Dipeptidyl Peptidase-4 Inhibitors versus Other Antidiabetic Drugs Added to Metformin Monotherapy in Diabetic Retinopathy Progression: A Real World-Based Cohort Study
Article information
Abstract
Background
To investigate the effects of dipeptidyl peptidase-4 inhibitor (DPP4i) as add-on medications to metformin on progression of diabetic retinopathy (DR) in patients with type 2 diabetes mellitus, compared with sulfonylurea (SU) or thiazolidinedione (TZD).
Methods
We identified 4,447 patients with DPP4i, 6,136 with SU, and 617 with TZD in addition to metformin therapy from the database of Korean National Health Insurance Service between January 2013 and December 2015. Cox proportional hazards regression models were used to calculate hazard ratios (HRs) for DR progression. The progression of DR was defined by the procedure code of panretinal photocoagulation, intravitreal injection or vitrectomy; or the addition of diagnostic code of vitreous hemorrhage, retinal detachment, or neovascular glaucoma.
Results
The age and sex-adjusted HR of DR progression was 0.74 for DPP4i add-on group compared with SU add-on group (95% confidence interval [CI], 0.62 to 0.89). This lower risk of DR progression remained significant after additional adjustments for comorbidities, duration of metformin therapy, intravitreal injections and calendar index year (HR, 0.80; 95% CI, 0.66 to 0.97).
Conclusion
This population-based cohort study showed that the use of DPP4i as add-on therapy to metformin did not increase the risk of DR progression compared to SU.
INTRODUCTION
Diabetic retinopathy (DR) is the most frequent microvascular complication of diabetes, leading to severe visual impairment in working-age population [1]. Retinal neovascularization and its accompanying complications such as vitreous hemorrhage and neovascular glaucoma occurring in proliferative stage, as well as diabetic edema, contribute to major sources of severe visual loss in DR [12]. These late-stage complications require treatments as laser photocoagulation or intravitreal injections of anti-vascular endothelial growth factor agents or corticosteroids, which need repeated treatments and result in high socioeconomic burden [345].
Metformin is commonly used antidiabetic drug as first-line therapy in United States as well as in Korea [678]. Sulfonylurea (SU) still remains the most commonly prescribed second-line agent, while the use of dipeptidyl peptidase-4 inhibitor (DPP4i) increased significantly over the past decade [678]. DPP4i have introduced at the end of 2008 in Korea and approved by the Ministry of Food and Drug Safety in 2012 [9], and their prescription has been increased to be 4-fold in 2013 (38.4% of total antidiabetic prescription) compared to 2009 [6]. The antidiabetic effect of DPP4i is based on the glucose-lowering activities of the gastrointestinal hormone, glucagon-like peptide-1 [10]. DPP4i seems to be protective in cardiovascular events as well as in nephropathy through previous studies [10111213], while few investigations have been performed on retinopathy.
We previously investigated the effect of DPP4i to the progression of DR as a pilot study, showing that DPP4i had protective effect on DR progression independently from glucoselowering effect [14]. However, there are controversies concerning the effect of DPP4i in DR [151617], which highlight further investigation for safety issue associated with DR and DPP4i. Accordingly, we investigated the risk of DR progression associated with DPP4i use based on real-world population-cohort study.
METHODS
Study design and participants
The study protocol was reviewed and approved by the Institutional Review Board of Severance Hospital at Yonsei University College of Medicine (IRB No. 4-2016-1005) and informed consent was waived because of the anonymous nature of the data. Data of approximately 50 million of Korean patients covered by the mandatory social National Health Insurance Service (NHIS) was investigated. The NHIS involves claim database including demographic information, diagnoses, prescriptions, and procedures. Diagnoses are coded using the International Classification of Diseases, 10th revision (ICD-10). The NHIS also requires all insured employees and self-employed individuals aged ≥40 years as well as their family dependents for general medical examination regularly every 2 years. This health screening data include body size, blood pressure, blood chemistry data including lipid profile, health behaviors, and personal and family histories of diseases. We used the NHIS database for primary analysis, and secondary analysis implied the health screening data of NHIS.
This study used NHIS data (NHIS-2017-1-054) made by NHIS of Korea. We first extracted patients with type 2 diabetes mellitus (E11 to E14, ICD-10 codes) who had received metformin monotherapy for at least 90 days between January 2009 and December 2012. The second line antidiabetic medications implied DPP4i, SU, or thiazolidinedione (TZD), and those initiated second line antidiabetic therapy from January 2013 to December 2015 were included. Patients treated with above mentioned medications for more than 90 consecutive days were included, while those with insulin treatment were excluded. Further inclusion and exclusion criteria are summarized in Fig. 1.
Outcomes and covariates
The presence of DR was determined on the diagnostic code of DR (H36.0, ICD-10 code). Among the patients already diagnosed with DR, the progression of DR was defined by (1) the procedure code of panretinal photocoagulation (S5160), intravitreal injection (S5070) or vitrectomy (S5160 and S5121-22); or (2) the addition of diagnostic code of vitreous hemorrhage (H43.1 and H45.0, ICD-10 codes), retinal detachment (H33.0, H33.4, and H33.5, ICD-10 codes), or neovascular glaucoma (H40.5 and H40.88, ICD-10 codes). The definition of DR progression was based on the protocol of the Diabetic Retinopathy Clinical Research Network [18].
Following covariates were subjected to serial statistical adjustments to minimize confounding effects: age, sex, duration of metformin therapy, hypertension, dyslipidemia, atrial fibrillation, chronic kidney disease, microvascular complications of diabetes (neuropathy, or nephropathy), the Charlson comorbidity score, intravitreal injections, and calendar index year. The Charlson comorbidity score is a weighted index that implies the number and the seriousness of comorbid disease, used to predict survival in patients with multiple comorbidities [19]. The calendar index year was used to adjust selection bias associated with calendar time for cohort studies [20].
Statistical analysis
All analysis were performed using SAS software version 9.4 (SAS Institute Inc., Cary, NC, USA). Baseline characteristics were compared among DPP4i users, SU users, and TZD users as second line medication. Cox proportional hazards regression models were used to calculate hazard ratios (HRs) and 95% confidence intervals (CIs) for DR progression. Potential confounders were sequentially adjusted in two statistical models as follows: model 1, age and sex; model 2, age, sex, duration of metformin therapy, the Charlson comorbidity score, intravitreal injections, calendar index year, and comorbidities (hypertension, dyslipidemia, atrial fibrillation, chronic kidney disease, and microvascular complications of diabetes).
For the analysis of the subgroup with health screening data, model 2 was adjusted for age, sex, duration of metformin therapy, body mass index, waist circumference, systolic blood pressure, total cholesterol, high density lipoprotein cholesterol, low density lipoprotein cholesterol, triglycerides, fasting glucose, serum creatinine level, smoking status, family history of stroke and heart disease, the Charlson comorbidity score, intravitreal injections, and calendar index year.
RESULTS
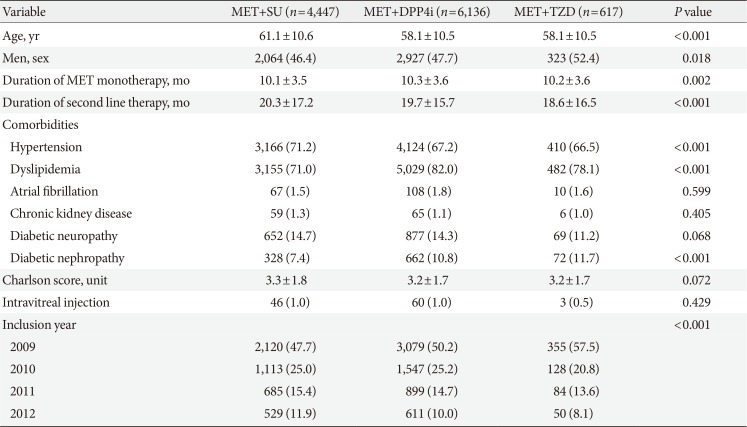
Among patients with DR who used second line drug along with metformin, those using DPP4i finally implied 4,447 patients, those with SU were 6,136 patients, and those with TZD were 617 patients (Fig. 1). The mean age of the study population was 59.3±10.6 years, and 47.4% were male. Baseline characteristics of these patients are presented in Table 1.
The age and sex-adjusted HR of DR progression was 0.74 (95% CI, 0.62 to 0.89; P=0.001) for DPP4i add-on group compared with SU add-on group (Table 2). This lower risk of DR progression remained significant after additional adjustments for comorbidities, duration of metformin therapy, intravitreal injections and calendar index year (HR, 0.80; 95% CI, 0.66 to 0.97; P=0.024).

Hazard ratios for aggravation of diabetic retinopathy by type of second-line antidiabetic medication
To adjust other confounding risk factors, we performed a subgroup analysis with additional adjustment for variables investigated at the health screening data of NHIS including fasting glucose level. Baseline characteristics of this subgroup are summarized in Supplementary Table 1. DPP4i add-on therapy was still associated with lower risk of DR progression although not statistically significant (HR, 0.89; 95% CI, 0.64 to 1.24; P=0.493) (Table 3).
DISCUSSION
In this study, less patients with DPP4i revealed DR progression than those with SU as add-on therapy. We previously reported a retrospective pilot study showing the protective effect of DPP4i on DR progression based on Early Treatment Diabetic Retinopathy Study (ETDRS) severity scale, which was independent of glycemic control [1421]. This study showed similar protective tendency, while the determination of DR progression was based on procedures required to treat complications or diagnoses associated with proliferative stage [18], not by the ETDRS severity scale. As the codes for procedures are mandatorily submitted in NHIS, less requirement of procedure codes with DPP4i use might reflect benefits for patients by less costs and saved time for treatment.
However, this protective effect of DPP4i was not evident in the subgroup analysis performed with those with available health screening data. The lower HR of DR progression with DPP4i was not statistically significant after adjusting variables including fasting glucose levels. It should be noted that the fasting glucose level was slightly higher in SU add-on group in Supplementary Table 1. Based on these data, there is a possibility that the benefit using DPP4i over SU in DR progression as shown in Table 1 might be due to better glycemic control since the glycemic control is important in DR progression [22]. A recent cohort study revealed that DPP4i did not increased overall risk of DR while a risk existed at early treatment phase, comparing ever-use and never-use cases of DPP4i [23]. Similarly, a cohort study with United States population aged 65 years or older reported that DPP4i use did not increase the risk of DR requiring treatments [24]. Taken together, it is reasonable to conclude that the use of DPP4i may not increase the risk of DR progression, compared to SU as add-on medication to metformin. DPP4i can be considered as second-line therapy in patient with type 2 diabetes mellitus, in safety from DR progression.
DR is one of major causes of visual impairment resulting in an important burden on health care systems [252627], so that protective factors other than glycemic control need to be considered in clinical practice. Retinopathy and nephropathy share common pathogenesis as microvascular complications of diabetes [12]. Renoprotective effect of DPP4i has been reported [282930], while there are few clinical studies on the effect of retinopathy [14]. Experimental studies on DR and DPP4i have revealed conflicting results. One study using linagliptin reported that loss of pericytes and retinal ganglion cells were prevented with the medication [16], while another study with sitagliptin also reported inhibition of blood-retinal barrier breakdown as well as decreased retinal inflammation and neuronal apoptosis [31]. Topical administration of DPP4i showed also protective effect by preventing neurodegeneration as well as vascular leakage in experimental diabetic retina [32]. However, there is still a study reporting increased vascular leakage with DPP4i suggesting possibility of DR progression [15].
Relatively small number of patients with TZD were included in this study compared to those with DPP4i or SU, which might be associated with low rate of TZD prescription in Korea [6]. Anti-angiogenic and anti-inflammatory effects of TZD in ischemic retina have been reported in experimental studies [3334], while a recent cohort study revealed no consistent evidence of DR progression in patients with TZD suggesting no association [35]. The low HR of DR aggravation in patients with TZD shown in this study was not further discussed here to avoid misinterpretation of the results, which need to be investigated with larger sample size of patients with TZD.
There are several limitations in this study. First, the presence of DR was defined by the presence of diagnostic code from NHIS database. Although the diagnostic code is mandatory for any patient, the accuracy of diagnoses are not adjudicated by medical records or laboratory tests. Second, bevacizumab is one of the widely used treatment for diabetic macular edema or complications of proliferative DR [3637], but intravitreal injection of bevacizumab was not identified in this analysis as this procedure is not covered by NHIS due to its off-label use in Korea. However, the ratio of patients treated intravitreally with other medications (ranibizumab, aflibercept, triamcinolone, or dexamethasone implants) were not different between groups, so that intravitreal bevacizumab injections may be also similar between groups as this may not be one-sided. Third, lack of glycemic control data is also critical, as intensive glycemia treatment and intensive combination treatment of dyslipidemia are well known to reduce the rate of DR aggravation [22]. We tried to overcome this limitation by adjusting fasting glucose levels instead in our subgroup analysis, although insufficient to reflect the whole study population. Lastly, follow-up period for DR aggravation was relatively short to compare the effect for second-line antidiabetic medications. However, above mentioned limitations might affect both DPP4i group and SU group, so that one-sided application might be prevented. Further study with larger sample size of patients with blood chemistry data would be helpful, so that more criteria would be available for the choice of second-line antidiabetic medications.
In conclusion, this population-based cohort study demonstrated that the use of DPP4i as second line medication did not increase the risk of DR progression compared with SU in patients with DR. This suggests that DPP4i can be considered as second line antidiabetic medication safely in patients with DR.
ACKNOWLEDGMENTS
This study used National Health Insurance Service (NHIS) data (NHIS-2018-1-348) made by NHIS of Korea. The authors declare no conflict of interest with NHIS. This study was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health and Welfare, Republic of Korea (grant number: HI13C0715). The funding organization had no role in the design or conduct of this research.
Notes
This study was presented as a poster at the European Association for Vision and Eye Research (EVER) 2018 Congress on October 4 to 6, 2018 in Nice, France.
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTIONS:
Conception or design: H.C.K., K.L., D.J.K.
Acquisition, analysis, or interpretation of data: K.H.H., S.J.P.
Drafting the work or revising: Y.R.C.
Final approval of the manuscript: K.L., D.J.K.
References
SUPPLEMENTARY MATERIALS
Supplementary materials related to this article can be found online at https://doi.org/10.4093/dmj.2018.0137.
Supplementary Table 1
Baseline characteristics of second-line drugs in a subgroup with available health screening data