Latent Autoimmune Diabetes in Adults: A Review on Clinical Implications and Management
Article information
Abstract
Latent autoimmune diabetes in adults (LADA) is a heterogeneous disease characterized by a less intensive autoimmune process and a broad clinical phenotype compared to classical type 1 diabetes mellitus (T1DM), sharing features with both type 2 diabetes mellitus (T2DM) and T1DM. Since patients affected by LADA are initially insulin independent and recognizable only by testing for islet-cell autoantibodies, it could be difficult to identify LADA in clinical setting and a high misdiagnosis rate still remains among patients with T2DM. Ideally, islet-cell autoantibodies screening should be performed in subjects with newly diagnosed T2DM, ensuring a closer monitoring of those resulted positive and avoiding treatment of hyperglycaemia which might increase the rate of β-cells loss. Thus, since the autoimmune process in LADA seems to be slower than in classical T1DM, there is a wider window for new therapeutic interventions that may slow down β-cell failure. This review summarizes the current understanding of LADA, by evaluating data from most recent studies, the actual gaps in diagnosis and management. Finally, we critically highlight and discuss novel findings and future perspectives on the therapeutic approach in LADA.
INTRODUCTION
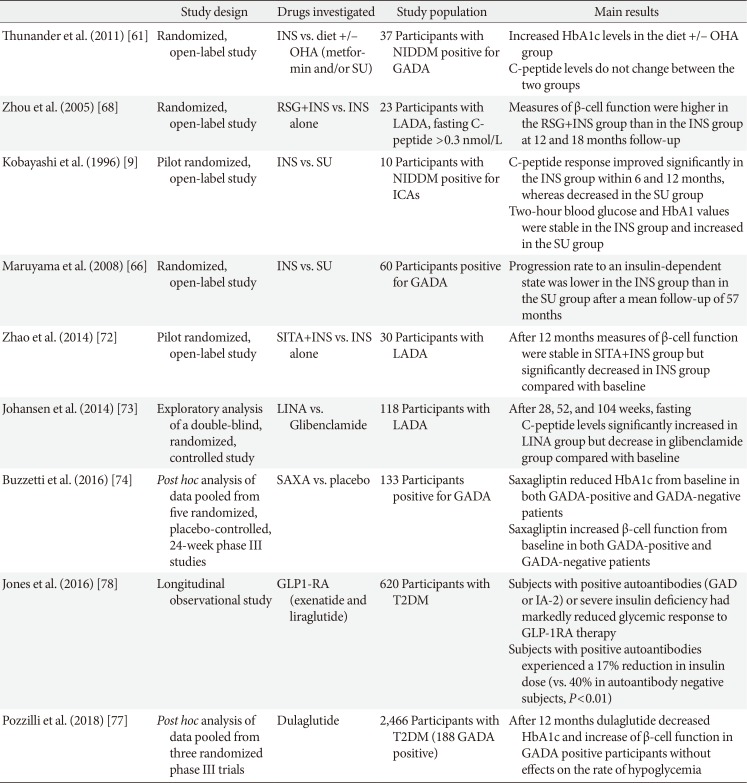
Autoimmune diabetes is characterized by the presence of specific autoantibodies directed against pancreatic β-cells islet and initial requirement of insulin therapy [1]. This condition is as prevalent in adulthood as in childhood [2]. However, a subgroup of subjects with newly diagnosis adult-onset autoimmune diabetes is initially insulin independent and shows clinical features more similar to individuals with type 2 diabetes mellitus (T2DM) than type 1 diabetes mellitus (T1DM), that could lead to misdiagnosis of T2DM [2]. These subjects are defined as affected by latent autoimmune diabetes in adults (LADA) [345] a specific form of autoimmune diabetes, more heterogeneous than young-onset T1DM and characterized by slower evolution towards β-cells failure and insulin therapy (Fig. 1) [678].
The heterogeneity of diabetes, with latent autoimmune diabetes in adults (LADA) that shares halfway clinical, genetic and immunological features between type 2 diabetes mellitus (T2DM) and type 1 diabetes mellitus (T1DM). GADA, glutamic acid decarboxylase autoantibody; HLA, human leukocyte antigen.
Alternative terms for the disease have been suggested in the past years. Kobayashi et al. [9] proposed the eponym “slowly progressive insulin-dependent type 1 diabetes” (SPIDDM) to describe subjects positive for glutamic acid decarboxylase (GAD) autoantibodies and/or islet cell antibodies, who are initially insulin independent and do not experience ketosis or ketoacidosis at the onset. In this context, patients who are long-term non-progressor have been defined as affected by NIR-SPIDDM [10]. However, although SPIDDM patients do not always develop to insulin-dependent, unlike those with LADA, it is reasonable to consider the terms LADA and SPIDDM synonymous, since they both identify a type of disease that has an autoimmune basis and necessitate a similar approach in clinical setting, which eventually will lead to insulin for its treatment.
According to the Immunology of Diabetes Society (IDS), patients diagnosed with LADA are defined by adult age of onset (>30 years) and insulin independence for at least 6 months after diagnosis plus positivity for circulating islet-cell autoantibodies, regardless of titre, number or epitope specificity [7]. Nonetheless, the exact definition of LADA is still under debate and no clear diagnostic guidelines are currently available. Thus, the population defined as having LADA is extremely heterogeneous in genetic, phenotypic and immunological features, showing an extensive variability in the pancreatic β-cell destruction's rate, insulin resistance and autoimmunity, probably due to differences in genetic and immune factors [111213]. As a consequence, LADA continues to be unnoticed in clinical setting and a high misdiagnosis rate (5% to 10%) still occurs among patients with T2DM [2]. Ideally, plasma C-peptide and autoantibody screening should be performed in subjects with newly diagnosed T2DM, ensuring a closer monitoring of those with autoantibody positivity [14]. However, this practice would be expensive and specific risk scores based on clinical parameters should be considered before requiring islet-cell autoantibodies tests in patients with recent evidence of diabetes. Nevertheless, the early detection of LADA among patients with T2DM remains crucial. In fact, since the autoimmune process in LADA seems to be slower than in classical T1DM, treatments that prevent β-cells failure are needed and should be implemented [15].
In this review we summarize and discuss the current understanding in pathophysiology, clinical features and implications of LADA, highlighting the heterogeneity among affected patients. Finally, we evaluate data from the most recent studies, the actual gaps in diagnosis and management for LADA as well as the novel pharmacological approaches for treatment.
EPIDEMIOLOGY OF LADA
T1DM has been classically considered as a childhood disease. However, epidemiological studies carried out in the last decade have demonstrated that approximately 30% of cases of T1DM are diagnosed after 30 years of age [21114161718], showing that adult-onset T1DM is more frequent than previously recognized. On that note, Naik et al. [19] observed that LADA may account for 2% to 12% of all cases of diabetes in adult population, proving that this form of adult-onset autoimmune diabetes is the most common one. Multicentre studies carried out in Europe, Asia and North America found that the occurrence of T1DM related autoantibodies among people with reported diagnosis of T2DM ranges between 3% and 12% [4519202122232425262728]. Nevertheless, the incidence of adult-onset autoimmune diabetes varies between different countries and ethnicity, and seems to be higher among Northern Europeans than African-American, Latinos, and Asians [4519202122232425262728]. Compared to Caucasians, data collected from Asian population have shown lower prevalence of LADA ranging from 2.6% in United Arab Emirates to 5.7% in China [29]. A frequency of 4.4% to 5.3% was reported among Korean population [182230], whereas data collected in India show a prevalence ranging between 2.6% and 3.2% [3132]. Interestingly, both Caucasians and Chinese people have shown different incidence of LADA between northern and southern areas, with higher prevalence of the disease in Northern region of both Europe (7% to 14%) and China (6.5%) [433]. However, differences in study design and selection criteria (such as number and type of autoantibodies tested), as well as different life-style and ethnicity might contribute to the worldwide variance observed.
GENETIC BACKGROUND OF LADA
Genome-wide association studies suggest that LADA shares the same genetic features as T2DM and childhood-onset T1DM, supporting the concept that LADA may be considered an admixture of the two major types of diabetes [34]. In this regard, Grant et al. [35] found a strong genetic linkage with T2DM within the transcription factor 7-like 2 (TCF7L2) gene, that appears to be associated with both adult-onset autoimmune diabetes and T2DM [36], whereas no association with classical childhood-onset T1DM has been detected [37]. On the other hand, human leukocyte antigen (HLA), protein tyrosine phosphatase, non-receptor type 22 (PTPN22), signal transducer and activator of transcription 4 (STAT4), cytotoxic T-lymphocyte–associated antigen 4 (CTLA4), interleukin 2 receptor alpha (IL2RA), and insulin (INS), which are strictly linked to young-onset T1DM, have been also associated with autoimmune diabetes in adults [12]. In particular, the detection of high-risk HLA genotypes in subjects with LADA is related to a higher risk of developing insulin dependence compared with the low-risk HLA [17]. In this regard, the DRB1*0301/DRB1*0401 heterozygosity confers the highest risk for LADA [38]. However, the prevalence of HLA-DQB1 risk genotype that is increased in LADA, is less common than in childhood-onset T1DM [39], as well as it has been observed for the Cyst1858Thr single-nucleotide polymorphism in the PTPN22 gene and the INS variable number tandem repeat (VNTR) I/I genotype expression [40]. Altogether, these data suggest a less pronounced effect of the same genes in LADA than in classical T1DM [111241]. In addition, the very low detection of HLA-DR3 and -DR4 heterozygotes in Chinese population might explain the low incidence of childhood-onset T1DM in Chinese subjects possessing moderate risk or protective HLA disease-associated variants [33]. In summary, it should be underlined that a genetic background that is exclusive for LADA is still lacking up to now. Further studies are needed in order to clarify the pathophysiology of LADA and allow safe and effective therapies.
AUTOANTIBODIES AND PATHOPHYSIOLOGY
Adult-onset autoimmune diabetes and classical T1DM are hardly distinguishable immunologically, although the young-onset T1DM has a greater immunogenetic load with faster impairment of β-cells [678], as demonstrated by the lower C-peptide levels and the faster C-peptide decrease [42]. Thus, LADA appears to be characterized by the same diabetes-associated autoantibody (DAA) detectable in classical T1DM. On that note, glutamic acid decarboxylase autoantibodies (GADAs) represent by far the most sensitive marker in both adult-onset T1DM and LADA even in China where it is less recurrent [433]. Otherwise, insulin autoantibodies (IAA), protein tyrosine phosphatase IA-2 (IA-2A), and islet-specific zinc transporter isoform 8 (ZnT8) autoantibodies, which are frequent in younger subjects with recent diagnosis of T1DM, are less prevalent in LADA patients [43]. However, mixed data have been reported in literature. Tiberti et al. [44] observed that the specific IA-2 construct 256–760 appears to be more frequent in LADA than previously reported, stressing the concept that the occurrence of other DAA could be also indicative of LADA.
With respect to the immunogenetic load, independent studies have observed fewer multiple DAAs in LADA than in young-onset T1DM. On that note, the Action LADA Study showed that the majority of subjects screened for DAA were positive for GADA, whereas only 24.1% of LADA patients were positive for at least two autoantibody types [4]. With regard to the pathogenesis of LADA, only a few and conflicting data are available in literature. Specifically, it is still unclear whether islet-cell autoantibodies are an epiphenomenon rather than acting as pathogenetic factors. However, the recent finding of a significant association between IA-2A positivity and increased body mass index (BMI) [45] has led to hypothesize two distinct pathogenetic mechanisms for LADA, one resulting from low-grade chronic inflammation in obese subjects with genetic susceptibility to T2DM, the other through specific immunological factors in leaner people with moderate genetic susceptibility to T1DM [15].
CLINICAL HETEROGENEITY OF LADA
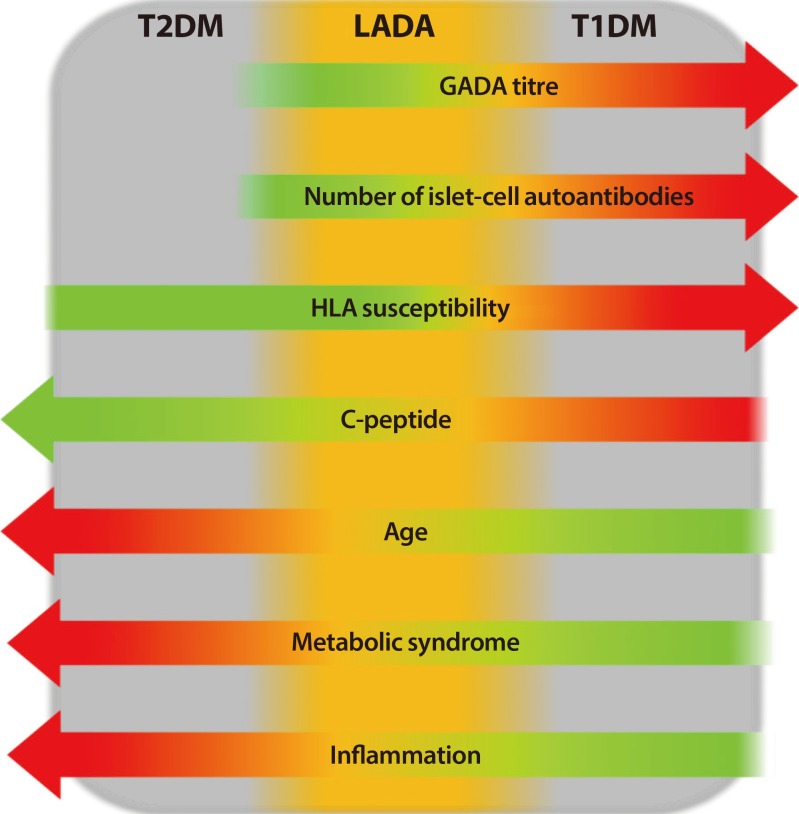
At diagnosis, the clinical presentation of autoimmune diabetes is extremely broad, ranging from diabetic ketoacidosis to hyperglycemia controlled with diet alone or hypoglycemic agents. In this context, subjects defined as having LADA who do not require insulin at first, encompass a wide spectrum of phenotypes from prevalent insulin resistance to prevalent insulin deficiency, sharing halfway clinical and metabolic features of T1DM and T2DM (Table 1). By comparing patients with T2DM with those affected by LADA, the latter tend to show fewer signs of metabolic syndrome, such as healthier lipid and blood pressure profiles, lower BMI and waist-to-hip ratio [530]. In this regard, the A Diabetes Outcome Progression Trial (ADOPT) found that among recently diagnosed individuals with T2DM, those who were positive for GADA had higher high-density lipoprotein cholesterol and lower triglyceride levels, as well as lower frequency of metabolic syndrome [28]. By contrast, the majority of studies reported that the features of metabolic syndrome appear to be more prevalent in LADA than in classical T1DM.
In 1999, the BOTNIA Study observed a worse metabolic profile in patients positive for GADA who did not need insulin therapy than in those with young-onset T1DM [26]. Later, similar conclusions were achieved independently by the Non-Insulin Requiring Autoimmune Diabetes (NIRAD) group [20] and the Action LADA 3 [46]. Another interesting finding emerges from the Nord-Tróndelag Health (HUNT) Study that demonstrated an increased risk of LADA among subjects with metabolic syndrome and a family history of diabetes [47].
However, the overlap for clinical features between these group of patients makes it difficult to distinguish LADA from T2DM based on clinical phenotype alone. Moreover, the definition of LADA itself, just as it was proposed by the IDS, is extremely broad, since it includes any adult who does not require insulin and who is positive for at least one DAA, regardless of titre or number [7]. For this reason, a great effort has been made nowadays to provide insight into how to correctly identify LADA patients, investigating the differences between LADA and T2DM with respect to genetic and immunological features. In this regard, recent studies suggested that the high heterogeneity observed within patients who are GADA positive, may be partly explained by different autoantibody profiles, as expression of a different degree of the autoimmune response [48]. The NIRAD Study demonstrated that LADA patients with high-titre GADA are younger and phenotypically more similar to classical T1DM [49]. Moreover, these individuals seem to have a higher risk of progression to insulin treatment and show lower C-peptide levels. Additionally, the same group found a higher prevalence of other organ-specific autoantibodies among high-titre GADA patients, such as anti-thyroid peroxidase autoantibodies, suggesting a broader autoimmune process in this group than in the low-titre GADA group. Otherwise, low-titre GADA subjects show less risk of ketosis as compared with high-titre GADA, but more pronounced traits of insulin resistance, as well as a higher prevalence of obesity, hypertension, dyslipidemia, and cardiovascular disease, both in Caucasians and non-Caucasians. In accordance with these findings, data collected among Korean population found that GADA levels were inversely associated with the age at disease onset, fasting and stimulated C-peptide levels as well as with BMI, total cholesterol and triglycerides [18]. Similarly, a recent nationwide survey carried out in Japan showed that GADA level ≥13.6 U/mL, age at onset <47 years, duration before diagnosis <5 years and fasting C-peptide <0.65 ng/mL predicted progression to insulin requiring diabetes among subjects with SPIDDM [10].
As noted above, since high-titre GADA tends to be associated with other organ-specific autoantibodies, it is not surprising that the United Kingdom Prospective Diabetes Study observed that among patients with LADA, the number of islet autoantibodies was directly proportional to the intensity of autoimmune response [25], suggesting a faster insulin insufficiency among patients with multiple DAAs [254348]. However, there are other immunological aspects to consider. Thus, some studies have found that the clinical phenotype of LADA subjects may be influenced even by different patterns of antibody positivity. In 2015, Buzzetti et al. [45] observed that IA-2A was the only islet-cell autoantibody appearing by increasing the degree of BMI in obese subjects with T2DM. Similarly, Maddaloni et al. [5] pointed out that the positivity for IA-2A only was related to a clinical phenotype closer to T2DM, whereas the concurrent positivity for both IA-2A and GADA was linked to clinical features closer to classical T1DM.
However, the debate remains whether specific autoantibody profiles correlate with significant differences in demographic and/or clinical parameters among LADA patients [4]. Moreover, conflicting data are available regarding the correlation between GADA titre and the time interval between diagnosis and insulin requirement [17265051].
LADA AND DIAGNOSTIC CHALLENGES
To identify patients affected by LADA, islet autoantibodies should be theoretically tested in all cases of newly diagnosis T2DM. In fact, the early identification of LADA as well as a personalized therapeutic approach might be essential in order to slow down the autoimmunity process and preserve β-cell function.
However, no general recommendations on islet antibodies testing for adult-onset diabetes are available right now. Currently, these assays are performed by physicians only if a strong suspect of LADA exists, usually on the basis of a normal or low BMI. From this perspective, normal-weight individuals are considered as potentially having LADA and may undergo immunological assays, whereas overweight and obese adults are supposed to have T2DM and are not investigated [52]. Unfortunately, this practice does not take into account the evidence that LADA can also occur in subjects with elevated BMI [4202628]. On the other hand, performing full autoantibody panel may not always be achievable because of high costs and difficulties in interpreting the results if carried out indiscriminately. As a consequence, still remains an issue what is the most proper strategy to identify diabetic patients who have an increased risk of LADA and need to be tested for DAAs. In this respect, authors suggest that the evaluation of C-peptide levels as well as the use of specific risk scores based on clinical parameters may be more cost-effective and should be considered before requiring autoantibody screening tests in all patients newly-diagnosed for diabetes (Fig. 2) [15].
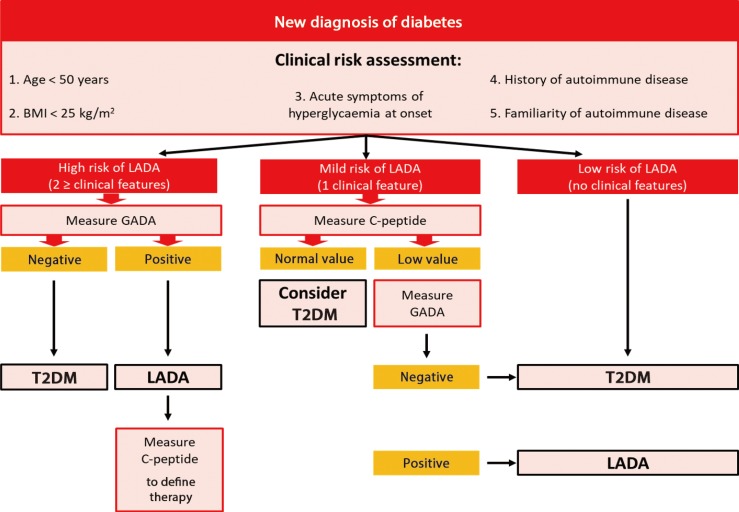
Proposal of algorithm for diagnosis latent autoimmune diabetes in adults (LADA). BMI, body mass index; GADA, glutamic acid decarboxylase autoantibody; T2DM, type 2 diabetes mellitus.
The role of clinical screening in diagnosing LADA
A clinically oriented approach is essential to identify patients with diabetes who have a high likelihood of LADA and therefore need islet-cell autoantibodies screening. However, reliable risk scores based on clinical parameters are still not established and most part of physicians suspect autoimmune diabetes only on the basis of age and BMI.
In a cohort-study the application of a screening tool, based on three clinical features (normal or low BMI; poor glycaemic control in spite of adequate compliance to treatment; weight loss during constant diet), allowed the identification of 75% of patients with LADA among a cohort of subjects with adult-onset diabetes [53]. Similarly, Fourlanos et al. [52] developed a “LADA clinical risk score” based on five clinical features which were found to be significantly more frequent in autoimmune diabetes compared with T2DM at diagnosis. These parameters included: age of onset <50 years; acute symptoms of hyperglycaemia before diagnosis; BMI <25 kg/m2; personal and family history of autoimmune disease. In this study, the authors demonstrated that the presence of at least two clinical features at diagnosis has 90% sensitivity and 71% specificity for detecting LADA, whereas a negative predictive value of 99% was detected for LADA clinical risk score <2. Taking together these data suggest that a systematic application of clinical risk score increases the efficiency of a screening programme for LADA. However, due to the broad heterogeneity of LADA, islet-cell antibodies measurement remains essential in order to decrease the number of misdiagnosis of diabetes.
The role of C-peptide in diagnosing LADA
C-peptide is mostly undetectable in classical T1DM and normal or high in patients with newly diagnosed T2DM, whereas individuals with LADA tend to have low but still detectable C-peptide values at the time of diagnosis. Furthermore, stimulated C-peptide levels are generally higher at all time-points following a mixed-meal tolerance test in LADA than in classical T1DM [13].
As a marker of endogenous insulin production and of the autoimmune process, C-peptide could be measured to differentiate LADA from T2DM [5455]. Bell and Ovalle [56] evaluated serum C-peptide in subjects with LADA and T2DM, demonstrating significant lower levels of C-peptide in patients affected by LADA than in those affected by T2DM. Similar findings emerged from both the NIRAD Study [20] and the Action LADA 9 [13]. These results demonstrate that in patients presenting with adult-onset diabetes, LADA can be ruled out by the presence of elevated C-peptide levels. In fact, since testing for islet autoantibodies may not always be indicated because of elevated costs, C-peptide measurement for screening purposes may be more cost-effective. Thus, islet autoantibodies screening, especially GADA, should be required as a second step for patients with adult-onset diabetes showing low serum C-peptide.
TREATMENT STRATEGIES FOR LADA
Even though well-established guidelines exist for the treatment of classical T1DM, there are only few data regarding therapy for LADA and no clear management strategy has been defined yet. To date, the therapeutic approach for people affected by LADA is extremely heterogeneous, depending on the clinical intuition and knowledge of the caregiver. Moreover, due to misdiagnosis, these patients are often treated with therapies commonly used in T2DM, which might further worsen the autoimmune process and accelerate β-cell loss, leading to a faster progression toward insulin dependency [15] especially in subjects with high-titre GADA who show clinical features closer to T1DM than T2DM [557]. Considering the autoimmune response in LADA, which generally lead to the β-cell impairment, insulin therapy should ideally represent the treatment of choice in these subjects. However, open debate remains whether diabetic patients positive for islet autoantibodies should be treated with insulin irrespective of the insulin requirement and predictive markers of insulin requirement among LADA patients who are insulin independent should be carefully considered. In fact, although the evidence that insulin therapy support β-cell function [5859], recent studies suggest that some hypoglycemic agents could play an important role in slowing down β-cell loss (Table 2). Thus, a personalized therapeutic strategy for LADA patients should be implemented in order to preserve residual β-cell function and reduce the risk of long term complications [60].
Insulin therapy
Insulin administration is essential in all cases of undetectable C-peptide, representing the only weapon to replace the β-cell insulin secretion in T1DM. However, people with LADA show slower progression towards absolute insulin dependency as demonstrated by higher C-peptide at the onset of the disease. Thus, the major issue remains whether insulin therapy is really indicated as initial treatment for LADA patients.
Most studies agree that insulin intervention is effective and safe for patients affected by LADA with residual β-cell function [61]. Results from preclinical studies suggest that the administration of exogenous insulin supports β-cell function and decreases the severity of insulitis [59] both because reduction of the glucotoxicity [58] and also suppression of islet-cell activity. Other findings support the hypothesis that the exposure to exogenous insulin favors the shift from Th1 to a Th2 immune response as well as the activation of insulin-specific regulatory T-cells (T-regs) [6263]. Furthermore, insulin therapy seems to suppress autoreactive T-cells through local release of regulatory cytokines [64]. To date, according to the outcomes of large randomized clinical trials [65], physicians tend to prescribe insulin therapy at an earlier stage in patients with LADA, regardless C-peptide levels and residual β-cell function. However, further studies are needed to clarify the pathophysiological basis of the effect of insulin therapy on β-cells.
Sulfonylureas
Two randomized controlled trials conducted in Japan compared glibenclamide and insulin therapy in patients with SPIDDM. In one pilot study the assay of stimulated C-peptide demonstrated that subjects treated with glibenclamide showed faster impairment of β-cell function compared with the insulin treated group at 30 months follow-up [9]. This finding was confirmed by the Tokyo Study [66], that evaluated 4,089 non-insulin-dependent patients affected by SPIDDM with a duration of disease ≤5 years. In this multicenter, randomized, non-blinded clinical trial, Maruyama et al. [66] demonstrated that the progression rate to an insulin-dependent state in the insulin group was lower than that observed in the sulfonylurea group, whereas C-peptide values during the oral glucose tolerance test were better preserved in patients treated with insulin. These data sustain the hypothesis that sulfonylureas might stimulate β-cell and potentially enhance the antigen expression of β-cells, exacerbating the autoimmune process. Therefore, it is recommended that sulfonylureas should be avoided in patients with LADA/SPIDDM [66].
Insulin sensitizers
As previously discussed, LADA shows a wide clinical phenotype, ranging from prevalent insulin deficiency to variable degrees of insulin resistance. Thus, insulin sensitizers might be helpful in subjects with LADA who share more pronounced insulin-resistant features.
In clinical setting, metformin is prescribed by physicians in order to reduce insulin resistance in selected cases of autoimmune diabetes. In fact, data showed that metformin added to insulin treatment results in significant reduction of daily insulin requirement and body weight in classical T1DM [67]. However, there are no controlled studies on the effects of metformin alone in LADA [65]. Only rosiglitazone has been investigated among LADA patients, suggesting the potential benefits of its use in preventing β-cell function [68], even though further studies are needed.
New pharmacological approaches to preserve β-cell function in LADA
Clinical management of LADA should aim to preserve β-cell function. Thus, a great effort has been made nowadays to elucidate what therapeutic approach might slow down β-cells loss in LADA subjects. In respect to this a great deal of interest is arising for in some drugs currently used for treatment of T2DM. These agents include dipeptidyl peptidase 4 (DPP-4) inhibitors and glucagon-like peptide-1 receptor agonists (GLP-1RAs). Recent evidences sustain their use in both childhood-onset and adult-onset autoimmune diabetes [65]. However further studies are requested in order to elucidate the role of these hypoglycemic agents.
DPP-4 inhibitors
DPP-4 inhibitors have been widely investigated in T2DM, providing evidence of a protective role on islet β-cells [697071]. With respect to LADA, interesting findings emerge from three recent trials, testing the role of sitagliptin, linagliprin, and saxagliptin in preserving β-cell function. A prospective study carried out in China [72] showed that treatment with Sitagliptin in addition to insulin glargine preserved C-peptide better than insulin alone in patients with LADA over a 1-year period. Similarly, Johansen et al. [73] reported that linagliptin slowed down the decline of C-peptide levels in LADA after a 2-year study period, whereas Buzzetti et al. [74] found that saxagliptin improved glycemic control and C-peptide secretion at 24 weeks follow-up in a post hoc analysis of five randomized, placebo-controlled studies.
Glucagon-like peptide-1 receptor agonist
Encouraging results of the use of GLP-1RA as an add-on therapy to insulin have been proved for overweight adult patients with T1DM. In a randomized, double-blind, placebo-controlled trial, Dejgaard et al. [75] observed that the administration of liraglutide as an add-on therapy to insulin for overweight adult patients with T1DM, was associated with reductions in hypoglycemic events and daily insulin requirement. Taking account the relationship between GLP-1RA therapy and its effect on β-cells, data available suggested that this pharmacological class reduces β-cell apoptosis and promotes β-cell neogenesis in animal models [76]. Nonetheless, no randomized controlled trials on the role of the GLP-1RA in preserving β-cell function in LADA are available. However, in a post hoc analysis of data pooled from three randomized phase 3 trials, we demonstrated a significant reduction of HbA1c and increase of β-cell function in GAD antibody positive individuals treated with dulaglutide, without effects on the rate of hypoglycemia, over a 12-month observation period [77]. To the best of our knowledge, this study is the first to demonstrate that Dulaglutide is an effective anti-hyperglycemic treatment in LADA. In contrast, in a subgroup analysis of an observational study, Jones et al. [78] found a poorer glycemic response to exenatide or liraglutide therapy among a group of 19 patients positive for GADA and/or IA-2 autoantibodies who had low fasting C-peptide levels (≤0.25 nmol/L) compared to T2DM group. However these findings are limited by the small size of LADA group. Moreover, the potential benefits of GLP-1RAs in terms of sustaining β-cell function has not been excluded, as suggested by the reduction in daily insulin requirement in both LADA and T2DM subjects [78].
On this basis, it can be supposed that DPP-4 and GLP-1RAs might have a significant therapeutic role in changing the natural history of LADA. However, before these therapies can be routinely used in clinical practice, large prospective randomized trials are required to clearly demonstrate whether these adjunct therapies translate into a reduced progression to insulin dependence and diabetic long-term complications [74].
CONCLUSIONS
Adult-onset autoimmune diabetes encompasses a broad spectrum of clinical and metabolic features, ranging from prevalent insulin resistance to prevalent insulin deficiency, probably due to differences in genetic and immunological factors [111213]. Patients affected by LADA show mid-way features between T1DM and T2DM. Although adults with high titre of GADA are phenotypically closer to those with classical T1DM than T2DM [20], an overlap exists between diabetic subjects and makes difficult to distinguish LADA from T2DM just on the basis of clinical features. As a consequence, this condition continues to be unnoticed in clinical setting and a high misdiagnosis rate (5% to 10%) remains among patients with T2DM [2]. Thus, despite some studies suggest that the systematic use of clinical risk score increases the efficiency of a screening programme for LADA [52], testing for islet-cell autoantibodies remains essential for not missing a correct diagnosis, therefore routine GADA screening should be considered [2]. However, since testing for islet-cell autoantibodies may not always be indicated because of high costs, C-peptide measurement may be a useful tool to rule out diagnosis of LADA in case of low clinical suspicion. Another outstanding issue concerns treatment strategy. Thus, due to the wide heterogeneity among patients with LADA it is difficult to establish an a priori algorithm for treatment [15] and a tailor-made therapeutic approach is needed to improve glycaemic control and insulin sensitivity, taking into account clinical and biochemical features of each patient. To date, evidence shows that patients with LADA should be treated with insulin at an earlier stage [966], whereas sulfonylureas are discouraged due to their effects on β-cells [66]. However, there is a wider window of other therapeutic interventions that may be of some clinical benefits when added to insulin treatment. Insulin sensitizers have been shown to be helpful in subjects with autoimmune diabetes who share more pronounced insulin-resistant traits [79]. Moreover, as LADA subjects generally show some degree of residual β-cell function, treatment should aim at both protect and stimulate β-cell regeneration. Thus, combined therapies to target different pathways could be a proper strategy. Based on the newest evidences, DPP-4 inhibitors and GLP-1RAs might be used in LADA as an add-on therapy to insulin to sustain residual β-cell function, especially in patients with a relative maintenance of C-peptide secretion [7477]. Therapeutic strategy in LADA should focus on the preservation of residual β-cell function as long as possible since its preservation is associated with reduction of long-term diabetic complications [15]. Further studies in this field of interest are strongly encourage.
Notes
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.