Anti-Obesity Drugs: A Review about Their Effects and Safety
Article information
Abstract
The current recommendations for the treatment of obese people include increased physical activity and reduced calories intake. When the behavioral approach is not sufficient, a pharmacologic treatment is recommended. In past years, numerous drugs have been approved for the treatment of obesity; however, most of them have been withdrawn from the market because of their adverse effects. In fact, amphetamine, rimonabant and sibutramine licenses have been withdrawn due to an increased risk of psychiatric disorders and non-fatal myocardial infarction or stroke. Even if orlistat is not as effective as other drugs in reducing body weight, orlistat is presently the only available choice for the treatment of obesity because of its safety for cardiovascular events and positive effects on diabetic control. Hopefully, more effective and better tolerated anti-obesity drugs will be developed through an improved understanding of the multiple mechanisms and complex physiological systems targeting appetite.
INTRODUCTION
Obesity is now a global problem [1] and is associated with a number of chronic conditions including osteoarthritis, obstructive sleep apnea, gallstones, fatty liver disease, reproductive and gastrointestinal cancers, dyslipidemia, hypertension, type 2 diabetes, heart failure, coronary artery disease, and stroke [2,3]. Lifestyle modifications such as diet and exercise intervention are essential for both prevention and management of obesity, and pharmacotherapy may be considered if the interventions are ineffective for individuals with a body mass index [BMI] ≥30 kg/m2 or for those with a BMI ≥27 kg/m2 when co-morbidities, such as hypertension or type 2 diabetes mellitus are present [4]. However, anti-obesity drugs are a frequent adjunct, because these interventions have limited long-term success [5] and the weight is regained when treatment is discontinued.
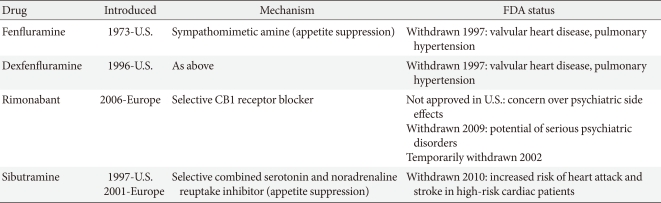
Many medications have been used to manage obesity over the years. However, most of the anti-obesity drugs that were approved and marketed have now been withdrawn due to serious adverse effects. In the 1990s, fenfluramine and dexfenfluramine were withdrawn from the market because of heart valve damage [6]. In 2000, the European Medicines Agency (EMA) recommended the market withdrawal of several anti-obesity drugs, including phentermine, diethylpropion, and mazindol, due to an unfavorable risk to benefit ratio [7]. The first selective CB1 receptor blocker, rimonabant, was available in 56 countries from 2006 but was never approved by the U.S. Food and Drug Administration (FDA) due to an increased risk of psychiatric adverse events, including depression, anxiety, and suicidal ideation [8]. Subsequently, rimonabant was withdrawn from the European market in 2009 (Table 1).
Recently, many newer agents have been tried, though only orlistat and sibutramine have been approved for long-term use. In October 2010, sibutramine, widely used after approval by the U.S. FDA in 1997, was withdrawn from the market because of an association with increased cardiovascular events and strokes [11], leaving only orlistat (Table 2). More recently, in February 2011, the U.S. FDA rejected approval of the bupropion/ naltrexone combination marketed as Contrave due to concerns over potential cardiovascular risks. The long-term safety and efficacy of newly-developed drugs should also be evaluated in the management of obesity, which often requires continuous treatment to achieve and maintain weight loss, though the rigidity of a regulatory committee for the approval of novel anti-obesity drugs and the regulatory guidelines for anti-obesity therapy represent a significant limitation to developing drugs. The present paper reviews the effects and safety of the medications which are available for the treatment of obesity including many recently withdrawn from the market, and discusses several newer treatments currently being investigated.
SYMPATHOMIMETIC DRUGS
Phentermine
Phentermine is one of the centrally acting appetite-suppressant drugs of the β-phenethylamine family, which was approved for short-term (up to 3 months) use in the treatment of obesity by the U.S. FDA in 1959 and remains available today. There is little data from large randomized controlled trials (RCTs) relating to the long-term efficacy or safety of phentermine, especially when used as monotherapy. The β-phenethylamine family drugs have limited use in the routine management of obesity and are not currently approved for long-term use. A study in 1968 was the only longer-term (36 weeks) controlled trial of phentermine and demonstrated a mean weight loss of 12.2 kg with phentermine compared to 4.8 kg with a placebo (P<0.001). A meta-analysis, which included 6 randomized trials lasting from 2 to 24 weeks, reported patients treated with phentermine lost an average of 3.6 kg of additional weight compared with a placebo [14]. There have been no new RCTs of phentermine since 1999, and our research team recently reported the efficacy and safety of a newly developed formulation of phentermine diffuse-controlled release (DCR) in obese Korean patients [15]. This was a randomized, double-blind, placebo-controlled trial of 12 weeks treatment with phentermine DCR 30 mg (n=37) or placebo (n=37) once daily in obese patients with controlled diabetes, hypertension, or dyslipidemia. The participants in the phentermine DCR group showed significant reductions in body weight (-9.3±3.4 kg vs. -1.8±3.1 kg, P<0.001) and waist circumference (7.2±0.5 cm vs. 2.1±0.6 cm, P<0.001) compared with the placebo group. Weight reductions of 5% or greater from the baseline (95.8% vs. 20.8%, P<0.001) and 10% or more (62.5% vs. 4.7%, P<0.001) were shown in the phentermine DCR group. Total cholesterol and low density lipoprotein cholesterol (LDL-C) levels were significantly improved in the phentermine DCR group. However, there were no significant differences in systolic and diastolic blood pressure between the groups, and pulse rate in the phentermine DCR group significantly increased compared with the placebo group (P=0.02). Dry mouth and insomnia were the most common adverse events, but were mild to moderate and transient. Although the long-term effects and safety of the new formulation of phentermine DCR cannot be evaluated because of Korea FDA regulations, short-term phentermine DCR treatment resulted in significant reduction of weight and improvement of metabolic parameters, including waist circumference and several lipid profiles, without clinically severe adverse events.
The authors of the present study did not identify any systematic reports of adverse events with phentermine [15]. However, because phentermine has sympathomimetic properties, possible side effects such as insomnia, dry mouth, dizziness, palpitation, hand tremor, and elevation in blood pressure and pulse rate should be considered. Although no serious adverse events were reported in the meta-analysis of RCTs on the use of phentermine for weight loss [14], the upper limit of the 1-sided 95% confidence interval [CI] given the number of patients studied who received phentermine was 1.5%, meaning the rate of serious adverse events could be as high as 15 per 1,000 [13]. Sympathomimetic drugs are also scheduled by the U.S. Drug Enforcement Agency, suggesting the government's view the drugs may be abused. If health professionals decide to use any of these drugs, physicians should monitor blood pressure and heart rate regularly to ensure acceptable levels are maintained in patients receiving phentermine.
Phentermine had been used in combination with fenfluramine. The combination therapy demonstrated significantly more weight loss than a placebo in a 28-week RCT (15.5% vs. 4.9%, P<0.001). However, fenfluramine was withdrawn from the market by the U.S. FDA in 1997 [10]. A preliminary report identified heart valve damage and pulmonary arterial hypertension in association with the use of fenfluramine [6]. Phentermine is currently under evaluation in combination with topiramate (see section below entitled "Combination treatments being developed").
Diethylpropion
Another amphetamine-like analogue, diethylpropion, is a phenylethylamine ring compound with minor sympathomimetic properties and with fewer stimulant effects than amphetamine. Although diethylpropion has been approved by the U.S. FDA for treatment of obesity since 1959, diethylpropion has been used off-label to achieve modest weight loss, and few studies have evaluated the long-term use of diethylpropion [7]. A meta-analysis that assessed the use of diethylpropion for weight loss in obese individuals identified 13 studies published between 1965 and 1983. Although most studies were less than 20 weeks, the duration of treatment with diethylpropion varied from 6 to 52 weeks, and obese patients treated with diethylpropion lost an average of 3.0 kg of additional weight compared with a placebo [14].
No new phentermine's RCTs have been conducted since 1983, but a recent report evaluates the efficacy of diethylpropion on a long-term basis, with an emphasis on cardiovascular and psychiatric safety aspects [16]. Sixty-nine obese healthy adults received a hypocaloric diet and were randomized to diethylpropion 50 mg twice daily or placebo for 6 months. After this period, all participants received diethylpropion in an open-label extension for an additional 6 months. After the initial 6 months, the diethylpropion group lost an average of 9.8% of initial body weight vs. 3.2% in the placebo group (P<0.0001). From baseline to month 12, the mean weight loss produced by diethylpropion was 10.6%. Participants in the placebo group who were switched to diethylpropion after 6 months lost an average of 7.0% of initial body weight. No differences in blood pressure, pulse rate, electrocardiogram, and psychiatric evaluation were observed. Dry mouth and insomnia were the most frequent adverse events, which were experienced in the first 3 months and became less apparent with continued treatment.
Common side effects of diethylpropion included insomnia, dry mouth, dizziness, headache, mild increases in blood pressure, palpitations and rash, which are similar to the pharmacologic effect of amphetamines. Diethylpropion and phentermine are classified by the U.S. Drug Enforcement Agency as schedule IV drugs, meaning they have a very low potential for drug abuse. Although no serious adverse events were reported in the RCTs of diethylpropion, the upper limit of the 1-sided 95% CI given the number of patients studied who received diethylpropion was 1.5%, meaning the rate of serious adverse events could be as high as 15 per 1,000 [13].
CANNABINOID-1 RECEPTORS ANTAGONISTS (RIMONABANT AND TARANABANT)
The endocannabinoid system has been identified as playing a significant role in the control of appetite as well as glucose metabolism [17]. Rimonabant was the first in a new class of agents that appears to work by selectively blocking the cannabinoid-1 receptors in the endocannabinoid system and has been extensively investigated in the Rimonabant in Obesity (RIO) program, comprised of 4 randomized, double-blinded, placebo-controlled phase 3 clinical trials recruiting more than 6,000 overweight or obese patients whose weight at the start of the studies was on average 94 to 104 kg [18-21]. Each of the 4 studies on rimonabant showed significant reductions in body weight and waist circumference over a 1 to 2-year period. Rimonabant also improved cardiometabolic risk factors, including triglycerides, blood pressure, insulin resistance, C-reactive protein levels, and high density lipoprotein cholesterol concentrations in both non-diabetic and type 2 diabetic overweight/obese patients. Rimonabant was generally well-tolerated. However, later reports showed the use of rimonabant was associated with psychiatric side effects, including anxiety, depression, and suicidal ideation. The adverse psychiatric events were observed in 26% of the participants in the rimonabant group compared with 14% in the placebo group in the same 4 studies [22], and the risk of depressive symptoms was estimated at 2.5 fold higher than in placebo-treated patients [8]. In spite of the extensive favorable clinical data, the U.S. FDA refused marketing authorization for rimonabant as a consequence of the risks. Rimonabant was marketed in 18 EU member states. However, in November 2008, the EMA subsequently withdrew authorization for rimonabant in Europe.
Taranabant, a second CB1 antagonist, has also been assessed in large scale clinical trials over a 52-week period and showed 4 kg placebo-adjusted significant weight reduction, similar to rimonabant [23]. However, psychiatric side effects were observed in all studies with taranabant using both high [23,24] and low doses [25]. For this reason, psychiatric side effects are considered a class issue for first generation CB1 antagonists. As a consequence, the development of other first generation CB1 antagonists, including the Pfizer CB1 antagonist, otenabant (CP-945,598), and the Bristol Myers Squibb compound, SLV-319, have also been halted.
SIBUTRAMINE
Sibutramine, a selective noradrenaline/serotonin reuptake inhibitor, was widely used after approval by the U.S FDA in 1997. In meta-analyses that included obese participants treated with sibutramine for at least 12 months, the mean placebo subtracted weight loss was 4.2 to 4.45 kg [26]. Among Korean obese patients receiving sibutramine, 68.2% of patients lost 5% or more of body weight compared with 13.0% in the placebo group with the mean absolute weight change of -5.9±3.8 kg in the sibutramine group and -1.6±2.6 kg in the placebo group after 12 weeks of treatment [27].
Because sibutramine led to a 4.5% body weight loss for long-term treatment (over 24 to 52 weeks) [13] and also showed potential benefits by improving cardiometabolic factors including plasma glucose, insulin, and lipid-profiles [28], sibutramine was the most effective anti-obesity drug marketed, though in a meta-analysis which included 10 studies of approximately 1,213 participants who were treated with sibutramine or placebo for at least 6 to 12 months, treatment was not associated with a significant decrease in total cholesterol after adjustment for weight loss [29].
Sibutramine was relatively well tolerated because common side effects included only constipation, headache, dry mouth, and insomnia. However, sibutramine was originally reviewed by the EMA in 1999 and 2002, following concerns over its safety, particularly cardiovascular side effects (increased blood pressure and heart rate), and was temporarily withdrawn from the Italian market on the basis of 47 adverse event reports (arrhythmias, primarily tachycardia, and hypertension) and 2 deaths from cardiovascular disease causes in that country [30]. At that time, the Agency's Committee for Medicinal Products for Human Use (CHMP) concluded the benefits of sibutramine for the management of obese and overweight patients outweighed the risks. However, the CHMP also requested Abbott Laboratories start a long-term study of sibutramine in patients with cardiovascular risk factors, with particular focus on the medicine's safety.
The 5-year Sibutramine Cardiovascular Outcomes (SCOUT) trial was a randomized, double-blind, placebo-controlled study involving 10,742 overweight or obese patients with cardiovascular disease, hypertension, or type 2 diabetes [31]. After a 6-week lead-in period, patients who received single-blind sibutramine had on average a 2.2 kg reduction of body weight, a 2.0 cm reduction of waist circumference, a 3.0 mm Hg decrease in systolic blood pressure, a 1.0 mm Hg decrease in diastolic blood pressure, and a 1.5 mm Hg decrease in pulse rate. In addition, sibutramine was found to be efficacious, tolerable, and safe in the 6-week single-blind period. In January 2010, a preliminary report of the SCOUT study, which showed sibutrasibutramine was associated with an increased risk of serious, nonfatal cardiovascular events such as myocardial infarction or stroke as compared with a placebo (11.4% vs. 10%; hazard ratio [HR], 1.16; 95% CI, 1.03 to 1.31), led to the recommendation to suspend the use of sibutramine by the CHMP of the EMA. Sibutramine has been subsequently withdrawn from the European market. The U.S. FDA requested healthcare professionals be notified sibutramine should not be used in patients with known cardiovascular disease. The full results of the SCOUT study were published in September 2010 [11]. Long-term sibutramine treatment was shown to increase the risk of nonfatal myocardial infarction and nonfatal stroke, but not of cardiovascular death or death from any cause in overweight or obese patients with pre-existing cardiovascular diseases. The U.S. FDA initially allowed sibutramine to be available and reviewed its potential benefits and risks [32], but asked for stronger warnings on the product labels. The warning recommended sibutramine not be used by people with a history of stroke or heart attacks and uncontrolled high blood pressure. A 3-year prospective observational study of 15,686 patients who were prescribed sibutramine in New Zealand has not demonstrated a higher risk of death from a cardiovascular event [33]. However, the U.S. FDA decided the drug may pose unnecessary cardiovascular risks to patients, and thus sibutramine was withdrawn on October 8, 2010.
ORLISTAT
Orlistat is a potent and reversible gastrointestinal lipase inhibitor preventing dietary fat absorption by 30% by inhibiting pancreatic and gastric lipase. Orlistat was approved in 1998 and is currently the only available drug for the long-term management of obesity. The prescribed dose is 120 mg capsule 3 times daily, and a half dose (60 mg) is available over-the-counter in some countries, including the U.S. The efficacy of orlistat for weight loss has been reported in several RCTs for the long-term management of obesity (approximately 4 years) [34,35]. In meta-analyses of 12 and 15 trials, the mean difference in weight loss due to orlistat was -2.59 kg (95% CI, -3.46 to -1.74 kg) at 6 months and -2.9 kg (-3.2 to -2.5 kg) at 12 months [13], which was more than the placebo. The beneficial effect on body weight is sufficient to improve several cardiometabolic parameters, including waist circumference, blood pressure, blood glucose levels, and lipid profiles [35,36]. In a meta-analysis which included 15 studies of approximately 10,995 participants who were treated with orlistat or placebo for at least 6 to 12 months, treatment with orlistat was associated with a significant decrease in total cholesterol after adjustment for weight loss [29], which indicates orlistat is a useful adjunctive tool for improving cardiovascular risk factor profiles in obese patients. Orlistat also reduced the incidence of type 2 diabetes from 9.0% to 6.2% (HR, 0.63; 95% CI, 0.46 to 0.86) in a longer 4-year trial [35].
Among Korean obese patients receiving orlistat for 24 weeks, the mean weight change from baseline was -2.8 kg. Treatment with orlistat also improved several metabolic parameters. There were significant improvements of glycemic control (HbA1c, -0.87%; P<0.01), fasting insulin, total cholesterol, LDL-C (P<0.001), waist circumference (-5.48±0.54 cm, P<0.001), systolic blood pressure and diastolic blood pressure (P=0.000), without serious side effects [37].
The most common side effects of orlistat are gastrointestinal and include diarrhea, fecal incontinence, oily spotting, flatulence, bloating, and dyspepsia [10,26,38]. As a result of the adverse effects, orlistat may not be well tolerated. However, the side effects tend to occur early and can be reduced as patients learn how to avoid fat-rich diets.
Recently, serious liver injury has been reported over the past 10 years. Between 1999 and 2008, the U.S. FDA received 32 reports of severe liver injury, including 6 cases of liver failure in patients using orlistat, which prompted the U.S. FDA to undertake a review of orlistat's treatment safety. The review identified a total of 13 cases of severe liver injury, reported between April 1999 and August 2009 out of an estimated 40 million people worldwide who had used Xenical or Alli. The U.S. FDA advised healthcare professionals to continue prescription of orlistat in August 2009, because severe liver injury was rare. However, a review in May 2010 led to a label revision and the addition of a warning of severe liver injury to educate the public regarding the signs and symptoms of liver injury.
MONOTHERAPY AND COMBINATION THERAPIES CURRENTLY UNDER INVESTIGATION
Lorcaserin
Lorcaserin is a selective serotonin 2C (5-HT2C) receptor agonist that reduces body weight by reducing food intake and is not thought to stimulate the 5-HT2B receptor associated with cardiac valvulopathy. Nonselective serotoninergic agents, including fenfluramine and dexfenfluramine, were withdrawn form the market in 1997 after they were reported to be associated with valvular heart disease [6].
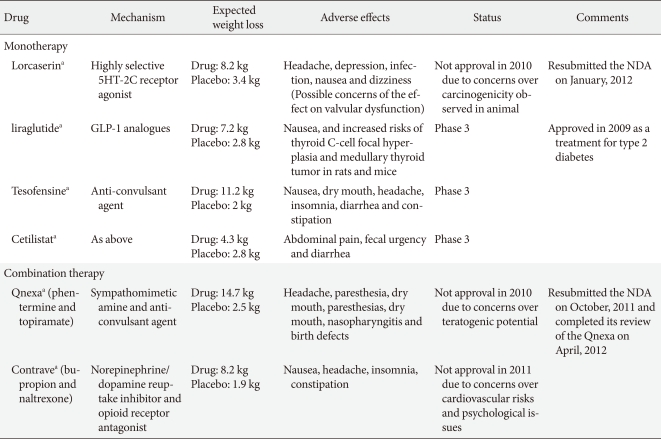
Two phase 3 studies have now been reported (Behavioral Modification and Lorcaserin for Overweight and Obesity Management [BLOOM] [39] and BLOSSOM [40]) and have shown a modest but significant placebo-adjusted weight reduction. In a 2-year, double-blind, placebo-controlled study, 3,182 obese and overweight patients who were treated with placebo or lorcaserin 10 mg/kg twice daily, obese patients lost 3.6 kg (3.6% body weight) more than controls at the end of the first year. Additionally, the weight reduction was maintained in more patients who continued to receive lorcaserin during year 2 (67.9%) than in patients who received a placebo during year 2 (50.3%, P<0.001), among the patients who received lorcaserin during year 1 and who had lost 5% or more of their baseline weight at 1 year [39]. A second trial of similar design over 1 year, termed "BLOSSOM" in which 4,008 patients were treated with lorcaserin 10 mg every day or twice daily, was designed to assess the efficacy and safety of a dose range of lorcaserin when administered in conjunction with a nutritional and physical exercise program to promote weight loss in obese patients and at-risk overweight patients. The participants who took lorcaserin 10 mg twice daily achieved an average weight loss of 5.9% of their body weight, compared to 2.8% with a placebo (P<0.0001). Similarly, patients who took lorcaserin 10 mg once daily achieved an average weight loss of 4.8% of their body weight at the end of 12 months (P<0.0001) [40]. The most frequent adverse events in the 2 phase 3 studies were fatigue, headache, dizziness, dry mouth, and nausea, which were not significantly different between treatment groups. In addition, there was no increase in the rate of cardiac valve disease after a 2-year treatment with lorcaserin [39]. As a result of the trials, a new drug application (NDA) for lorcaserin was filed with the U.S. FDA in December 2009. However, the advisors recommended against approval in September 2010, as they did not conclude the potential benefits of the drug outweighed the risks [41]. Arena Pharmaceuticals Inc. (San Diego, CA, USA) reported results from a third phase 3 clinical trial for lorcaserin, Behavioral Modification and Lorcaserin for Overweight and Obesity Management in Diabetes Mellitus (BLOOM-DM), which evaluated the safety and efficacy of lorcaserin for weight management in obese and overweight patients with type 2 diabetes and planned to submit the final study report from the BLOOM-DM trial as a supplement to the NDA for lorcaserin to the U.S. FDA [42]. The BLOOM-DM study evaluated 604 obese and overweight patients with type 2 diabetes. Patients were randomized to lorcaserin 10 mg twice daily (n=256), lorcaserin 10 mg dosed once daily (QD) (n=95) or placebo (n=253). To expedite enrollment, randomization to the lorcaserin 10 mg once daily dose was discontinued after approximately 300 patients were enrolled in the trial. The 3 primary efficacy endpoints at week 52 were as follows: the proportion of patients who lost at least 5% of their baseline body weight, change from baseline in body weight, and the proportion of patients who lost at least 10% of their baseline body weight. Using Modified Intent-to-Treat Last Observation Carried Forward (MITT-LOCF) analysis, lorcaserin 10 mg twice daily met the 3 primary efficacy endpoints by producing statistically significant weight loss compared to placebo (P<0.0001). At week 52, 37.5% of patients treated with lorcaserin 10 mg twice daily achieved at least 5% weight loss, more than double the 16.1% of patients taking a placebo. Patients treated with lorcaserin 10 mg twice daily achieved a mean weight loss of 4.5% (4.7 kg), compared to 1.5% (1.6 kg) with the placebo. Additionally, at week 52, 16.3% of lorcaserin 10 mg twice daily patients achieved at least 10% weight loss compared to 4.4% of patients taking a placebo. BLOOM-DM also evaluated multiple secondary endpoints at week 52. Five families of endpoints have been or are being evaluated: glycemic, lipid, blood pressure, body composition, and quality of life (QOL). Data from the first 3 families are available and analysis of body composition and QOL are pending. Within the glycemic, lipid and blood pressure families, lorcaserin patients achieved statistically significant improvements relative to placebo in HbA1c and fasting glucose. Lorcaserin 10 mg twice daily patients achieved a 0.9% reduction in HbA1c, compared to a 0.4% reduction for the placebo group (P<0.0001). However, the FDA did not approve the application for lorcaserin due to unexplained preclinical carcinogenicity signals in rats, specifically, an increase in breast tumors. In January 2012, Arena Pharmaceuticals submitted a response to the Complete Response Letter issued by the FDA following review of the lorcaserin NDA and the company expects by the end of January, the FDA will confirm acceptance of the response and assign a new Prescription Drug User Fee Act (PDUFA) date.
Glucagon-like peptide-1 (GLP-1) analogues
Anorexigenic GLP-1 are gut hormones that increase secretion of insulin in pancreatic β-cells. GPL-1 analogues such as exenatide and liraglutide are approved for the treatment of type 2 diabetes, and the long-term use of the GLP-1 analogues leads to a decrease in HbA1c level and blood pressure [43,44]. Meta- analysis of clinical trials also revealed an average weight loss of 2.13 kg in exenatide-treated groups more than the placebo, and a 4.76 kg weight loss compared with insulin [45]. Exenatide is currently only in phase 2 trials for obesity [46]. A recent 32-week open-label extension study of liraglutide in 564 non-diabetic obese patients following the 20 week dose-ranging placebo-controlled phase 2 study of liraglutide in comparison with orlistat treatment (a mean weight loss of approximately 6.0 kg with liraglutide compared with 2.8 kg with a placebo and 4.1 kg with orlistat) [47] demonstrated that high-dose liraglutide led to a 5.5 to 6.0 kg placebo-adjusted weight loss at 52 weeks [48]. Novo Nordisk plans to initiate additional phase 3 trials of liraglutide for anti-obesity treatment in 2011.
Tesofensine
Tesofensine is an inhibitor of noradrenaline, dopamine and serotonin reuptake that reportedly also indirectly stimulates the cholinergic system and a sympathomimetic in the family of sibutramine. The drug was initially developed for the treatment of Parkinson's disease or Alzheimer's disease. Although its efficacy was limited for this application, significant weight loss was evident [49]. A proof of concept phase 2 study, a 24-week randomized double-blind placebo-controlled study, showed the proportion of patients achieving ≥5 kg (4.9%) was 59%, 87%, and 91% for 0.25, 0.5, and 1 mg groups, respectively, compared with 29% of controls. However, heart rate was significantly elevated in all tesofensine groups, and the highest dose of tesofensine (1 mg daily) showed a significant increase in blood pressure and the highest frequency of mood change [50]. Reportedly, the phase 3 program agreed with the FDA which assessed the 0.5 and 0.25 mg dose levels only [51]. Given the results, further phase 3 trials may limit the dose to 0.25 and 0.5 mg in order to reduce the impact on heart rate and blood pressure. NeuroSearch plans to initiate a clinical phase 3 program endorsed by the FDA and EMA. The trial consists of 3 trials: 2 traditional 1-year obesity trials with a mixed population with and without co-morbidities such as type 2 diabetes and dyslipidemia and 1 cardiovascular outcome study with more than 2 years of treatment in patients with a history of cardiovascular disease. The cardiovascular study is planned to enroll approximately 6,000 patients.
Cetilistat
Cetilistat is an inhibitor of pancreatic lipase, an enzyme that breaks down triglycerides in the intestine. Without this enzyme, triglycerides from the diet are prevented from being hydrolyzed into absorbable free fatty acids and are left to be excreted undigested. This drug, while similar to the currently FDA-approved drug orlistat, may have a more tolerable side-effect profile due to a different molecular structure. A phase 2 trial studied 612 obese, diabetic subjects with a BMI of 28 to 45 kg/m2 over a 12-week treatment period. Cetilistat 80 and 120 mg promoted significant weight loss compared with placebo (3.85 kg and 4.32 kg vs. 2.86 kg, respectively). Cetilistat-induced weight loss was similar to the weight loss achieved with orlistat (3.78 kg). Cetilistat was well tolerated and showed fewer discontinuations due to adverse events than in the placebo and orlistat groups. Given that discontinuation in the orlistat group was significantly worse than in the 120 mg cetilistat and placebo groups, and was entirely due to gastrointestinal adverse events, cetilistat may become a preferred lipase inhibitor for achieving weight loss [52]. Phase 3 trials of cetilistat are currently in progress in Japan.
Combination treatments being developed
Combination treatments of anti-obesity drugs showed disappointing results. The first important clinical study for weight reduction combining drugs used phentermine and fenfluramine as previously mentioned. The trial showed a highly significant weight reduction. However, fenfluramine was withdrawn from the market worldwide on September 15, 1997, because of heart valve damage [6]. Combination treatment of orlistat and sibutramine, which had been approved only for long-term use, did not induce any further weight loss [53]. Because none of the single-agent drugs that have been approved or appear close to approval have consistently been able to achieve a weight loss of more than approximately 10% of body weight [54], several other combinations of existing drugs are now under development and may be the next therapeutic option for treatment of obesity.
Qnexa (Vivus Pharmaceuticals, Mountain View, CA, USA), a combination of low-dose phentermine and the antiepileptic agent topiramate, has recently been shown to be effective for the long-term treatment of obesity, though topiramate remains unlicensed for obesity. The efficacy and safety of this combination drug as a treatment for obesity was recently evaluated in 3 phase 3 trials (EQUATE, EQUIP, CONQUER) [55,56] in over 4,500 obese patients and the U.S FDA acknowledged the effect of weight reduction. However, In October 2010, the FDA did not approve Qnexa and requested more evidence that the elevated heart rate does not increase cardiovascular risk. In addition, Vivus Pharmaceuticals had concerns regarding the drug's teratogenic potential. In January 2011, the agency announced the FDA had requested additional information regarding teratogenicity and the FDA's endocrinologic and metabolic drugs advisory committee is scheduled to review the NDA for Qnexa for the treatment of obesity in February 2012. The company resubmitted the NDA on October 2011 seeking approval to market Qnexa in the U.S. and the FDA accepted the NDA for review in November 2011. The target date for the FDA to complete review of the Qnexa NDA is April 2012.
Contrave (Orexigen Pharmaceuticals, La Jolla, CA, USA) is a fixed-dose combination of naltrexone sustained-release (SR) and bupropion SR. Bupropion, a dopamine and norepinephrine reuptake inhibitor was approved for depression and smoking cessation and showed modest weight loss. Naltrexone, approved for the treatment of opioid addiction and alcoholism, is not associated with weight reduction. However, the combination with bupropion and naltrexone leads to a synergistic effect on weight control, although the mechanism by which the naltrexone/bupropion combination induces weight loss is not entirely understood. Several phase 3 trials (COR, COR-BMOD, and COR-Diabetes) have been completed to evaluate this combination in both diabetic and non-diabetic obese patients [57-59].
The Contrave group showed significant weight reduction and improvement in cardiometabolic markers compared to the placebo group in all studies. The COR-Diabetes trial showed overweight or obese patients with type 2 diabetes lost significantly more weight and achieved greater improvement in glycemic control than patients treated with a placebo after 56 weeks. The Contrave group lost significantly more weight (5.0% vs. 1.8%, P<0.001) at 56 weeks in 44.5% of patients (≥5% loss of body weight) compared to 18.9% using the placebo. Baseline A1C, the standard test for monitoring glycemic control, was significantly reduced by 0.6% with Contrave compared to 0.1% with the placebo [57]. A specific 56-week, randomized, placebo-controlled trial examined the efficacy and safety of naltrexone plus bupropion as an adjunct to intensive behavior modification (BMOD) in obese and overweight patients with major depressive illness because of recent concerns regarding psychiatric side effect issues and the known potential for bupropion to induce a depressive mood. The study concluded Contrave significantly improved body weight and ameliorated depressive symptoms as scored by the MADRS [58]. Contrave was submitted for U.S. regulatory approval in March 2010. The original submission was based on multiple clinical trials that evaluated Contrave in more than 4,500 patients. However, the U.S. FDA rejected Contrave in January 31, 2011 due to concerns regarding the cardiovascular safety profile of naltrexone/bupropion when used long-term in a population of overweight and obese subjects [8].
SUMMARY
Obesity can be an incurable chronic disease that increases the risk for cardiovascular diseases such as diabetes and hypertension. Because lifestyle interventions alone rarely result in long-term weight loss and the large proportion of patients return to baseline weight within 3 to 5 years, pharmacotherapy as a lifestyle modification adjunct to improve the induction and maintenance of weight loss may be considered for individuals with a BMI ≥30 kg/m2 or for individuals with a BMI ≥27 kg/m2 and co-morbidities [4]. Early administration of anti-obesity pharmacotherapy could provide significant benefit in the reduction of both weight and the risk for the development of comorbidities. However, despite promising results on reduction of body weight and improvement of several cardio-metabolic factors, many drugs that have been effective weight loss medications were withdrawn from the market in the last few years due to serious adverse effects. The positive data observed for surrogate markers was found not necessarily corresponding to positive clinical outcomes, though the anti-obesity drugs were expected to reduce cardiovascular outcome, beyond weight control. Treatments that reduce weight but do not improve cardiovascular outcome are thought to be of cosmetic benefit only and would thereby be less likely to gain approval for clinical use. Because orlistat is currently the only anti-obesity drug approved for long-term use, the development of new anti-obesity drugs is therefore urgently needed. This reasoning has led to the development of a number of new treatments for obesity in which multiple mechanisms are targeted, either by a single drug, such as tesofensine, or through drug combinations such as Qnexa, Contrave and Empatic (Table 3). However, the previous withdrawals also suggest regulatory committees are increasingly reluctant to approve a recommendation for the treatment of obesity using novel drugs, including lorcaserin, Qnexa, and Contrave without data that clearly supports long-term safety. The emphasis that both the U.S. FDA and EMA have placed on metabolic indices is based on recognition the drugs impact the onset of cardiovascular outcome more directly than weight loss alone. Although the guidelines for approval and market withdrawal are considerable barriers to the development of new anti-obesity drugs, the long-term safety and efficacy of newly developed drugs should also be evaluated in the management of obesity, which often requires ongoing therapy to achieve and maintain weight loss. Finally, government policy should support social intervention as a means of managing obesity because obese patients often return to toxic environments and behaviors. When considering anti-obesity therapy, in addition to scientific and regulatory considerations, patients' financial barriers also need to be taken into account.
In conclusion, it is expected that more effective and better tolerated anti-obesity drugs will be developed through a better understanding of the multiple mechanisms and complex physiological systems targeting appetite. Additionally, a lifestyle modification will hopefully be supported alongside all other treatments for obesity, because it remains the cornerstone of the medical management of obesity.
Notes
No potential conflict of interest relevant to this article was reported.