutrient intakes, and carbohydrate from various sources due to the moderate low-carbohydrate diet. We compared levels at baseline and after 6 months.
We computed Spearman's correlation coefficients (rs) to examine correlations of Δtotal cabohydrate (g/day) or Δcarbohydrate (g/day) from sources with ΔHbA1c. We considered the associations to be strong when rs was ≥0.5, moderate when rs was ≥0.3 to <0.5 and weak when rs was <0.3.
Multiple regression analyses were performed to examine associations of Δtotal carbohydrate or Δcarbohydrate from the sources (g/day) with ΔHbA1c according to two models: adjustment for age and leisure time physical activities (Model 1), for covariates in Model 1 plus ΔBMI, for covariates in Model 1 plus ΔBMI and Δtotal energy intake (Model 2). Regarding Δcar-bohydrate from non-staple foods, adjustment for Δcarbohyd-rate from staple foods (rice, total noodles, and bread) was also conducted to eliminate the possible effects of Δcarbohydrate due to them on ΔHbA1c.
P values less than 0.05 were considered statistically significant. Data are shown as mean±SD. All statistical analyses were performed using SPSS version 25.0 (IBM Co., Armonk, NY, USA).
RESULTS
HbA1c levels and clinical variables at baseline, changes in them during 6 months and changes in HbA1c according to selected baseline characteristics by sex
Tables 1 and 2 summarizes clinical variables at baseline, their changes and changes in HbA1c according to the selected baseline characteristics in men (n=138) and women (n=107). Compared to baseline, the mean HbA1c levels significantly decreased over 6 months in both sexes, by 1.5%±1.6% (15.6±16.1 mmol/mol) from 8.3%±1.7% (64.2±17.0 mmol/mol) at baseline in men, and by 0.9%±1.3% (9.5±13.0 mmol/mol) from 7.8%±1.5% (59.6±7.0 mmol/mol) at baseline in women (Table 1). The higher HbA1c, total energy intake and total carbohydrate intake at baseline, the greater were reductions in HbA1c in both sexes (Table 2). Men aged less than 65 years had greater decreases in HbA1c compared with older men.
We investigated changes in leisure-time physical activities in 83 patients (47 male and 36 female) who were registered after July 2016. The 47 male patients had significantly higher mean leisure-time physical activities (8.4±13.5 Mets hr/wk) than the other male patients (n=91, 4.8±1.0 Mets hr/wk) at baseline and significantly increased them during the 6 months (11.2±16.9 Mets hr/wk; P=0.017), whereas the 36 female patients had leisure-time physical activities of 4.4±0.9 Mets hr/wk at baseline that were not significantly different (P=0.126) compared with the other female patients (n=71, 5.7±1.3 Mets hr/wk) and did not change them (4.4±0.8 Mets hr/wk; P=0.490).
The mean BMI, plasma glucose levels, fasting insulin levels, HOMA-IR, serum HDL-C and triglyceride levels significantly improved in both sexes, and LDL-C levels significantly decreased in men but not women (Table 1).
Total carbohydrate, carbohydrate from sources and their changes during 6 months by sex
Tables 3 (men) and 4 (women) show macronutrients, total carbohydrate and carbohydrate from sources at baseline and after 6 months and their changes. The average total carbohydrate (g/day) significantly decreased in both sexes, from 285.0±93.7 to 170.0±57.8 g (−115.3±103.7 g) in men and from 229.9±67.3 to 166.3±42.6 g (−63.6±71.1 g) in women. Tables 3 and 4 also show percentages of patients who consumed each carbohydrate source (%). Since the percentage of patients who consumed the following carbohydrate sources was <30% at baseline, we eliminated them from the analysis: soba, spaghetti, bananas, apples, fruits juice, chuhai and wine in men; soba, spaghetti, bananas, fruits juice, beer, chuhai, and wine in women.
Intake from 14 carbohydrate sources significantly decreased over 6 months in men (rice, total noodles, Chinese soup noodles, bread, sugar and sweetenings, total fruits, citrus fruits, dairy, confectionery, soft drinks, total alcoholic drinks, beer, sake, and seasonings) (Table 3). Δcarbohydrate from rice was greatest and accounted for about 50% of Δtotal carbohydrate, followed by that from soft drinks, confectionery, Chinese soup noodles, beer and bread, and means of Δcarbohydrate from these sources were much smaller, 1/5 to 1/10 of that from rice.
Intake from seven carbohydrate sources significantly decreased in women (rice, bread, potatoes, sugar and sweetenings, total fruits, dairy, and confectionery) (Table 4). Δcarbo-hydrate from rice accounted for about 50% of Δtotal carbohydrate, followed by that from confectionery, soft drinks, bread and total fruits, and means of Δcarbohydrate from these sources were small, 1/3 to 1/10 of that from rice.
Associations of Δtotal carbohydrate and Δcarbohydrate from sources with ΔHbA1c in men
In men, Spearman correlation coefficients indicated positive correlations with ΔHbA1c for Δtotal carbohydrate and Δcar-bohydrate from five sources (Table 5 and Supplementary Fig. 1A–F). The coefficient was greatest for Δtotal carbohydrate (rs= 0.584), followed by Δcarbohydrate from soft drinks (rs=0.368), confectionery (rs=0.361), rice (rs=0.325), bread (rs=0.221), and Chinese soup noodles (rs=0.199) in descending order. There was a strong association for Δtotal carbohydrate and associations were moderate for Δcarbohydrate from soft drinks, confectionery and rice and weak for Δcarbohydrate from bread and Chinese soup noodles.

Association of changes in intake of carbohydrate and its sources with changes in glycosylated hemoglobin (%) in men (n=138)
According to multiple regression analyses with adjustment for age and leisure-time physical activity (Model 1), reducing total carbohydrate by 50 g was associated with a decrease in HbA1c of 0.43% and reducing carbohydrate from soft drinks, confectionery, rice, bread, and Chinese soup noodles by 50 g was correlated with decreases of 1.33%, 0.88%, 0.34%, 0.63%, and 0.82%, respectively (Table 5). With adjustment for the covariates in Model 1 plus ΔBMI, the results were almost the same: Δtotal carbohydrate, β=0.419, SE=0.055, P<0.001; Δcarbohydrate from soft drinks, β=1.313, SE=0.208, P<0.001; that from confectionery, β=0.854, SE=0.248, P=0.002; that from rice, β=0.312, SE=0.110, P=0.005; that from bread, β= 0.604, SE=0.292, P=0.041; that from Chinese soup noodles, β=0.873, SE=0.216, P<0.001. Changes in β compared with the basic Model 1 ranged from 0.007% to 0.051%.
With the adjustments in Model 2 (covariates in Model 1 plus ΔBMI and Δtotal energy intake), the positive correlations for Δcarbohydrate from rice and bread were no longer significant, but correlations remained significant for Δtotal carbohydrate and Δcarbohydrate from soft drinks, confectionery and Chinese soup noodles (Table 5).
Regarding soft drinks and confectionery, we conducted the analysis with adjustment for Δcarbohydrate from the staple foods (rice, total noodles, and bread). The positive correlations of Δcarbohydrate from soft drinks (β=1.286, SE=0.202, P< 0.001) and confectionery (β=0.542, SE=0.270, P=0.047) remained significant.
Association of Δtotal carbohydrate and Δcarbohydrate from sources with ΔHbA1c in women
In women, Δtotal carbohydrate and Δcarbohydrate from two sources had positive associations with ΔHbA1c (Table 6 and Supplementary Fig. 1G–I) according to Spearman's correlation coefficients. The coefficient was greatest for Δtotal carbohydrate (rs=0.547) followed by Δcarbohydrate from rice (rs= 0.376), and confectionery (rs=0.195) in descending order. Thus, there was a strong association for Δtotal carbohydrate, a moderate association for Δcarbohydrate from rice and a weak association for Δcarbohydrate from confectionery.

Association of changes in intake of carbohydrate and its sources with changes in glycosylated hemoglobin (%) in women (n=107)
Multiple regression analyses with adjustment for age and leisure-time physical activities (Model 1) revealed that reducing total carbohydrate by 50 g was associated with a decrease in HbA1c of 0.45% and that reducing carbohydrate from rice and confectionery by 50 g was associated with a decrease in HbA1c of 0.34% and 0.67%, respectively (Table 6). With adjustment for the covariates in Model 1 plus ΔBMI, the significance for carbohydrate sources did not change, but the regression coefficient for Δcarbohydrate from confectionery slightly decreased: Δtotal carbohydrate, β=0.416, SE=0.075, P<0.001; Δcarbo-hydrate from rice, β=0.322, SE=0.140, P=0.023; that from confectionery, β=0.528, SE=0.250, P=0.037. Changes in β compared with the basic Model 1 ranged from 0.018% to 0.142%.
With adjustment for the covariates in Model 1 plus ΔBMI and Δtotal energy intake (Model 2), the positive correlations were no longer significant for Δcarbohydrate from rice and confectionery, but remained significant for Δtotal carbohydrate (Table 6).
With adjustment for Δcarbohydrate from the staple foods, the significant positive correlation disappeared for Δcarbohyd-rate from confectionery (β=0.427, SE=0.250, P=0.09).
DISCUSSION
The current study showed that: (1) the mean reductions in HbA1c were 1.5% in men and 0.9% in women, while those in total carbohydrate were 115.3 g/day in men and 63.6 g/day in women; (2) Δtotal carbohydrate was positively and strongly correlated with ΔHbA1c in both sexes and reducing total carbohydrate by 50 g was associated with a decrease in HbA1c of 0.43% in men and 0.45% in women; (3) there were positive, moderate or weak correlations of Δcarbohydrate from soft drinks, confectionery, rice, bread, and Chinese soup noodles with ΔHbA1c in men and reducing carbohydrate from these sources by 50 g was associated with decreases in HbA1c of 1.33%, 0.88%, 0.34%, 0.63%, and 0.82%, respectively; (4) there were positive, moderate, or weak correlations of Δcarbohydrate from rice and confectionery with ΔHbA1c and reducing carbohydrate from rice and confectionery by 50 g was associated with a decrease in HbA1c of 0.34% and 0.67%, respectively, in women; (5) the mean reductions in carbohydrate from soft drinks, confectionery, bread, and Chinese soup noodles were very small, 1/5 to 1/10 of that from rice in men and that from confectionery was also small in women, 1/3 of that from rice.
There are two confounding factors to be considered from a clinical point of view. First, with respect to ΔBMI, the significance for carbohydrate sources did not change as compared with the basic Model 1 when adjustment was carried out with ΔBMI added to the covariates in both sexes, although the regression coefficient for Δcarbohydrate from confectionery slightly decreased in women. As the effects of carbohydrate reductions on decreases in HbA1c are partly mediated by weight loss, ΔBMI is statistically regarded as an intermediate variable. We therefore considered regression coefficients from the basic Model 1 without ΔBMI as clinically relevant. Second, the significance of Δcarbohydrate from rice disappeared with the additional adjustment for Δtotal energy intake in Model 2, which may be relevant to the reduction in total energy intake of 10% to 20% during the study period. The reduction in total energy was due to reduction in carbohydrate intakes because only these intakes decreased; mean protein and fat intakes increased during the study period in both sexes. Rice is the major carbohydrate source in Japan and the reduction in carbohydrate from rice accounted for up to 50% of reduction in total carbohydrate in the current study. Our purpose is to obtain clinically useful information for dietary instruction given to patients in East and South Asia including Japan where the major carbohydrate source is rice. We want to know decreases in HbA1c per unit reduction in various carbohydrate sources even if the decrease is partially due to the reduction in energy intake. In this regard, adjustment for Δtotal energy intake would lead to underestimation of the regression coefficients for associations of ΔHbA1c with Δcarbohydrate from staple foods, especially rice. We, therefore, considered the regression coefficient from the basic Model 1 (without ΔBMI and Δtotal energy intake) as a clinically relevant measure.
Patients consume many types of carbohydrate sources, usually three staple ones, i.e., rice, noodles and bread, that account for 60% of total carbohydrate, together with non-staple carbohydrate sources. In this regard, Δcarbohydrate from soft drinks and confectionery still remained significant after adjustment for Δcarbohydrate from the three staple foods in men. This suggests that the effects of reducing carbohydrate from the above non-staple carbohydrate sources are independent of carbohydrate from the three staple foods.
In spite of the small reductions, the regression coefficient of the regression lines for these carbohydrate sources was highest for Δcarbohydrate from soft drinks, followed by that from confectionery, Chinese soup noodles, bread, and rice in descending order in men. The effects of Δcarbohydrate from the sources on ΔHbA1c were 4-fold greater for soft drinks and 2.5-fold greater for confectionery, respectively, compared with rice. This is a reason why some patients who followed our previous moderate low-carbohydrate diet that ignored carbohydrate sources achieved a greater reduction in HbA1c levels than we expected [9]. We frequently experience rapid deterioration in HbA1c levels with excess consumption of soft drinks [17], and our results clearly explain the reason for this. Large-scale long-term cohort studies have indicated increases in mortality from cancer or cardiovascular diseases in people with strict carbohydrate restrictions [181920] and therefore, patients need a diet with less strict carbohydrate restriction, namely a moderate low-carbohydrate diet. Notably, 41% of the male patients and 59% female patients were consuming amounts (i.e., over 40% of total carbohydrate intake) of carbohydrate from non-staple foods, such as soft drinks and confectionery, at baseline that could not be ignored. In the clinical setting, patients should preferentially reduce carbohydrate from soft drinks or confectionery over that from rice and our results may enable them to achieve effective decreases in HbA1c by reducing carbohydrate from its sources by small amounts.
Although values of Δcarbohydrate from bread and Chinese soup noodles were small, 1/7 to 1/10 of that from rice, and their associations with ΔHbA1c were weak, reducing carbohydrate from bread and Chinese soup noodles by 50 g was associated with decreases in HbA1c of 0.63% and 0.82%, respectively, in men, which were 2- and 2.5-fold greater than that from rice, respectively. As a possible explanation, single servings of Chinese soup noodles (7 g salt) and bread (2 g salt) have a higher sodium chloride concentration than rice (0 g salt). Glucose absorption taking place in small intestinal cells is mediated by the sodium-glucose co-transporter 1 (SGLT1), which is driven by sodium extrusion [21]. Also, animal experiments have demonstrated that dietary sodium chloride increases intestinal glucose absorption by increasing the expression of SGLT1 in the intestine [22]. Although there is no clinical data on the effects of dietary sodium chloride on blood glucose, it is theoretically possible that foods containing more sodium chloride may have a stronger hyperglycemic effect. Therefore, in this regard, further studies on the effects of carbohydrate sources containing a large amount of salt on HbA1c are needed.
The question is: why were the associations of Δcarbohydrate from soft drinks and confectionery with ΔHbA1c stronger than for Δcarbohydrate from rice in men, although the means of Δcarbohydrate from soft drinks and confectionery were very small, about one fifth of Δcarbohydrate from rice? From a statistical point of view, despite the lower means of Δcarbohydrate from soft drinks and confectionery, there was greater inter-individual variation while the reverse was so for Δcarbohydrate from rice. This could possibly have led to the stronger correlations. Specifically, there was no carbohydrate intake from soft drinks or only a small amount of intake (<5.0 g/day) in 73% of men, who had lower HbA1c levels at baseline, and later exhibited only a small reduction in carbohydrate from soft drinks with little change in HbA1c. In contrast, the remaining 27% had a greater carbohydrate intake from soft drinks and higher HbA1c levels at baseline, and they had a greater reduction in carbohydrate from soft drinks and greater decreases in HbA1c levels. From a clinical point of view, the glycemic index (GI) is a measure of the relative impact of carbohydrate-containing foods on serum glucose. Consuming carbohydrates with a lower GI results in HbA1c reductions of 0.2% to 0.5% [2324]. The differential effects of various carbohydrate sources on HbA1c in our study may be partly explained by the differences in the GI of each source. Since the GI of rice is higher than that of soft drinks or confectionery, it was expected that Δcarbo-hydrate from rice would have a greater impact on ΔHbA1c than that from soft drinks or confectionery. However, the results were opposite to this. Although the reasons are unclear, it is interesting that our results cannot be explained in terms of GI alone. They suggest that reducing carbohydrate from several sources would be more practical and useful than consuming foods with a lower GI.
Regarding Δcarbohydrate from some sources (total fruits and dairy in men; other wheat-based foods, sugar and sweetenings, soft drinks, and total alcoholic drinks in women), the results from analysis using Spearman's correlation coefficients were inconsistent with those from multiple regression analyses. One explanation is that this may have been due to outliers; however, there is the risk that eliminating some outliers would be arbitrary because outliers were not statistically defined. Another explanation is that although many patients usually consumed these sources, the mean reductions in them were very small (0.4 to 5.3 g/day). Further studies are needed for evaluating effects of the carbohydrate sources with small amounts or those with seasonal variations, like fruits, on HbA1c.
There were sex differences in the associations of reductions in various carbohydrate sources with decreases in HbA1c. Positive, moderate or weak correlations of Δcarbohydrate from soft drinks, confectionery, rice, bread, and Chinese soup noodles with ΔHbA1c were observed in men, while associations of Δcarbohydrate from rice and confectionery with ΔHbA1c were seen in women. These differences could be explained by differences in dietary habits between men and women. In women, there were only two carbohydrate sources that satisfied the two conditions of: a decrease in carbohydrate of more than 5.0 g/day after 6 months, the decrease after 6 months was statistically significant (rice and confectionery). In contrast, there were six carbohydrate sources that satisfied both of these conditions in men (rice, Chinese soup noodles, bread, confectionery, soft drinks, and beer).
The first strength of our study is that it included only patients with T2DM not taking any antidiabetic medications from 3 months before baseline to the end of study. Taking anti-diabetic medication would lead to a mistaken understanding of net changes in HbA1c levels due to dietary therapy. Another strength is its use of dietary records to analyze intake from carbohydrate sources. The food frequency questionnaire or 24-hour recall frequently used in dietary management of T2DM have been less valid means of estimating amounts of carbohydrate intake compared with dietary records [252627].
The first limitation of our study is that 3-day dietary records may not be enough for obtaining precise information about carbohydrate from sources that are not consumed as frequently as staple foods. Longer duration dietary records would be necessary. The second limitation is that the average level of carbohydrate intake at baseline in our patients was somewhat lower than in the general Japanese population [28]. They may already have been taking care regarding carbohydrate intake before their first visit or they might have under-reported consumption of carbohydrate-rich foods in the dietary records. The third limitation is that we did not take changes in leisure-time physical activities into account. Data on changes in leisure-time physical activities during the 6 months were available only for 47 men and 36 women, about 1/3 of all patients. Although patients were requested to maintain their usual level of physical activity throughout the study, the 47 male patients increased their level of activity; however, there was no change for female patients. This number of patients is too small to conduct regression analysis. Thus, we need a further study to fully examine the confounding influence of changes in leisure-time physical activities in all patients. The fourth limitation is that our results will be valuable for patients with T2DM in East and Southern Asia where the major carbohydrate source is rice, but less so for patients in Western countries where the major carbohydrate source is not rice.
In summary, in 245 outpatients with T2DM not taking antidiabetic medications who followed a moderate low-carbohydrate diet for 6 months, focusing on the differential effects of carbohydrate sources on HbA1c, reducing carbohydrate from soft drinks, confectionery, bread, and Chinese soup noodles achieved a decrease in HbA1c that was 2- to 4-fold greater than that for the same reduction in rice in men, while reducing carbohydrate from confectionery achieved a decrease in HbA1c that was 2-fold greater in women. Dietary information provided to patients based on our results would enable them to reduce HbA1c efficiently.
Notes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTIONS
Conception or design: H.H., S.W., K.M.
Acquisition, analysis, or interpretation of data: H.H., S.W., T.M., K.W.
Drafting the work or revising: H.H.
Final approval of the manuscript: H.H., S.W., K.M., T.M., K.W.
FUNDING
This study was partly supported by a grant from Chukyo Longevity Medical Research and Promotion Foundation. The study sponsor was not involved in the design of the study; the collection, analysis, and interpretation of data; writing the report; or the decision to submit the report for publication.
Acknowledgements
The authors would like to thank the nurses at Haimoto Clinic for their assistance and excellent patient care.
References
SUPPLEMENTARY MATERIALS
Supplementary materials related to this article can be found online at https://doi.org/10.4093/dmj.2020.0033.
Supplementary Fig. 1
Spearman correlation coefficients of Δtotal carbohydrate or Δcarbohydrate from sources with ΔHbA1c in men and women. Numbers along vertical axes indicate ΔHbA1c (%) and those along horizontal axes Δcarbohydrate from various sources (g/day). Dotted lines are linear regression lines. We obtained positive correlations for Δtotal carbohydrate and Δcarbohydrate from five sources in men. The correlations were strong for Δtotal carbohydrate (A), moderate for Δcarbohydrate from soft drinks (B), confectionery (C) and rice (D), and weak for Δcarbohydrate from bread (E) and Chinese soup noodles (F). In women, we obtained positive correlations for Δtotal carbohydrate and Δcarbohydrate from two sources. The correlations were strong for Δtotal carbohydrate (G), moderate for Δcarbohydrate from rice (H), and weak for Δcarbohydrate from confectionery (I). HbA1c, glycosylated hemoglobin.