Umbilical Cord-Mesenchymal Stem Cell-Conditioned Medium Improves Insulin Resistance in C2C12 Cell
Article information
Abstract
Background
Umbilical cord-mesenchymal stem cell-conditioned medium (UC-MSC-CM) has emerged as a promising cell-free therapy. The aim of this study was to explore the therapeutic effects of UC-MSC-CM on insulin resistance in C2C12 cell.
Methods
Insulin resistance was induced by palmitate. Effects of UC-MSC-CM on insulin resistance were evaluated using glucose uptake, glucose transporter type 4 (GLUT4) translocation, the insulin-signaling pathway, and mitochondrial contents and functions in C2C12 cell.
Results
Glucose uptake was improved by UC-MSC-CM. UC-MSC-CM treatment increased only in membranous GLUT4 expression, not in cytosolic GLUT4 expression. It restored the insulin-signaling pathway in insulin receptor substrate 1 and protein kinase B. Mitochondrial contents evaluated by mitochondrial transcription factor A, mitochondrial DNA copy number, and peroxisome proliferator-activated receptor gamma coactivator 1-alpha were increased by UC-MSC-CM. In addition, UC-MSC-CM significantly decreased mitochondrial reactive oxygen species and increased fatty acid oxidation and mitochondrial membrane potential. There was no improvement in adenosine triphosphate (ATP) contents, but ATP synthesis was improved by UC-MSC-CM. Cytokine and active factor analysis of UC-MSC-CM showed that it contained many regulators inhibiting insulin resistance.
Conclusion
UC-MSC-CM improves insulin resistance with multiple mechanisms in C2C12 cell.
INTRODUCTION
How do we manage type 2 diabetes mellitus (T2DM) more appropriately? This is a very difficult problem, because T2DM has a complex pathophysiology [123]. Although we have various anti-diabetic drugs to treat patients with T2DM, half of them have not achieved the glycemic target yet [456]. Unmet medical needs still exist in this field.
Stem cells are an attractive candidate for treatment of many diseases because of their properties, such as division, renewal, differentiation, homing, and engraftment [78]. Umbilical cord-mesenchymal stem cells (UC-MSC) could be the best choice if one considers their high potential to differentiate into other cells and low immunogenicity [9]. Although decreased insulin secretion and increased insulin resistance are two major defects in T2DM, most studies using stem cells were focused on increasing insulin secretion [8]. Some studies have shown that UC-MSC treatment improved insulin resistance in patients with T2DM [1011].
Therapeutic usage of UC-MSC, however, is not easy, because of entrapment in filtering organs and potential tumorigenicity [12]. After it was revealed that the beneficial effects of UC-MSC depend mostly on their paracrine activity, a conditioned medium (CM) that consists of bioactive factors and cytokines has emerged as a promising cell-free therapy [12]. Nevertheless, UC-MSC-CM has not been studied for the treatment of T2DM so far.
Muscles play a major role in insulin resistance, but there have been no anti-diabetic drugs targeted at reducing insulin resistance in muscles [13141516]. If we can improve insulin resistance in the muscles, that will be helpful for patients with T2DM in a way different from already existing anti-diabetic drugs. The aim of this study was to explore the potential therapeutic effects of UC-MSC-CM on insulin resistance in C2C12 cell.
METHODS
Cell culture
Mouse skeletal-muscle cells (CRL-1772, passage #6) were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA) and maintained in Dulbecco's Modified Eagle's Medium (DMEM; Hyclone laboratories Inc., Logan, UT, USA) supplemented with 10% fetal bovine serum (FBS; Gibco, Carlsbad, CA, USA) and 1% penicillin-streptomycin (P/S; Hyclone, Northumberland, PA, USA). To differentiate the myotube, we cultured C2C12 myoblasts in DMEM containing 2% horse serum (Gibco, Auckland, New Zealand) for 4 days, with media changed every 2 days. Written informed consent was obtained from all patients. This study was approved by the CHA University Institutional Review Board (201803-BR-015-03).
Palmitate solution preparation and induction of insulin resistance
Palmitate (PA; Sigma, St. Louis, MO, USA) was dissolved in 99.9% ethanol by heating at 95℃ and adjusted to a concentration of 100 mM. After filtration, 100 mM PA was diluted in DMEM mixed with 2% bovine serum albumin (BSA; Bio Basic, Markham, ON, Canada) at a ratio of 1:100. The final concentration of PA solution was 1 mM, which was then incubated in a 37℃ water bath for 1 hour and stored at −20℃.
Preparation of UC-MSC-CM
UC-MSC was thawed at a density of 1×106 cells in a T-175 flask. When cell density reached 80% to 90%, they were replaced with fresh serum-free Minimum Essential Medium Eagle Alpha Modification (α-MEM; Hyclone) supplemented with 1% P/S. After 37℃ incubation for 48 hours, we harvested them from the medium and centrifuged them at 1,500 rpm for 5 minutes. We filtered them with a 0.2 µM filter and stored the supernatant at −80℃ until use.
2-Deoxy glucose uptake
For quantifying glucose uptake in cells, we treated differentiated C2C12 cell with 1 mM PA with or without UC-MSC-CM for 24 hours. Prior to the assay, cells were washed with phosphate buffered saline twice and starved in Krebs-Ringer-Phosphate-HEPES buffer for 40 minutes. Cells were then stimulated with 100 nM insulin (Eli Lilly Nederland, Indianapolis, IN, USA) for 30 minutes. We measured glucose uptake by C2C12 cells using the Glucose Uptake Assay kit (ab136955, Abcam, Cambridge, UK) following the manufacturer's instructions. Glucose levels were quantified using Microplate reader (BioTek Inc., Winooski, VT, USA).
Membrane protein extraction
Cytosol and membrane were separated by a membrane protein extraction kit (Thermo, Rockford, IL, USA) and differential centrifuge. Additionally, we added a protease inhibitor (PI; Quartett Immunodiagnostika, Berlin, Germany) to the permeabilization and solubilization buffer. Briefly, we centrifuged at 300 ×g for 5 minutes and washed the cells twice using wash buffer. After permeabilization buffer treatment, we centrifuged the permeabilized cells for 10 minutes at 4℃ with constant mixing and for 15 minutes at 16,000 ×g to transfer the supernatant containing cytosolic proteins to a new tube. To isolate membrane-associated protein, cell pellets were resuspended with solubilization buffer, were incubated at 4℃ for 30 minutes with constant mixing, and were centrifuged for 15 minutes at 16,000 ×g.
Western blot analysis
Cell were harvested and centrifuged at 1,500 rpm for 3 minutes and lysed with radioimmunoprecipitation assay buffer including a PI. The concentration of protein extraction was evaluated by using a bicinchoninic acid protein assay kit (Thermo) at 562 nm with a microplate reader. Western blot for glucose transporter type 4 (GLUT4) protein was carried for the membrane fraction and the cytosol fraction. We analyzed other markers using total proteins. Each protein sample were migrated by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to a polyvinylidene difluoride membrane (Millipore, Billerica, MA, USA). The membrane was next blocked for an hour with 5% BSA in 1X-Tris buffered saline with Tween 20 (TBST) at room temperature and incubated overnight at 4℃ with constant mixing with insulin receptor substrate 1 (IRS-1; Cell Signaling Technology, Danvers, MA, USA; #2386S, #2381S), phosphoinositide 3-kinase (PI3K; Cell Signaling Technology, #4223), protein kinase B (AKT1; Cell Signaling Technology, #9271), GLUT4 (Abcam, ab654), peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) (Santa Cruz Biotechnology, Santa Cruz, CA, USA; sc-13067), mitochondrial transcription factor A (mtTFA; Santa Cruz Biotechnology, sc-376672), and β-actin (Santa Cruz Biotechnology, sc-47778). After washing with 1x TBST, we visualized specific secondary antibodies by incubation with 1:2,000 dilution of goat anti-rabbit immunoglobulin G (IgG; Santa Cruz; sc-2004), goat anti-mouse IgG (Santa Cruz, sc-2005). We confirmed the band using enhanced chemiluminescence (ECL component of Pierce Clarity and Western ECL Substrate, Bio-Rad Laboratories, Hercules, CA, USA) with an LAS-4000 imager (Fujifilm Inc., Tokyo, Japan).
Mitochondrial DNA analysis
The relative level of mitochondrial DNA (mtDNA) copy number was calculated using reverse transcription polymerase chain reaction (PCR) by measuring the ratio of Cox2 (mitochondrial-encoded gene) versus Rsp18 (nuclear-encoded gene). Prepare 1×106 cells and extract RNA using RNA extraction kit (iNTRON, Seongnam, Korea). Then, complementary DNA (cDNA) is synthesized by PCR with a primer (Supplementary Table 1) and master mix using a cDNA synthesis kit (iNTRON).
Measurement of reactive oxygen species production and fatty acid oxidation
Intracellular reactive oxygen species (ROS) was measured using chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate (CM-DCFDA, Molecular Probes; Invitrogen, Carlsbad, CA, USA). For positive control, cells were treated with H2O2 (1 mM) for 10 minutes and then incubated with 10 µM of DCFDA dye concentration for 30 minutes. Intracellular ROS was measured at excitation 485 nm and emission 535 nm. Mitochondrial ROS was measured using the fluorescent dye MitoSox Red, a mitochondrial superoxide indicator (Invitrogen). Cells were incubated with 5 µM of MitoSox dye concentration at 37℃ for 30 minutes. The dye was activated at excitation 510 nm and emission 528 nm. Results from the fluorescence images showed similar trends. Fatty acid oxidation was measured by the non-radioactive fatty acid oxidation assay (Biomedical Research Service Center, E-141).
Analysis of inner mitochondrial membrane potential
To confirm the mitochondrial function and viability, cells were stained with JC-1 dye (Invitrogen), which assesses the mitochondrial membrane potential. For positive control, we used carbonyl cyanide-p-trifluoromethoxyphenylhydrazone (CCCP at 50 µM for 30 minutes; Sigma). We dissolved it in dimethyl sulfoxide to make 1 mM stock, and then diluted the solution in DMEM to a concentration of 50 µM and incubated at 37℃ for 30 minutes. After incubation, monomer signal (green) was activated at excitation 488 nm, emission 528 nm, and the aggregate signal (red) was activated at excitation 530 nm, emission 590 nm.
Measurement of ATP contents and ATP synthesis
Intracellular adenosine triphosphate (ATP) was measured with a CellTiter-Glo 2.0 assay kit (Promega, Madison, WI, USA). We prepared a 1:1 solution containing fresh culture media and CellTier-Glo luminescence test reagent and inserted the mixture (200 µL) into each well. After incubation for 30 minutes at room temperature, we read luminescent signal using a luminescence microplate reader. ATP concentration was calculated from a standard curve and normalized by total protein. ATP synthesis was measured after adding adenosine diphosphate (ADP) as a substrate.
Cytokine assay
Our group has already analyzed UC-MSC-CM in a previous study [17]. To summarize briefly, cytokine, growth factor, and active protein contents were analyzed using an antibody-based protein array capable of simultaneously detecting 507 different factors (Ray Biotech Inc., Norcross, GA, USA).
Statistical analysis
All in vitro experiments were independently performed at least three times. Data were analyzed using the SigmaPlot version 11.0 software (Systat Software, San Jose, CA, USA). Quantitative results are shown as the mean±standard deviation for each experiment, and P<0.05 was considered statistically significant.
RESULTS
The effect of UC-MSC-CM on glucose uptake in C2C12 myotubes
To investigate the effect of UC-MSC-CM on insulin resistance in C2C12 myotube, we induced insulin resistance by PA and measured 2-deoxy glucose (2-DG) uptake. UC-MSC-CM significantly improved 2-DG uptake in comparison with the PA-treated group. However, there was no improvement of 2-DG uptake in the experiment using C2C12-CM to verify the interference effect (Fig. 1).
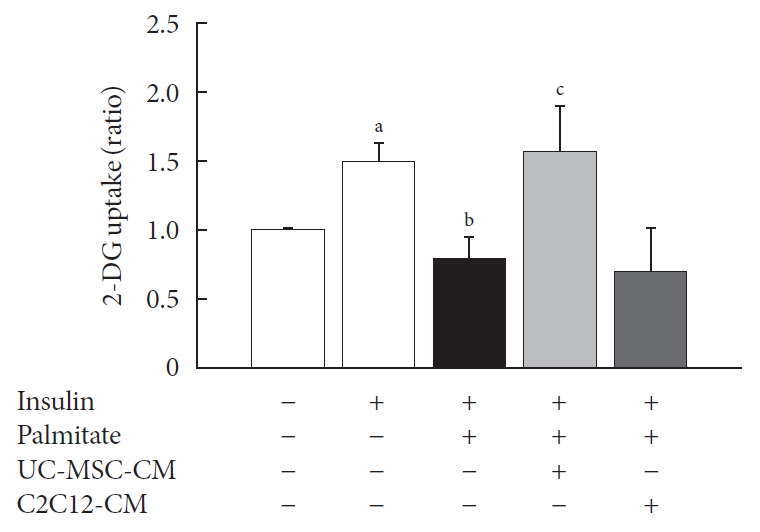
The effect of umbilical cord-mesenchymal stem cell-conditioned medium (UC-MSC-CM) on glucose uptake in C2C12 myotubes. The results are expressed as the fold change of the mean±standard error (n≥3). 2-DG, 2-deoxy glucose. aP<0.05, vs. insulin-unstimulated group, bP<0.05, vs. insulin-stimulated group, cP<0.05, vs. insulin and palmitate treated group.
The effect of UC-MSC-CM on protein expression of GLUT4
GLUT4 is a membranous channel that transports glucose into cells. Insulin resistance is influenced by translocation to the cell membrane of GLUT4, not the presence of GLUT4 in cytosol. PA significantly reduced the expression of membranous GLUT4, and UC-MSC-CM restored it in C2C12 myotube. The level of cytosolic GLUT4 expression was not changed by UC-MSC-CM treatment (Fig. 2).
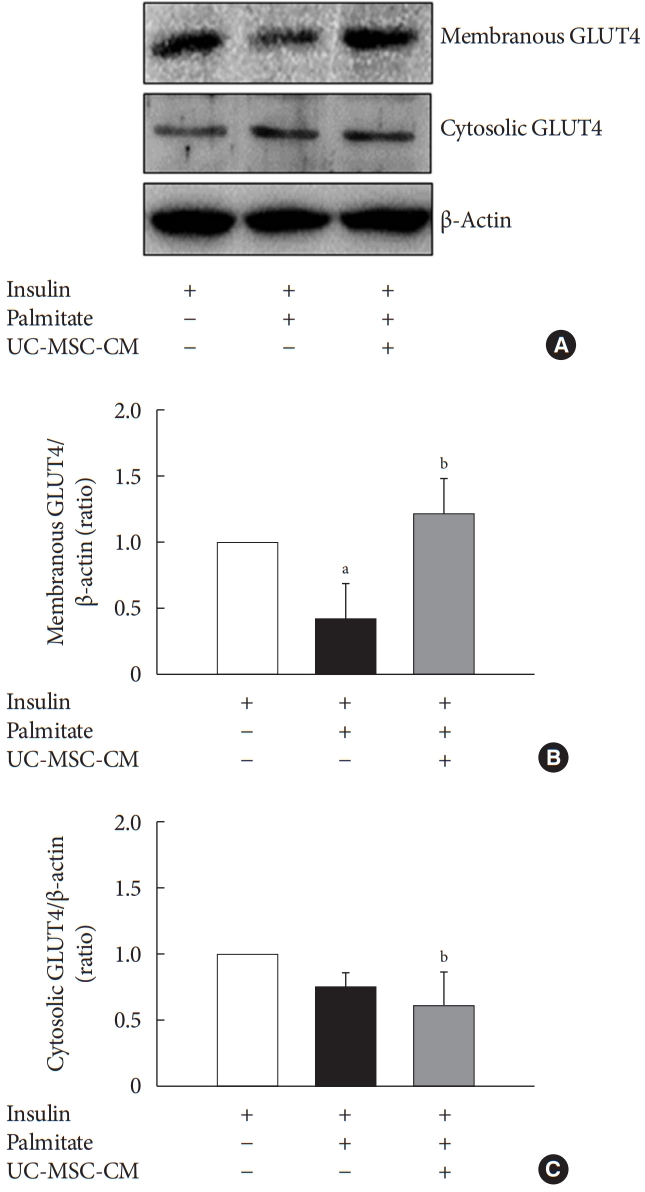
The effect of umbilical cord-mesenchymal stem cell-conditioned medium (UC-MSC-CM) on protein expression of glucose transporter type 4 (GLUT4). (A) Western blot. (B) Protein expression of membranous GLUT4. (C) Protein expression of cytosolic GLUT4. The results are expressed as the fold change of the mean±standard error (n≥3). aP<0.05, vs. insulin-stimulated group, bP<0.05, vs. insulin and palmitate treated group.
The effect of UC-MSC-CM on the insulin-signaling pathway
IRS-1 plays a key role in transmitting signals from the insulin and insulin-like growth factor receptor to intracellular pathways PI3K/AKT kinase pathway. Phosphorylation of IRS-1 at ser612 results in an inhibition of insulin signaling in the cell and phosphorylation at Ser307 activates dissociation of insulin receptor [18]. IRS1 contains multiple tyrosine phosphorylation sites, which acts as docking sites for PI3K. PI3K elicit AKT phosphorylation causing translocate GLUT4 onto the cell membrane [19]. Consequently, insulin-dependent transport of glucose is initiated. UC-MSC-CM decreased the phosphorylation of IRS1 at Ser612 and Ser307 (Fig. 3A–C). However, although the phosphorylation of PI3K was increased by PA treatment, there was no change by UC-MSC-CM treatment (Fig. 3D). The expression of phosphorylated AKT1 at Ser473 was up-regulated by UC-MSC-CM (Fig. 3E).
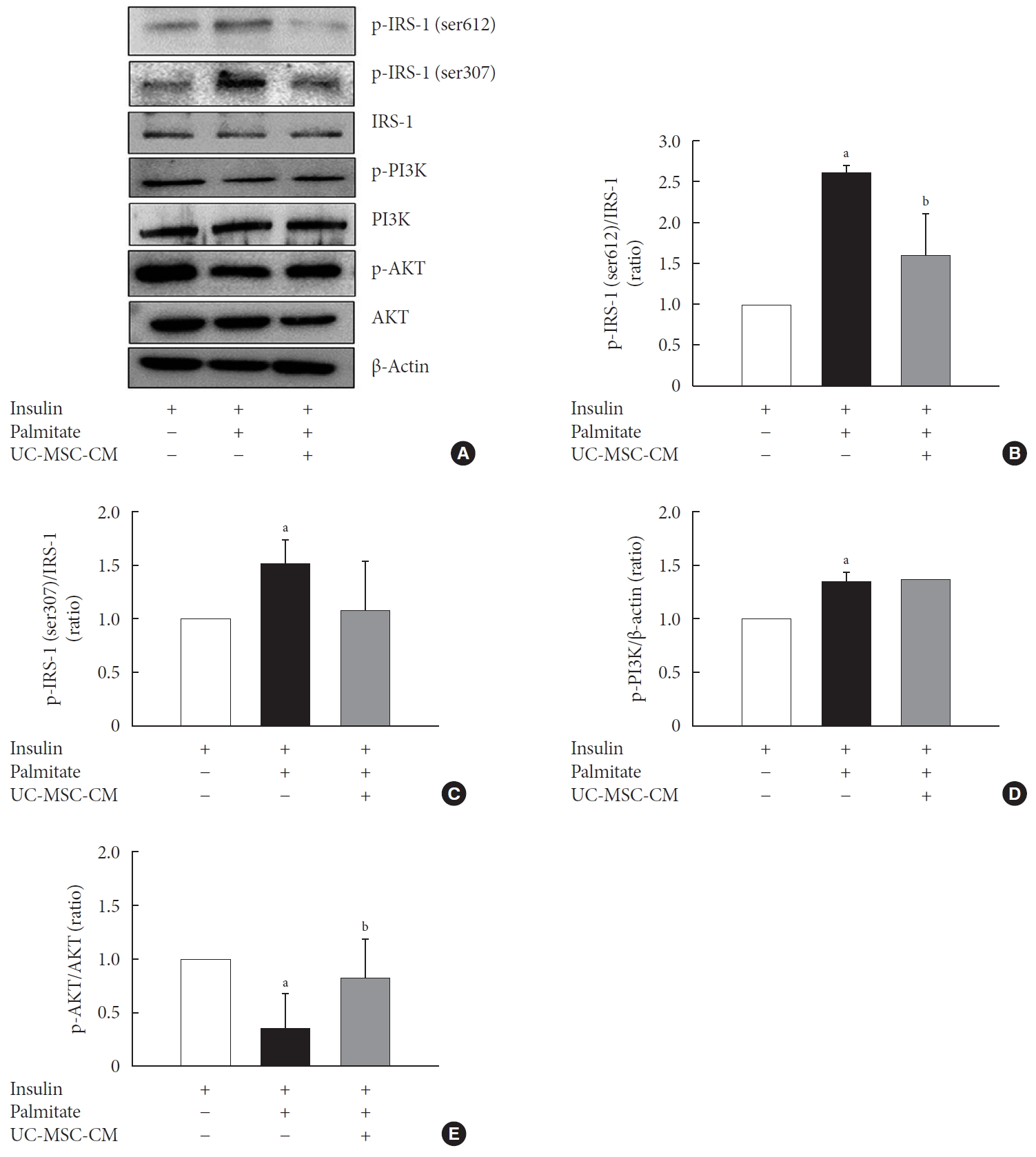
The effect of umbilical cord-mesenchymal stem cell-conditioned medium (UC-MSC-CM) on the insulin-signaling pathway. (A) Representative Western blots for figure B–E. (B, C) Protein expression of insulin receptor substrate 1 (IRS1) at Ser612 (B) and Ser307 (C). (D) Protein expression of phosphoinositide 3-kinase (PI3K). (E) Protein expression of phosphorylated protein kinase B (p-AKT). The results are expressed as the fold change of the mean±standard error (n≥3). aP<0.05, vs. insulin-stimulated group, bP<0.05, vs. insulin and palmitate treated group.
The effect of UC-MSC-CM on mitochondria Contents
mtTFA plays a key role for the regulation of mtDNA replication and its level is proportional to mtDNA [20]. The protein level of mtTFA was restored by UC-MSC-CM treatment (P<0.05) (Fig. 4A). In addition, UC-MSC-CM treatment increased the relative level of mtDNA copy number (Fig. 4B). PGC-1α is widely regarded as the master regulator of mitochondrial biogenesis [2122]. There was no difference in content of PGC-1α between the control and PA-treated C2C12 myotubes. Treatment with UC-MSC-CM increased the content of PGC-1α (Fig. 4C).
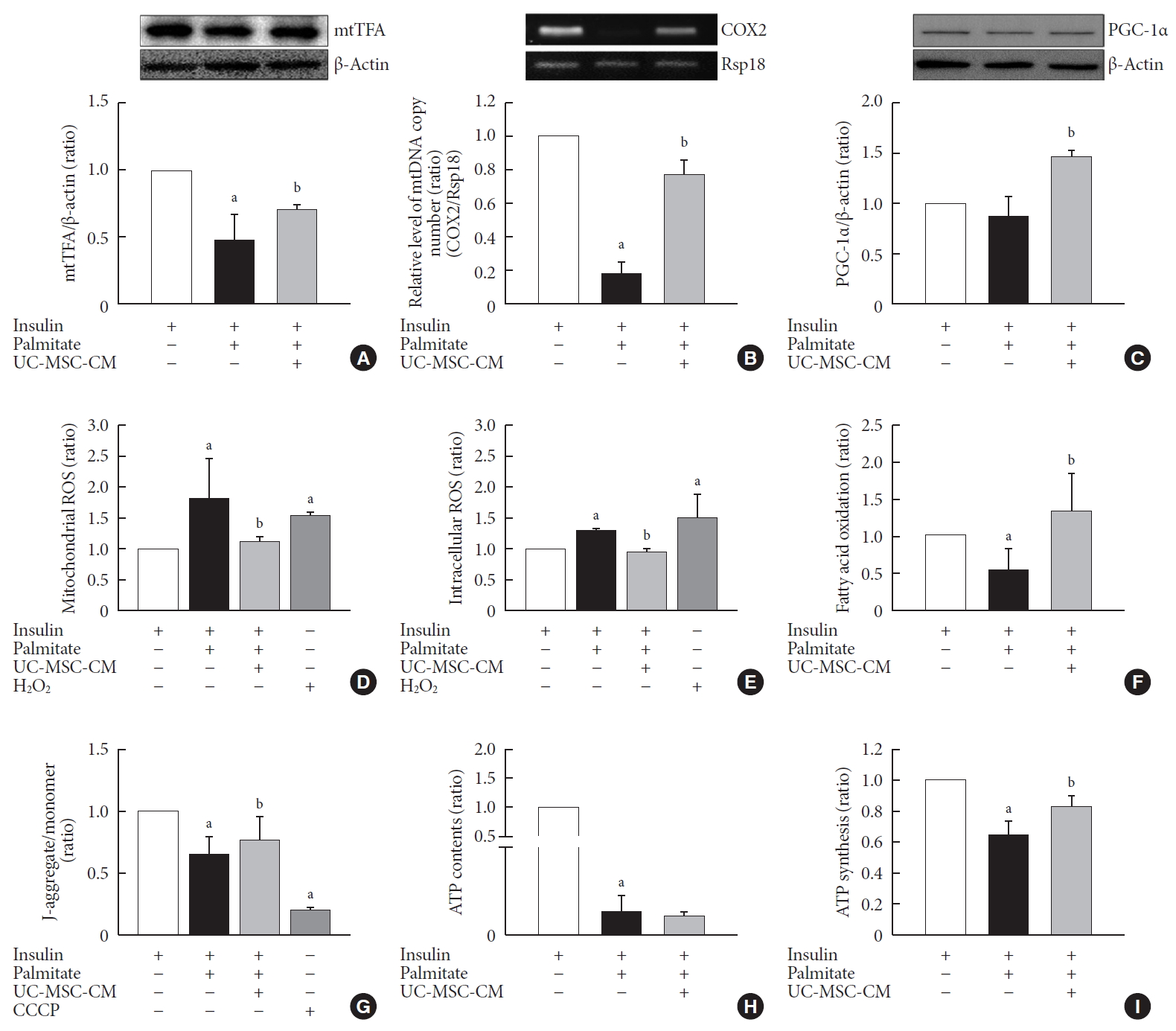
The effect of umbilical cord-mesenchymal stem cell-conditioned medium (UC-MSC-CM) on mitochondrial contents and functions. (A) Protein level of mitochondrial transcription factor A (mtTFA). (B) The relative level of mitochondrial DNA (mtDNA) copy number. (C) Protein level of peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α). (D) Mitochondrial reactive oxygen species (ROS). (E) Intracellular ROS. (F) Fatty acid oxidation. (G) Mitochondrial membrane potential. (H) Adenosine triphosphate (ATP) contents. (I) ATP synthesis. The results are expressed as the fold change of the mean±standard error (n≥3). CCCP, carbonyl cyanide-p-trifluoromethoxyphenylhydrazone. aP<0.05, vs. insulin-stimulated group, bP<0.05, vs. insulin and palmitate treated group.
Function
Oxidative stress plays an important role in glucotoxicity and insulin resistance [23]. In this study, we found that UC-MSC-CM significantly decreased the level of mitochondrial ROS as well as intracellular ROS, in contrast to PA-treated C2C12 myotubes (Fig. 4D and E). Fatty acid oxidation increases glucose extraction and glycogen synthesis, so incomplete fatty acid oxidation contributes to muscle insulin resistance. UC-MSC-CM increased fatty acid oxidation (Fig. 4F). Mitochondrial function is usually monitored by mitochondria membrane potential (MMP) as well as intracellular ATP contents [24]. The MMP was not as low as CCCP, but it was decreased by PA. Treatment with UC-MSC-CM increased MMP (Fig. 4G). After ATP contents were diminished by PA, there was no improvement by UC-MSC-CM treatment (Fig. 4H). However, ATP synthesis was increased by UC-MSC-CM treatment when ATP was measured after adding ADP as a substrate (Fig. 4I).
Analysis of secreted factors in UC-MSC-CM
There were many cytokines, growth factors, and active protein contents affecting insulin resistance and mitochondrial function in UC-MSC-CM. Among them, tissue inhibitor of metalloproteinases-2 (TIMP-2), secreted protein acidic and rich in cysteine (SPARC), monocyte chemoattractant protein-1 (MCP-1), vascular endothelial growth factor (VEGF), growth differentiation factor-15 (GDF-15), and angiopoietin-1 are known regulators inhibiting insulin resistance (Fig. 5).
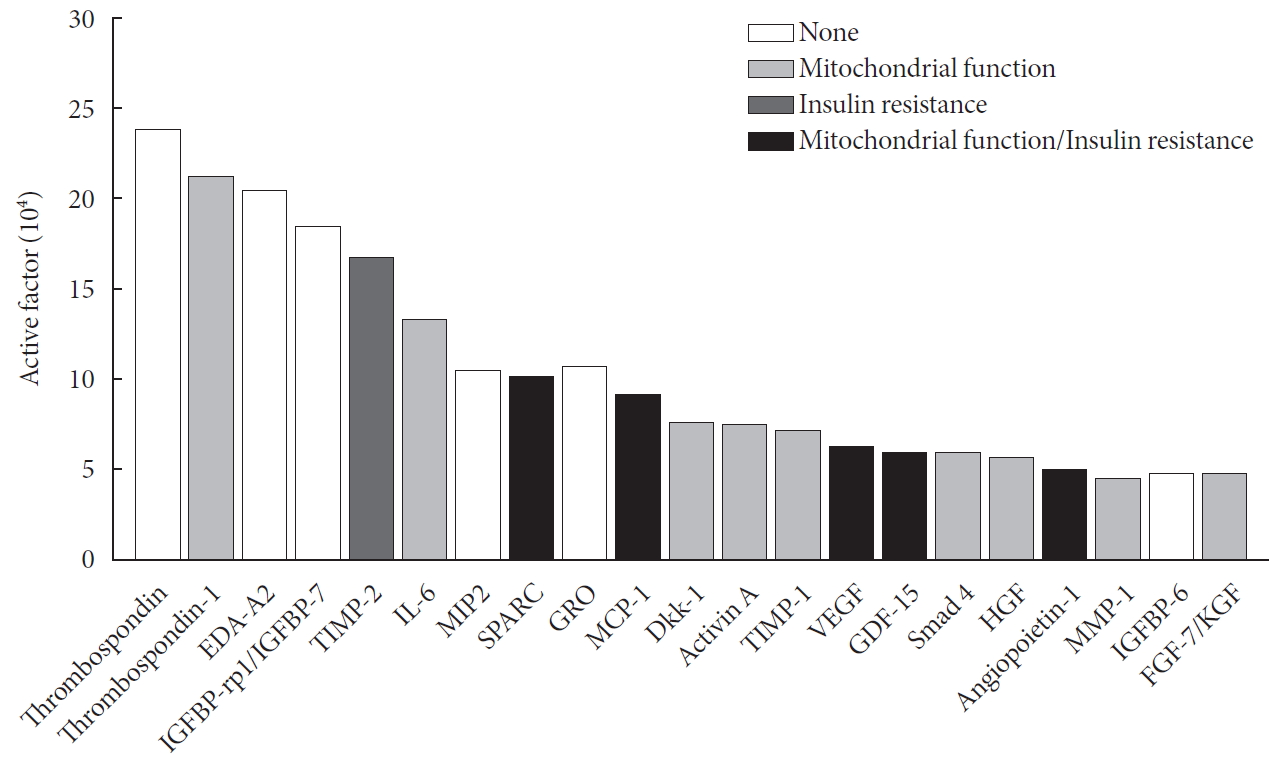
Analysis of secreted factors in umbilical cord mesenchymal stem cell-conditioned medium. EDA-A2, ectodysplasin-A2; IGFBP, insulin-like growth factor binding protein; TIMP, tissfATCC, Manassas, VA, USA) and maintained in Dulbeccoue inhibitor of metalloproteinases; IL-6, interleukin-6; MIP2, macrophage inflammatory protein 2; SPARC, secreted protein acidic and rich in cysteine; GRO, growth related oncogene; MCP, monocyte chemoattractant protein; Dkk, Dickkopf-related protein; VEGF, vascular endothelial growth factor; GDF, growth differentiation factor; HGF, hepatocyte growth factor; MMP-1, matrix metalloproteinase-1; FGF, fibroblast growth factor; KGF, keratinocyte growth factor.
DISCUSSION
T2DM has become one of the greatest challenges to human health nowadays. More and more people are suffering from T2DM, and many drugs are being used to treat T2DM, which is not easy to treat, because of its complex pathophysiology, and we still need other treatment options [123]. UC-MSC-CM is an attractive candidate for treatment of T2DM and can overcome the problem of cell therapy [89101112]. However, there were few studies to investigate whether UC-MSC-CM could improve insulin resistance in muscles. In this study, we demonstrated that UC-MSC-CM improved insulin resistance in C2C12 cells. Possible mechanisms we have identified were improving GLUT4 translocation, the insulin-signaling pathway, and mitochondrial contents and functions.
MSCs exert beneficial effects on T2DM through differentiation into insulin-producing cells, promotion of islet cell regeneration, protection of endogenous islet cells, and ameliorating insulin resistance [8]. In fact, many studies have focused on improving insulin secretory function by MSCs [825]. Some studies investigated whether UC-MSC improved insulin resistance in T2DM [1011]. Xie et al. [26] showed that UC-MSC alleviated insulin resistance by directing macrophages into an alternatively activated phenotype (anti-inflammatory, M2) in adipose tissues of T2DM rats. A study in 22 patients with T2DM demonstrated that treatment with UC-MSC can improve glucose profiles and beta-cell function by improving systemic inflammation and/or immunological regulation [10]. However, direct use of MSC has several problems, such as potential tumorigenicity, entrapment in filtering organs, scarce overall availability, and low survival rate [12]. UC-MSC-CM is a charming candidate for treating T2DM, because it is not a cell therapy and its beneficial effects depend mostly on their paracrine activity [12]. Shree and Bhonde [27] demonstrated that adipose-derived MSC-CM restored insulin resistance in C2C12 cells by improving inflammation and insulin signaling. UC-MSC-CM suppressed ROS generation and improved muscle atrophy in atrophied muscles [1728]. There is no study about the effects of UC-MSC-CM on insulin resistance in skeletal muscles yet. In this study, UC-MSC-CM improved glucose uptake in PA-treated C2C12 myotubes. The expression of membranous GLUT4 was increased and insulin signaling, such as IRS1 and AKT1, was improved when UC-MSC-CM was used. In addition, mitochondrial contents and functions were also improved by UC-MSC-CM treatment. To the best of our knowledge, this is the first study to show that UC-MSC-CM improved insulin resistance via multiple mechanisms in C2C12 cell.
Mitochondria are organelles that are a critical contributor to cellular and organismal homeostasis [29]. Mitochondrial biogenesis might be associated with insulin resistance through GLUT4 translocation, the insulin-signaling pathway, and inflammation [303132]. However, whether mitochondrial dysfunction is a cause or a consequence of insulin resistance is not clear. There were many efforts to improve insulin resistance by restoring mitochondrial dysfunction [33], but there has been no study investigating the effects of UC-MSC-CM on mitochondrial dysfunction. In this study, mitochondrial contents assessed by mtTFA, the relative level of mtDNA copy number, and PGC-1α were increased by UC-MSC-CM treatment. In terms of mitochondrial function, UC-MSC-CM treatment decreased mitochondrial ROS and increased MMP in C2C12 cells. ATP contents were not increased by UC-MSC-CM treatment, but ATP synthesis was increased. Because ATP generation is a complex process and is associated with various factors, UC-MSC-CM treatment could not restore it completely. However, because ATP synthesis was increased after adding ADP as a substrate, so we could assume that UC-MSC-CM influenced the improvement of ATP synthesis. Based on previous studies, these improvements for mitochondrial dysfunction might be associated with improving insulin resistance.
UC-MSC-CM consists of various cytokines, growth factors, and active proteins [17]. Among them, TIMP-2, SPARC, MCP-1, VEGF, GDF-15, and angiopoietin-1 are known regulators that improve insulin resistance [343536]. Thrombospondin-1, interleukin-6 (IL-6), SPARC, MCP-1, Dickkopf-related protein-1 (Dkk-1), activin A, TIMP-1, VEGF, GDF-15, smad-4, hepatocyte growth factor (HGF), angiopoietin-1, matrix metalloproteinase-1 (MMP-1), and fibroblast growth factor-7 (FGF-7) were factors that influenced mitochondria [373839]. There would also be active factors that aggravated insulin resistance and mitochondrial dysfunction. We could not find out which factors have mainly beneficial effects on insulin resistance. However, we could assume that UC-MSC-CM is a mixture that has beneficial effects on insulin resistance, although we do not know the exact ratio of active factors. One possible explanation might be that UC-MSC-CM is made when UC-MSCs are cultured, so it should have beneficial effects in all aspects. Furthermore, many of these were known factors, but an unknown one that could have beneficial effects on insulin resistance and mitochondrial dysfunction still might exist.
In conclusion, our findings demonstrated that UC-MSC-CM improves insulin resistance in C2C12 cells. GLUT4 translocation and the insulin-signaling pathway were improved by UC-MSC-CM treatment. Mitochondrial contents and functions were also improved, which is a novel finding, never reported previously. All these effects might be beneficial for insulin resistance in C2C12 cell. Further study is warranted to fully elucidate the effects of UC-MSC-CM on insulin resistance in animals or humans.
SUPPLEMENTARY MATERIAL
Supplementary materials related to this article can be found online at https://doi.org/10.4093/dmj.2019.0191.
Supplementary Table 1
Primer sequences used in this study
Notes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTIONS
Conception or design: K.S.K., Y.S.C., Y.W.C.
Acquisition, analysis, or interpretation of data: K.S.K., Y.K.C., M.J.K., J.W.H., K.M., S.Y.J., S.K.K., Y.S.C., Y.W.C.
Drafting the work or revising: K.S.K., Y.K.C., M.J.K., Y.S.C., Y.W.C.
Final approval of the manuscript: K.S.K, Y.S.C., Y.W.C.
FUNDING
This work was supported by a grant (Kyung-Soo Kim, 2016F2) from the Korean Diabetes Association and the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (NRF-2018R1C1B5042633).
ACKNOWLEDGMENTS
None