Association between Non-Alcoholic Steatohepatitis and Left Ventricular Diastolic Dysfunction in Type 2 Diabetes Mellitus
Article information
Abstract
Background
Impaired diastolic heart function has been observed in persons with non-alcoholic fatty liver disease (NAFLD) and/or with type 2 diabetes mellitus (T2DM). However, it is unclear whether NAFLD fibrotic progression, i.e., non-alcoholic steatohepatitis, poses an independent risk for diastolic dysfunction in T2DM. We investigated the association between liver fibrosis and left ventricular (LV) diastolic dysfunction in T2DM.
Methods
We analyzed 606 patients with T2DM, aged ≥50 years, who had undergone liver ultrasonography and pulsed-wave Doppler echocardiography. Insulin sensitivity was measured by short insulin tolerance test. Presence of NAFLD and/or advanced liver fibrosis was determined by abdominal ultrasonography and NAFLD fibrosis score (NFS). LV diastolic dysfunction was defined according to transmitral peak early to late ventricular filling (E/A) ratio and deceleration time, using echocardiography.
Results
LV diastolic dysfunction was significantly more prevalent in the NAFLD versus non-NAFLD group (59.7% vs. 49.0%, P=0.011). When NAFLD was stratified by NFS, subjects with advanced liver fibrosis exhibited a higher prevalence of diastolic dysfunction (49.0%, 50.7%, 61.8%; none, simple steatosis, advanced fibrosis, respectively; P for trend=0.003). In multivariable logistic regression, liver fibrosis was independently associated with diastolic dysfunction (odds ratio [OR], 1.58; 95% confidence interval [CI], 1.07 to 2.34; P=0.022) after adjusting for insulin resistance and cardiometabolic risk factors. This association remained significant in patients without insulin resistance (OR, 4.32; 95% CI, 1.73 to 11.51; P=0.002).
Conclusions
Liver fibrosis was associated with LV diastolic dysfunction in patients with T2DM and may be an independent risk factor for diastolic dysfunction, especially in patients without systemic insulin resistance.
INTRODUCTION
Non-alcoholic fatty liver disease (NAFLD) is increasingly a global public health epidemic, a consequence of rising rates of obesity, diabetes mellitus, and metabolic syndrome [1]. More than one-third of the United States adult population is estimated to have NAFLD [2], and prevalence reaches 30% in Europe and Asia [3]. The NAFLD spectrum ranges from simple steatosis to non-alcoholic steatohepatitis (NASH), a progressive necroinflammatory form that can lead to liver cirrhosis and hepatocellular carcinoma. Various inflammatory reactions including oxidative stress, release of proinflammatory cytokines, and lipotoxicity are closely related to development of NASH [4]. NASH is the second most common cause for liver transplantation in the United States, and is predicted to become the first over the next decade [5].
Notwithstanding, the leading cause of death in persons with NAFLD is cardiovascular disease (CVD) [6], presumably due to shared risk factors composing obesity and metabolic syndrome [78]. Previously NAFLD was linked to a higher prevalence of coronary artery disease [9] and subclinical atherosclerosis, demonstrated by increased carotid artery wall thickness and impaired endothelial flow-mediated vasodilatation [10]. In addition, persons with NAFLD had altered left ventricular (LV) geometry and early features of LV diastolic dysfunction by echocardiography [111213141516]. Another study demonstrated decreased LV energy metabolism in subjects with newly diagnosed NAFLD, despite normal LV structure and function [17]. The presence of concomitant liver fibrosis poses an even greater risk for cardiovascular mortality [18]. Recently, biopsy-proven NASH was associated with altered diastolic indices [1920], although studies with histopathologic assessment of liver fibrosis were limited in size, owing to the invasive nature of liver biopsy.
It is recognized that persons with type 2 diabetes mellitus (T2DM) are at increased risk for heart failure, following cardiac abnormalities including LV hypertrophy and diastolic dysfunction, collectively termed as diabetic cardiomyopathy [21]. The pathogenesis of diabetic cardiomyopathy encompasses an array of metabolic derangements mainly attributable to insulin resistance [22]. NAFLD—and especially NASH—is highly prevalent in persons with T2DM and is considered a hepatic manifestation of insulin resistance. However, it is unclear whether NAFLD or NASH in patients with T2DM conveys an independent risk for heart failure, aside from contributions by diabetes mellitus and insulin resistance. The aim of this study was to elucidate whether hepatic steatosis and fibrosis in T2DM were independently associated with abnormal LV diastolic function, and whether such a relationship is subject to systemic insulin resistance.
METHODS
The study population
We included 689 individuals with T2DM, aged ≥50 years who had undergone short insulin tolerance test (SITT), liver ultrasonography, and pulsed-wave Doppler echocardiography from 2002 to 2016 at Huh's Diabetes Center, Seoul, Korea. Subjects with previous histories of coronary artery disease or ischemic stroke, alcohol consumption greater than 140 g/week, or liver cirrhosis by ultrasonography were excluded (n=83). A total of 606 subjects were analyzed. The study protocol was approved by the Institutional Review Board of Severance Hospital, Seoul, Korea (IRB 2017-0760-001), and the requirement for informed consent was waived.
Anthropometric and biochemical measurements
Demographics, medical history, and social habits including smoking, alcohol consumption, and physical exercise, were obtained via self-reported questionnaire at the first visit. Smoking status was categorized into never, past, or current smoking. Height and body weight were measured and body mass index (BMI) was calculated as weight divided by the square of the height (kg/m2). Obesity was defined according to the criteria of the Asia-Pacific region (BMI ≥25 kg/m2). Waist circumference was measured at the midpoint between the lower ribs and the iliac crest after normal expiration. Blood pressure was read by mercury sphygmomanometer after at least 5 minutes of rest. Hypertension was defined as blood pressure ≥140/90 mm Hg or the use of antihypertensive drugs. Blood samples were collected after overnight fasting, and measured for fasting plasma glucose, glycosylated hemoglobin (HbA1c), C-peptide, insulin, total cholesterol, high density lipoprotein cholesterol (HDL-C), triglycerides, platelets, aspartate aminotransferase (AST), and alanine aminotransferase (ALT). Low density lipoprotein cholesterol was calculated per the Friedewald formula. Plasma glucose concentration was measured by Beckman Glucose Analyzer II (Beckman Instruments, Fullerton, CA, USA). HbA1c was quantified by high performance liquid chromatography (Variant II; Bio-Rad, Hercules, CA, USA). Metabolic syndrome was defined as the presence of ≥3 of the following criteria: waist circumference ≥90 cm (male) or ≥80 cm (female) using Asia-Pacific abdominal obesity criteria; serum triglycerides ≥1.7 mmol/L or on lipid lowering agents; HDL-C <1.03 mmol/L (male) or <1.3 mmol/L (female); blood pressure ≥130/85 mm Hg or taking antihypertensive medications; and serum glucose ≥100 mg/dL or the use of antidiabetic drugs.
Assessment of insulin sensitivity
Insulin sensitivity was determined by the rate constant for disappearance of plasma glucose during SITT (KITT, %/min) as previously described [23]. Plasma glucose concentration was measured from venous blood collected at 0, 3, 6, 9, 12, and 15 minutes following intravenous injection of regular insulin (Humulin R; Eli Lilly, Indianapolis, IN, USA) at a dose of 0.1 U/kg after overnight fast. The KITT was derived from the linear slope of log-transformed plasma glucose between 3 and 15 minutes. Insulin resistance was defined as KITT <2.5%/min [24].
Abdominal ultrasonography
Liver sonography was performed via a high-resolution ultrasound system (LOGIQ 7; GE, Milwaukee, WI, USA) by a single radiologist blinded to laboratory and clinical data. Fatty liver was assessed semi-quantitatively and described as absent, mild, moderate, or severe based on hepato-renal echo contrast, liver brightness, deep attenuation, and vascular blurring. NAFLD was defined as presence of hepatic steatosis on ultrasound. Among subjects with NAFLD, presence of advanced liver fibrosis was determined by NAFLD fibrosis score (NFS): −1.675+0.037×age+0.094×BMI+1.13×(impaired fasting glucose or diabetes mellitus)+0.99×AST/ALT−0.013×platelet−0.66×albumin ≥−1.445 [25]. Simple steatosis was defined as ultrasonographically detected fatty liver without fibrosis predicted by NFS.
Echocardiography
All subjects underwent pulsed-wave Doppler echocardiography (LOGIQ 7) conducted by a single experienced sonographer. LV and atrial dimensions and wall thicknesses were obtained from the parasternal long axis view. Left ventricular ejection fraction (LVEF) was calculated by the Teicholz formula. LV mass was estimated using the validated formula by Devereux et al. [26]. On the apical four-chamber view, transmitral Doppler peak flow velocities of early (E-wave) and late diastolic filling (A-wave), and the E-wave deceleration time (DT) were obtained from the average of three measurements. Diastolic dysfunction was defined as one of the following transmitral flow patterns by modification of previously used definitions [27]: impaired relaxation, E/A <1 or DT >240 ms (age <55 years) or E/A <0.8 and DT >240 ms (age ≥55 years); pseudonormal, E/A 1 to 1.5 and DT >240 ms; or restrictive, DT <160 ms with ≥1 of the following: E/A >1.5 or LA diameter >5 cm.
Statistical analyses
Continuous variables were assumed to be normally distributed and reported as mean±standard deviation. Categorical variables were reported as frequency (percentage). Intergroup comparisons were by Student's t-test or analysis of variance (ANOVA) for continuous variables, and chi-square test for categorical variables. Odds ratios (ORs) and 95% confidence interval (CI) for diastolic dysfunction were calculated by multivariable logistic regression. The regression model was: unadjusted in model 1; adjusted for age, sex, and BMI in model 2; further adjusted for hypertension, smoking status, duration of diabetes mellitus, fasting glucose, triglyceride, HDL-C, and ALT in model 3; and further adjusted for insulin resistance in model 4. Subgroup analyses and their interactions were adjusted by model 4. P value <0.05 was considered statistically significant. All statistical analyses were performed by R version 3.4.3 (R Foundation for Statistical Computing, http://www.R-project.org).
RESULTS
Clinical and echocardiographic parameters
Clinical characteristics and echocardiographic parameters were stratified by presence of NAFLD as summarized in Table 1. Mean age was 63.1±7.0 years, 143 participants (23.6%) were male, and mean duration of T2DM was 8.4±7.0 years. NAFLD was present in 355 subjects (58.6%). Patients in the NAFLD group were younger (P=0.013) and more recently diagnosed with T2DM (P=0.001) compared with the non-NAFLD group. Subjects with NAFLD had significantly greater BMI and waist circumferences and were more likely to have metabolic syndrome (all P<0.001). While fasting glucose and HbA1c were similar between the groups, subjects with NAFLD exhibited significantly greater insulin resistance, demonstrated by higher fasting insulin and lower KITT (all P<0.001). Serum cholesterol, triglycerides, and liver transaminases were also significantly elevated in the NAFLD group compared with the non-NAFLD group. On echocardiography, LVEF was within normal range in both groups, whereas LV mass index (P=0.014) and LA diameter (P=0.007) were significantly greater in the NAFLD group. Significantly lower E/A ratio (P=0.001) and longer DT (P=0.034) were seen in the NAFLD group. Accordingly, LV diastolic dysfunction was present in 335 participants (55.3%), and its prevalence was significantly higher in the NAFLD group compared with the non-NAFLD group (59.7% vs. 49.0%, P=0.011).
Association of hepatic steatosis with LV diastolic dysfunction in T2DM
The prevalence of diastolic dysfunction gradually increased with severity of fatty liver as shown in Fig. 1A (49.0%, 54.8%, and 66.7%; none, mild, and moderate-severe, respectively; P for trend=0.001). When NAFLD was stratified by NFS in Fig. 1B, subjects with advanced liver fibrosis exhibited a significantly higher prevalence of LV diastolic dysfunction compared with those without steatosis (49.0%, 50.7%, 61.8%; none, simple steatosis, advanced fibrosis, respectively; P=0.011 for advanced liver fibrosis vs. none, P for trend=0.003).
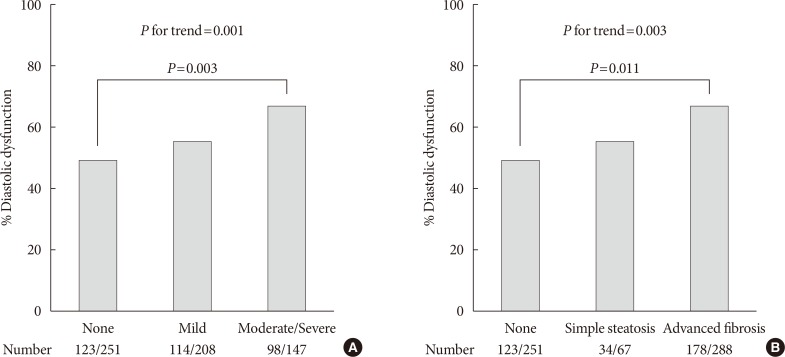
Prevalence of left ventricular diastolic dysfunction. (A) Prevalence according to sonographic grade of steatosis. (B) Prevalence according to presence of liver fibrosis predicted by non-alcoholic fatty liver disease fibrosis score. P for trend by chi-square test for linear-by-linear association. Pairwise comparisons corrected by Holm-Bonferroni method.
To assess whether NAFLD is independently associated with LV diastolic dysfunction in patients with T2DM, multivariable logistic regression was performed. Presence of NAFLD was associated with greater odds for diastolic dysfunction (OR, 1.53; 95% CI, 1.05 to 2.22; P=0.027) after adjusting for age, sex, BMI, hypertension, smoking status, duration of diabetes mellitus, fasting glucose, triglyceride, HDL-C, and ALT (Fig. 2A, model 3) compared with those without NAFLD. This association remained significant but was attenuated when the model was further adjusted for insulin resistance (OR, 1.48; 95% CI, 1.02 to 2.17; P=0.041) (Fig. 2A, model 4). Other significantly associated factors in model 4 included age (OR, 1.07; 95% CI, 1.04 to 1.10; P<0.001) and BMI (OR, 1.06; 95% CI, 1.01 to 1.13; P=0.017). In subgroup analyses, there was no heterogeneous effect of NAFLD on diastolic dysfunction depending on HbA1c, duration of diabetes mellitus, hypertension, BMI, age, and sex (all P for interaction >0.05) (Fig. 2B). Of note, the association was significant only in patients without insulin resistance (OR, 3.77; 95% CI, 1.54 to 9.77; P=0.005), but not in those with insulin resistance (P=0.327 and P for interaction=0.042).
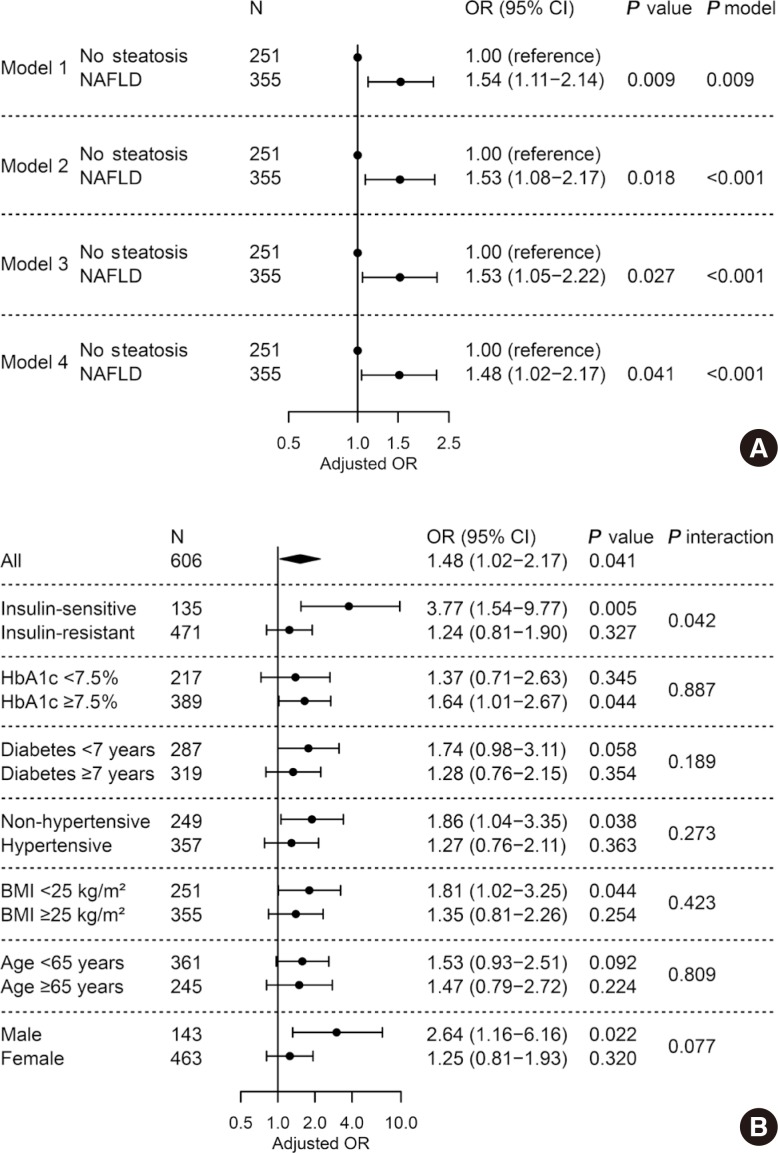
Adjusted odds ratio for left ventricular diastolic dysfunction by presence of non-alcoholic fatty liver disease (NAFLD). (A) Multivariable logistic regression in all subjects. Model 1, unadjusted; model 2, adjusted for age, sex, and body mass index (BMI); model 3, further adjusted for hypertension, smoking status, diabetes mellitus duration, fasting glucose, triglyceride, high density lipoprotein cholesterol, and alanine transaminase; model 4, further adjusted for insulin resistance. (B) Subgroup analyses and their interactions with NAFLD. Multivariable logistic regression with full model (model 4). OR, odds ratio; CI, confidence interval; HbA1c, glycosylated hemoglobin.
Association of advanced liver fibrosis with LV diastolic dysfunction in T2DM
As shown in Fig. 3A, we investigated the relationship between presence of advanced liver fibrosis with NAFLD and LV diastolic dysfunction. When NAFLD was stratified by NFS, advanced liver fibrosis was significantly associated with diastolic dysfunction (OR, 1.58; 95% CI, 1.07 to 2.34; P=0.022) after adjusting for confounders and insulin resistance, while simple steatosis alone did not show a statistical significance compared with patients without steatosis (Fig. 3A, model 4). Other independent factors in model 4 were age (OR, 1.07; 95% CI, 1.04 to 1.10; P<0.001) and BMI (OR, 1.06; 95% CI, 1.01 to 1.12; P=0.024). No significant heterogeneity was found in various subgroups of glycemic control, duration of diabetes mellitus, presence of hypertension, BMI, age, and sex (All P for interaction >0.05) (Fig. 3B). However, the association disappeared in patients with insulin resistance (P=0.275), while it was maintained in those without insulin resistance (OR, 4.32; 95% CI, 1.73 to 11.51; P=0.002, P for interaction=0.031).

Adjusted odds ratio for left ventricular diastolic dysfunction by presence of liver fibrosis predicted by non-alcoholic fatty liver disease (NAFLD) fibrosis score. (A) Multivariable logistic regression in all subjects. Model 1, unadjusted; model 2, adjusted for age, sex, and body mass index (BMI); model 3, further adjusted for hypertension, smoking status, diabetes mellitus duration, fasting glucose, triglyceride, high-density lipoprotein-cholesterol, and alanine transaminase; model 4, further adjusted for insulin resistance. (B) Subgroup analyses and their interactions with liver fibrosis. Multivariable logistic regression with full model (model 4). OR, odds ratio; CI, confidence interval; HbA1c, glycosylated hemoglobin.
DISCUSSION
The present study demonstrates that, among persons with T2DM, those with NAFLD exhibited altered LV structure and diastolic function demonstrated by greater LV mass index, lower E/A ratio, and longer DT by echocardiography compared with non-NAFLD. Prevalence of LV diastolic dysfunction increased with severity of fatty liver. Furthermore, advanced liver fibrosis with steatosis, indicating NASH, but not simple steatosis, was significantly associated with LV diastolic dysfunction after adjustment for cardiovascular risk factors and insulin resistance. The association between liver fibrosis and diastolic dysfunction was especially significant in patients without insulin resistance. Therefore, in T2DM without severe insulin resistance, the presence of NASH may strongly suggest the coexistence of several cardiometabolic risk factors and subclinical heart failure.
NAFLD has been linked to LV diastolic dysfunction and remodeling in a number of studies in which subjects with NAFLD had greater LV mass, lower E/A ratio, longer DT, and lower e' [111213141516], while having similar LVEF compared with normal controls. NASH was also associated with altered cardiac indices in small studies incorporating liver biopsy [1920]. However, most previous studies largely relied on univariate analyses and only a few observed an independent effect of NAFLD after adjusting for hypertension, diabetes mellitus, and/or obesity in a sufficient number of subjects [1314]. For patients with diabetes mellitus, only two studies with western individuals from a single group have reported a significant association between NAFLD and diastolic dysfunction [1516]. However, in contrast to our study, no information was available regarding liver fibrosis, which is increasingly suggested as an important risk factor for CVD [18]. Moreover, no study thus far has directly controlled for the effect of insulin resistance on cardiac dysfunction in patients with T2DM.
In a previous study comparing T2DM, non-diabetic NAFLD without advanced fibrosis, and healthy controls, only the diabetes mellitus group exhibited diastolic dysfunction, indicating a significant relationship between glycemic control and cardiac function [28]. These authors suggested that NAFLD-only subjects who had high liver fat demonstrated higher endocardial strain and structural compensation for maintaining cardiac function by first hit, and that progression to diabetes mellitus with hyperglycemia may lead to impairment in cardiac function by second hit. Prediabetes has also been associated with impaired myocardial glucose uptake [29]; therefore, changes in glucose level and presence of diabetes mellitus itself may play a crucial role in altering cardiac function. However, in the present study, since glucose levels and HbA1c were comparable with or without NAFLD, and the effect of glucose level was controlled in logistic regression, we were able to assess the impact of hepatic steatosis or fibrosis on cardiac function in T2DM aside from glycemic control.
Recently, Lee et al. [30] reported that diastolic dysfunction was independently associated with liver fibrosis, but not with steatosis after adjustment for visceral adiposity in non-cirrhotic subjects. However, in a subset of patients with diabetes mellitus, the association was insignificant, likely due to small sample size. In this regard, we found in the present study that, among 606 T2DM patients, presence of advanced hepatic fibrosis with steatosis—not simple steatosis—was significantly associated with LV diastolic dysfunction. Furthermore, in subgroup analysis, the association was significant only in patients without systemic insulin resistance, and there was a significant interaction between liver fibrosis and insulin resistance, indicating that the association between NASH and diastolic dysfunction was modified by insulin-resistance.
Several mechanisms have been implicated in the pathophysiology linking NASH to diastolic dysfunction. Liver fibrosis was associated with increased epicardial fat thickness [19] which, in turn, is linked to altered diastolic function and cardiac geometry [171931]. Epicardial fat may exert a paracrine effect on cardiomyocytes and may contribute to lipotoxicity [32]. Growing evidence also suggests that fatty liver, and especially necroinflammation, releases proinflammatory cytokines (e.g., tumor necrosis factor-α, interleukin 6, monocyte chemoattractant protein 1, etc.), procoagulant factors, and adhesion molecules that may be involved in myocardial oxidative stress, atherogenic dyslipidemia, and endothelial dysfunction [33]. Myocardial tissue alterations, including deposition of advanced glycation end-products, fibrosis, and increased resting tension in cardiomyocytes may lead to LV diastolic stiffness [34]. In addition, dysregulated secretion of hepatokines (e.g., fetuin A, LECT2, RBP4, etc.) may in turn promote inflammatory pathways and cardiac dysfunction [35]. Altered gut microbiota, which is increasingly reported in association with NAFLD, may contribute to the chronic inflammatory state [36]. All of these processes may also eventually lead to systemic insulin resistance, causing cardiac dysfunction by accumulation of free fatty acid and lipid metabolites in cardiomyocytes and impairment in mitochondrial function [22].
Our study has several strengths. It was the first to investigate the relationship between severity of hepatic steatosis and/or presence of advanced liver fibrosis and LV diastolic dysfunction in patients with T2DM. Compared with previous studies, our sufficient sample size allowed us to further explore the effect of liver fibrosis in subgroup analyses (especially according to insulin resistance). Moreover, our assessment of insulin resistance by SITT, which is a simple but reliable alternative to the standard glucose clamp technique, provides a more direct measure compared with steady-state markers.
However, we also acknowledge that our study has some limitations. First, the cross-sectional design precludes a causal inference between NAFLD and incident cardiac dysfunction. Second, due to unavailability of tissue Doppler imaging (TDI) in our echocardiography, characterization of LV diastolic function primarily relied on E/A ratio and DT without E/e'. Many studies have relied on transmitral flow indices for defining LV diastolic dysfunction prior to the availability of TDI [273738394041], but an important limitation of E/A ratio is its dependence on age. Notwithstanding, NAFLD-related changes in E/A ratio and DT in our study were not only in agreement with previous reports, but also remained significant after adjusting for confounders, including age. Third, presence of steatohepatitis was not confirmed by liver biopsy due to its invasive nature. Instead, we used NFS, which has been well validated and is widely used for screening of advanced liver fibrosis in NAFLD. Lastly, this is a single center study from a diabetes clinic in Korea, and should be interpreted with caution when generalized to different clinical settings.
In conclusion, this cross-sectional study from T2DM patients with results from SITT, liver ultrasound, and echocardiography demonstrated a significant association between liver fibrosis and LV diastolic dysfunction even after adjustment for known cardiovascular risk factors and insulin resistance. The presence of NASH, rather than simple steatosis, may be indicative of diminishing diastolic function in patients with T2DM, and insulin resistance may play a significant role. Moreover, in the presence of T2DM, individuals with simple fatty liver should be aware of the progression to NASH and subsequent cardiac dysfunction. These findings, in light of current views on the effect of NAFLD on CVD pathogenesis in patients with T2DM, warrant further investigation of the mechanism and potential new targets to prevent CVD in T2DM with NAFLD.
ACKNOWLEDGMENTS
This work was supported by the National Research Foundation of Korea (NRF) grant from the Ministry of Science and ICT (NRF-2016R1A5A1010764) and by the Korea Healthcare Technology R&D Project, Ministry of Health and Welfare (HI17C0913). The authors would like to thank Caron Modeas, University of North Carolina at Chapel Hill, for proofreading.
Notes
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.


