Triple Combination Therapy Using Metformin, Thiazolidinedione, and a GLP-1 Analog or DPP-IV Inhibitor in Patients with Type 2 Diabetes Mellitus
Article information
Abstract
Although there is no HbA1c threshold for cardiovascular risk, the American Diabetic Association-recommended goal of HbA1c < 7.0% appears to be unacceptably high. To achieve an optimal HbA1c level goal of 6.0% or less, a high dosage of sulfonylureas and insulin would be required; the trade-off would be the common adverse effects of hypoglycemia and weight gain. In contrast, hypoglycemia is uncommon with insulin sensitizers and GLP-1 analogs, allowing the physician to titrate these drugs to maximum dosage to reduce HbA1c levels below 6.0% and they have been shown to preserve β-cell function. Lastly, weight gain is common with sulfonylurea and insulin therapy, whereas GLP-1 analogs induce weight loss and offset the weight gain associated with TZDs. A treatment paradigm shift is recommended in which combination therapy is initiated with diet/exercise, metformin (which has antiatherogenic effects and improves hepatic insulin sensitivity), a TZD (which improves insulin sensitivity and preserves β-cell function with proven durability), and a GLP-1 analog (which improves β, α-cell function and promotes weight loss) or a dipeptidyl peptidase IV inhibitor in patients with type 2 diabetes mellitus.
INTRODUCTION
Beta cell failure and peripheral insulin resistance are the basic pathophysiologic defects of type 2 diabetes mellitus. Many studies, including the United Kingdom Prospective Diabetes Study (UKPDS), Steno-2, Action to Control Cardiovascular Risk in Diabetes (ACCORD), and Veterans Affairs Diabetes Trial (VADT) have demonstrated the limited efficacy of antidiabetic agents in terms of durability of blood glucose control and reduction in cardiovascular mortality. Antidiabetic agents also have their own treatment barriers, such as hypoglycemia and weight gain, which may be partly responsible for increased cardiovascular risk, especially in the late stages of type 2 diabetes mellitus.
An early and proactive approach to treating patients with type 2 diabetes mellitus using a triple combination of metformin, thiazolidinedione (TZD), and a glucagon-like peptide-1 (GLP-1) analog (or dipeptidyl peptidase IV [DPP-IV] inhibitor) is presented here. The goal of this treatment is to target insulin resistance in the liver and muscle and islet dysfunction, and to cover the basic defects and shortcomings of the stepwise American Diabetes Association (ADA)'s theoretical treatment algorithm. Basic clinical investigations and studies substantiating the suggestion of a paradigm shift in the treatment of type 2 diabetes mellitus will also be discussed briefly.
LIMITED EFFICACY OF METFORMIN AND SULFONYLUREA
The UKPDS clearly demonstrated that sulfonylureas had no protective effect on progressive β-cell failure in newly-diagnosed type 2 diabetic patients over the 15-year study duration [1]. Moreover, sulfonylureas were shown not to have a significant protective effect against atherosclerotic cardiovascular complications, and some studies even gave notion that sulfonylureas may accelerate the atherogenic process [2]. Similarly, metformin-treated patients also experienced a progressive deterioration in glycemic control [3]. Although, metformin was shown to reduce macrovascular events in the UKPDS, evidence that metformin actually modifies β-cell deterioration or reduces cardiovascular risk is very limited. The relentless rise in hemoglobin A1c (HbA1c) levels observed with both sulfonylureas and metformin resulted from a progressive decline in β-cell function, and by 3 years, approximately 50% of diabetic patients required an additional pharmacological agent to maintain HbA1c levels below 7.0%. Although the add-on treatment improved glycemic control, after the initial decline in HbA1c concentrations, progressive β-cell failure continued and HbA1c values rose progressively. Thus, most clinical evidence shows that the glucose-lowering effect of sulfonylureas and metformin is not durable and that the loss of glycemic control is associated with progressive β-cell failure.
Metformin is traditionally known for its metabolic effects on the liver; and other metformin target tissues include skeletal muscle and adipose tissue. Metformin is a useful adjuvant to lifestyle modification in overweight and obese patients with type 2 diabetes mellitus, metabolic syndrome, or impaired glucose tolerance (IGT). AMP-activated protein kinase (AMPK), a serine-threonine kinase that functions as an intracellular energy sensor, has been involved in the molecular mechanisms of metformin's actions in the liver, muscle, endothelium, and the ovaries [4]. Although metformin failure may occur rapidly in clinical practice, initiating treatment soon after diabetes diagnosis and while HbA1c levels are low might preserve β-cell function, prolonging the effectiveness of metformin.
DURABILITY OF GLUCOSE CONTROL WITH THIAZOLIDINEDIONE
The best evidence that retardation or arrest of β-cell loss can be achieved comes from interventions that reduce excess body adiposity or change its biology. Weight loss in the Diabetes Prevention Program and the Finnish Diabetes Prevention Study was associated with ongoing reductions in the rate of type 2 diabetes mellitus each year. This pattern would be expected if weight loss were slowing, or even stopping, the progression of β-cell deterioration. The same phenomenon has been observed repeatedly with TZDs, which change lipid distribution and adipose-tissue biology to ameliorate some adverse metabolic effects of obesity, including insulin resistance.
There are five studies in subjects with IGT demonstrating that TZDs prevent the progression of IGT to type 2 diabetes mellitus. The Diabetes Reduction Assessment with Ramipril and Rosiglitazone Medication study showed a 62% decrease in the development of type 2 diabetes mellitus with rosiglitazone, while the Actos Now for Prevention of Diabetes study showed an 81% reduction in the conversion of IGT to type 2 diabetes mellitus with pioglitazone. All five of these studies showed that, in addition to their insulin sensitizing effect, TZDs have a major action to preserve β-cell function. Many in vivo and in vitro studies with human and rodent islets have shown that TZDs exert a protective effect on β-cell function. Direct measurement of β-cell function in the Troglitazone in Prevention of Diabetes and Pioglitazone in Prevention of Diabetes studies proved that TZDs can slow or even stop the loss of β-cell function. The mechanism appeared to be a reduction in the secretory demand for insulin that occurs when insulin resistance is treated [5].
In contrast to the sulfonylureas, eight long-term (> 1.5 years), active-comparator or double-blind placebo-controlled studies with TZDs present a very different picture [6-8]. After an initial decline in HbA1c levels, durability of glycemic control is maintained because of the preservation of β-cell function in type 2 diabetes mellitus patients.
EFFICACY AND SAFETY OF GLP-1 ANALOGS
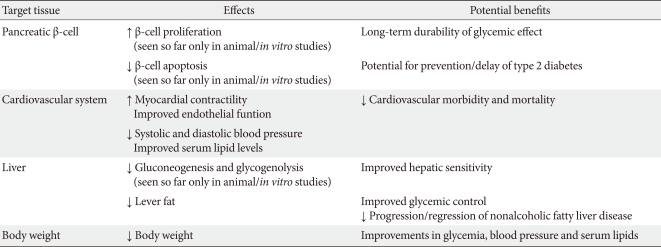
Incretins have been shown to improve β-cell function and maintain durability of glycemic control. In addition, the GLP-1 analogs exenatide and liraglutide consistently lower body weight, and, in some studies, have favorable cardiovascular benefits, including lowering blood pressure, beneficial effects on serum lipids, and the potential to improve myocardial contractility and endothelial function (effects seen with GLP-1 infusions) (Table 1) [9]. Bunck et al. [10] demonstrated improved beta cell function in patients treated with exenatide compared with a glargine-treated group. First and second phase insulin secretion and C-peptide response to a hyperglycemic clamp were measured before and after 1 year of treatment; insulin secretion increased 1.5- and 2.9-fold, respectively, and glargine increased the ratio of the C-peptide response during the hyperglycemic clamp by 31%. In contrast, exenatide increased the ratio more than threefold.
The addition of exenatide to metformin and/or sulfonylurea treatment for 3 years in type 2 diabetes mellitus patients resulted in sustained improvements in glycemic control, cardiovascular risk factors, and hepatic biomarkers, coupled with progressive weight reduction. A subset of these subjects was followed-up on for 3.5 years, and the decline in HbA1c levels was shown to persist [11].
In addition to their effects on β- and α-cells, GLP-1 analogs reduce hepatic glucose production, appetite, and gastric motility, which are responsible for weight loss. More importantly, stimulation of insulin secretion and suppression of glucagon by exenatides is strictly dependent on plasma glucose concentrations, thereby minimizing the adverse effect of hypoglycemia. The expectation that incretin therapy may not only improve β-cell function but also increase β-cell mass in patients with type 2 diabetes mellitus was not substantiated, in marked contrast to findings in rodents [12].
SUGGESTIVE EXTRA-ISLET ACTION OF DPP-IV INHIBITORS
Although there are no studies of the long-term efficacy of DPP-IV inhibitors on β-cell function, in short-term studies ranging from several months to 1 year, both sitagliptin and vildagliptin demonstrated a reduction in postprandial plasma glucose concentrations while maintaining plasma insulin response, indicating a positive effect on β-cell function [13-15]. But this enhancement in insulin secretion does not necessarily mean preservation of β-cell function on a long-term basis. DPP-IV inhibitors also decrease glucagon secretion, and in concert with a rise in plasma insulin, lead to a reduction in basal hepatic glucose production [16]. Hypoglycemia does not occur with the DPP-IV inhibitors, but they do not suppress appetite or cause weight loss.
It is well known that GLP-1 (7-36) (and GLP-1 [7-37]) is a primary insulinotropic hormone on the β-cell mediated by the G-protein coupled receptor, GLP-1R, which activates cAMP-dependent PKA and PI3 kinase-dependent Akt signal pathways. GLP-1 (9-36) amide (and GLP-1 [9-37]) is the cleavage product of GLP-1 (7-36) amide (and GLP-1 [7-37] formed by DPP-IV within minutes. Those cleavage products (GLP-1 [7-36] amide) have only weak insulinotropic agonist activities on the GLP-1 receptor; however, they also have extra-islet actions, such as suppression of hepatic glucose production, antioxidant cardioprotection, and reduction of oxidative stress in vascular tissues through binding to another receptor. Tomas and Habener [17] recently suggested a dual receptor hypothesis, which describes two hypothetical cell signaling pathways by which GLP-1 and its cleavage product exert insulinomimetic actions on insulin-sensitive target tissues. Prolonged inhibition of DPP may prevent the formation of GLP-1 (9-36) amide, and might ultimately augment oxidative stress in the heart, vasculature, and liver, and promote hepatic glucose production, which would be undesirable in the treatment of obesity-related diabetes. Therefore, potent, short-acting DPP-IV inhibitors taken at meal time might provide brief stimulation of insulin secretion by increasing levels of insulinotropic GLP-1 (7-36) amide, allowing escape from DPP-IV inhibition and the intermittent production of insulinomimetic GLP-1 (9-36) amide during interprandial periods. However, no clinical evidence exists demonstrating better outcomes using potent, short-acting DPP-IV inhibitors instead of longer-acting DPP-IV inhibitors, nor has there been a large study on the efficacy of the cleavage products of GLP-1 (7-36) (and GLP-1 [9-37]).
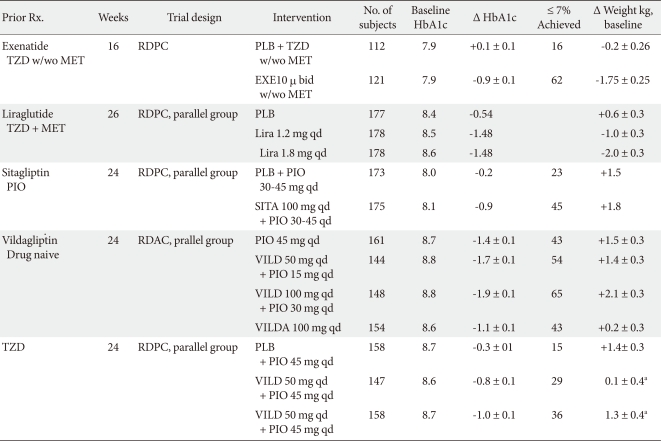
COMBINATION OF THIAZOLIDINEDIONE AND DPPIV INHIBITOR (Table 2)
In 175 patients (placebo group, n = 178) with type 2 diabetes mellitus who had not achieved adequate glycemic control with pioglitazone alone, 100 mg Sitagliptin was added once daily for 24 weeks. The addition resulted in effective glycemic control (between-treatment difference in least squares mean change from baseline, -0.70%) and the changes were well tolerated, with no increased risk of hypoglycemia compared with the placebo (2 vs. 0 patients, respectively) [18].
A 24-week, multicenter, double-blind, randomized, parallel-group study comparing the effects of vildagliptin (50 or 100 mg daily) with a placebo as an add-on therapy to pioglitazone (45 mg daily) was conducted with 463 patients with type 2 diabetes mellitus that was inadequately controlled by prior TZD monotherapy. The patients showed an adjusted mean change (AMΔ) in HbA1c levels, from baseline to endpoint, of -0.8 ± 0.1% (P = 0.001 vs. placebo) and -1.0 ± 0.1% (P < 0.001 vs. placebo), for daily dosages of 50 and 100 mg of vildagliptin, respectively. Mild hypoglycemia was reported by 0, 0.6, and 1.9% of patients receiving vildagliptin 50 mg/day, 100 mg/day, or a placebo, respectively [19].
A previous study compared the efficacy and tolerability of an initial combination therapy with vildagliptin/pioglitazone to component monotherapy [20]. The first-line treatment with vildagliptin/pioglitazone (both high- and low-dose regimens) provided better glycemic control than each component monotherapy with minimal hypoglycemia. The high-dose combination (100/30 mg qd) allowed 65% of patients to achieve target HbA1c levels (< 7%), with a tolerability profile comparable with pioglitazone (30 mg qd) monotherapy. The low-dose combination (50/15 mg qd) provided both efficacy and tolerability benefits over pioglitazone 30 mg qd. By addressing complementary mechanisms, the initial combination of vildagliptin and pioglitazone appears to be an effective means of achieving good glycemic control while attenuating drug-related adverse effects.
Another study evaluated the efficacy and safety of alogliptin in type 2 diabetes mellitus patients with a mean duration of 7.6 years and inadequate glycemic control using therapy with a thiazolidinedione and metformin/sulfonylurea. This study demonstrated a significant improvement in glycemic control after 26 weeks without clinically significant effects on the incidence of hypoglycemia, which occurred most often in patients who also were taking a sulfonylurea [21].
Recently, Gupta et al. [22] observed that the GIP receptor contains a functional PPAR-γ response element in the promoter region of the gene in INS-1 cells. Interventions that decreased or increased PPAR-γ activity (including exposure to a thiazolidinedione) resulted in corresponding changes in GIP receptor expression in in vivo rat models. Solomon et al. [23] suggested improved beta cell function following lifestyle-induced weight loss may partly be mediated by enhanced GIP secretion and action in older, obese, diabetic subjects. Whether treating patients with type 2 diabetes mellitus with a thiazolidinedione will enhance incretin effects mediated by GIP is not known, but is an intriguing possibility [24].
TRIPLE COMBINATION - METFORMIN, THIAZOLIDINEDIONE AND GLP-1 ANALOGS (Table 2)
Exenatide 10 µg bid therapy for 6 weeks improved glycemic control (mean difference, -0.98%), reduced body weight (mean difference, -1.51 kg), and caused gastrointestinal symptoms more than placebo in 233 patients (exenatide group 121, placebo group 112) with type 2 diabetes mellitus that was suboptimally controlled with TZD ± metformin [25].
A triple combination therapy of liraglutide, metformin, and TZD for 26 weeks in 533 subjects (1:1:1) randomized to once-daily liraglutide (1.2 or 1.8 mg) or liraglutide placebo in combination with metformin (1 g twice daily) and rosiglitazone (4 mg twice daily) demonstrated significantly greater mean ΔHbA1c values in the liraglutide groups versus placebo (mean ± SE, -1.5 ± 0.1% for both 1.2 and 1.8 mg liraglutide and -0.5 ± 0.1% for placebo). Minor hypoglycemia occurred more frequently with liraglutide, but there was no major hypoglycemia. Gastrointestinal adverse events were more common with liraglutide, but most occurred early in the study period and were transient. Investigators concluded that the triple combination of liraglutide, metformin, and TZD is an effective and safe treatment for patients with type 2 diabetes mellitus [26]. In a 14-week extension study of Liraglutide Effect and Action in Diabetes 6 trial, patients switched from 10 µg twice-daily exenatide to 1.8 mg liraglutide once-daily or 40 weeks of continued liraglutide. Liraglutide improved HbA1c levels (Δ 0.32%), fasting plasma glucose, and homeostasis model of β-cell function values more so than exenatide, with less persistent nausea and hypoglycemia. Over 40 weeks, liraglutide reduced HbA1c levels by 1.3% [27].
A 20-week study to observe the effects of exenatide plus rosiglitazone on β-cell function and insulin sensitivity using hyperglycemic and euglycemic insulin clamp techniques in type 2 diabetes mellitus patients currently taking metformin has been conducted [28]. Exenatide and rosiglitazone significantly improved the M value at 20 weeks (P < 0.05), while exenatide had no significant effect on insulin-stimulated glucose disposal. After 80 to 90 minutes of the hyperglycemic clamp the disposition index increased significantly and similarly with exenatide and rosiglitazone (both P < 0.001) but not with rosiglitazone [28]. These results demonstrate that exenatide has a positive influence on β-cell function but does not exert any significant insulin-sensitizing action, as determined by the M value during the insulin clamp. Rosiglitazone, however, caused a two-fold increase in insulin sensitivity, measured as M/I during the euglycemic insulin clamp. Conclusively, therapy with exenatide and rosiglitazone, in addition to metformin, offset the weight gain observed with rosiglitazone and elicited an additive effect on glycemic control with significant improvements in β-cell function and insulin sensitivity.
CONCLUSION
The stepped ADA algorithm of metformin/sulfonylurea therapy in type 2 diabetes mellitus has been proven ineffective in achieving durable glycemic control even in newly-diagnosed patients. However, TZDs and GLP-1 analogs, when used as monotherapy, have been shown to have a more durable effect. When used in combination, one would expect an even more durable effect on preservation of β-cell function and reduction in HbA1c levels, although this remains to be proven in a longer-term study. In contrast to sulfonylurea and metformin, both TZDs and GLP-1 analogs have been shown to preserve β-cell function. Although there is no HbA1c threshold for cardiovascular risk, the ADA-recommended goal of HbA1c < 7.0% appears to be unacceptably high. To achieve an optimal HbA1c level goal of 6.0% or less, a high dosage of sulfonylureas and insulin would be required; the trade-off would be the common adverse effects of hypoglycemia and weight gain. In contrast, hypoglycemia is uncommon with insulin sensitizers and GLP-1 analogs, allowing the physician to titrate these drugs to maximum dosage to reduce HbA1c levels below 6.0%. Lastly, weight gain is common with sulfonylurea and insulin therapy, whereas GLP-1 analogs induce weight loss and offset the weight gain associated with TZDs.
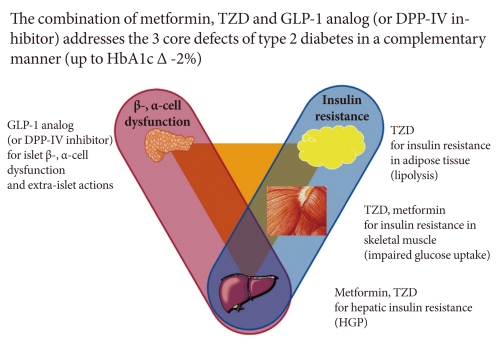
A treatment paradigm shift is recommended in which combination therapy is initiated with diet/exercise, metformin (which has antiatherogenic effects and improves hepatic insulin sensitivity), a TZD (which improves insulin sensitivity and preserves β-cell function with proven durability), and a GLP-1 analog (which improves β, α-cell function and promotes weight loss) or a DPP-IV inhibitor (Fig. 1) [29,30].