Higher Prevalence and Progression Rate of Chronic Kidney Disease in Elderly Patients with Type 2 Diabetes Mellitus
Article information
Abstract
Background
To evaluate the prevalence of chronic kidney disease (CKD) and progression rate to CKD in elderly patients with type 2 diabetes mellitus (T2DM).
Methods
We investigated the medical records of 190 elderly patients (65 years or older) with T2DM from 2005 to 2011 in 6-month increments. Mean follow-up duration was 64.5 months. CKD was defined as estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2 and/or the presence of albuminuria.
Results
The mean age was 70.4 years and mean diabetes duration was 10.6 years. Among all the participants, 113 patients (59.5%) had CKD. The eGFR was significantly decreased between baseline (65.7±15.0 mL/min/1.73 m2) and the end of follow-up (52.7±17.5 mL/min/1.73 m2, P<0.001). At the end of follow-up, the prevalence of eGFR <60 mL/min/1.73 m2 had increased by 61.6% (at baseline, 44.2%). Furthermore, in patients with eGFR ≥60 mL/min/1.73 m2, the progression rate to more than CKD stage 3 was 39.6% at the end of follow-up; 30.2% of elderly diabetic patients had progressed to albuminuria from normoalbuminuria. Kaplan-Meier analysis showed that the time interval to worsening nephropathy was significantly shorter in elderly patients with diabetes duration ≥10 years than in those with diabetes duration <5 years (P=0.018).
Conclusion
CKD was commonly observed in older patients with T2DM, and the progression rate to CKD is also high. Consequently, it is important to identify and manage CKD as early as possible in elderly patients with T2DM, especially in those with diabetes duration ≥10 years.
INTRODUCTION
Chronic kidney disease (CKD) is a global public health problem, and its prevalence is gradually increasing, mainly due to an increase in the number of patients with type 2 diabetes mellitus (T2DM) [123]. CKD develops in approximately 35% of patients with T2DM and is associated with increased mortality [456]. In addition, CKD is one of several conditions that are common in older people and are associated with an increased risk of cardiovascular disease [678]. Because renal function declines naturally with aging, CKD develops more frequently in older people [910]. According to data from the Korean National Health and Nutrition Examination Survey in 2005 and 2007, CKD prevalence reached 22.6% in participants aged 60 to 74 years and 44.5% in those aged 75 years or more [11]. Furthermore, elderly patients with T2DM have higher CKD prevalence than those without T2DM [12131415]. The prevalence of CKD in older diabetic patients (aged ≥65 years) increased from 27.3% between 1988 and 1994 to 40.6% between 2009 and 2014, based on the National Health and Nutrition Examination Survey (NHANES) [14]. A Spanish study has shown that CKD prevalence in older patients with T2DM (mean age, 67.9 years) controlled in primary care was 34.6% [15].
Well-documented risk factors for CKD are advanced age, obesity, hypertension, and fasting glucose in patients with T2DM [16171819]. However, most previous studies were not conducted in the older population. Older patients with T2DM have a high risk of CKD not only because of their age but also because they have diabetes [16202122]. Therefore, older diabetic patients without CKD have a higher risk of progression to CKD than do young patients. Nevertheless, there is limited data on the prevalence of CKD and progression rate to CKD in older patients with T2DM. The aim of this study is to evaluate the prevalence of CKD and the progression rate to CKD in older patients with T2DM.
METHODS
Participants and design
A retrospective cohort study was conducted by reviewing the medical records of older patients with T2DM who visited the diabetes clinic at the CHA Bundang Medical Center, Seongnam, Korea. The study population included T2DM patients ≥65 years of age who had an estimated glomerular filtration rate (eGFR) ≥30 mL/min/1.73 m2. We included the patients who had visited the clinic at least one time every 6 months from the baseline visit. Baseline visit period was from January 1, 2005, to December 31, 2005. We excluded subjects who had type 1 diabetes mellitus (T1DM), acute disease, known inherited kidney diseases, cancer, or acute renal failure caused by other circumstances (for example, drug use, contrast media, and so on). Follow-up ended on December 31, 2011, or earlier if either death or follow-up loss occurred. Among 433 older patients with T2DM, we enrolled 190 patients who visited regularly after the baseline visit. Both at baseline and at follow-up, anthropometric assessments, blood pressure, and laboratory tests were reviewed in all study participants. During the study, eGFR was calculated using the modification of diet in renal disease (MDRD) Study equation every 6 months. This study was approved by the CHA University Institutional Review Board (2016-12-041), and informed consent was obtained from all study participants.
Measurements and definitions
Body weight and height were measured in participants wearing a hospital gown and without shoes on a calibrated balance beam scale and stadiometer, respectively. Blood pressure was measured using a mercury sphygmomanometer while the subjects were seated. All blood samples were obtained after an overnight fast of at least 8 hours, and fasting plasma glucose, total cholesterol, triglyceride, high density lipoprotein cholesterol, and creatinine levels were measured. Albuminuria was assessed only once in a spot morning urine sample. Glycosylated hemoglobin (HbA1c) was analyzed using high-performance liquid chromatography (Variant II; Bio-Rad, Hercules, CA, USA).
CKD staging was done according to the Kidney Disease Outcomes Quality Initiative guidelines of National Kidney Foundation [23]. Stages of CKD were defined as follows: stage 1 (eGFR ≥90 mL/min/1.73 m2); stage 2 (eGFR 60 to 89 mL/min/1.73 m2); stage 3 (eGFR 30 to 59 mL/min/1.73 m2); stage 4 (eGFR 15 to 29 mL/min/1.73 m2); and stage 5 (eGFR <15 mL/min/1.73 m2). Albuminuria was defined by the urinary albumin-to-creatinine ratio as microalbuminuria (30 to 300 mg/g Cr) or macroalbuminuria (>300 mg/g Cr). CKD was defined as eGFR <60 mL/min/1.73 m2 and/or the presence of albuminuria.
The renal outcome was worsening nephropathy, defined as death from renal disease; the initiation of renal-replacement therapy; progression to macroalbuminuria; or a doubling of the serum creatinine level, accompanied by an eGFR of <45 mL/min/1.73 m2; or worsening of CKD stage at the end of follow-up than at baseline.
Statistical analysis
Data for continuous variables is presented as mean±standard deviation, and categorical factors are reported as percentages. The significance of differences in measurements among groups was tested using the independent sample t-test, paired t-test, or Mann-Whitney U test for continuous measures and chi-square tests for categorical measures. The odds ratio and 95% confidence interval were calculated using logistic regression analysis with or without adjustment for age, sex, body mass index (BMI), hypertension, diabetes duration, and HbA1c. Kaplan-Meier analysis and the log rank test were used to compare the difference in cumulative renal survival. A P<0.05 was considered significant. All statistical analyses were performed using IBM SPSS Statistics version 19.0 (IBM Co., Armonk, NY, USA).
RESULTS
The baseline characteristics of the study population according to the presence of CKD are presented in Table 1. Among the 190 elderly patients with T2DM, 113 patients (59.5%) had CKD at baseline. The mean age was 70.4 years and mean diabetes duration was 10.6 years. Although the average duration of diabetes was longer in patients with CKD than in those without CKD, mean HbA1c was not different in both groups.

Baseline characteristics of the study population according to the presence of chronic kidney disease at baseline
Duration of diabetes was the independent risk factor for CKD in older patients with T2DM (Table 2). Age, sex, BMI, hypertension, and HbA1c were not associated with the presence of CKD in these patients. After adjustment for age, sex, BMI, hypertension, diabetes duration, and HbA1c, longer duration of diabetes was still associated with CKD.
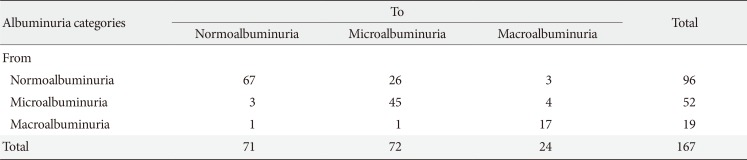
Mean follow-up duration was 64.5 months, and eGFR significantly declined between baseline (65.7±15.0 mL/min/1.73 m2) and the end of follow-up (52.7±17.5 mL/min/1.73 m2, P<0.001) (Fig. 1). The annual rate of GFR decline was 2.42 mL/min/1.73 m2. Table 3 shows the transition for the stage of CKD from baseline to the end of follow-up. At the end of follow-up, the prevalence of eGFR <60 mL/min/1.73 m2 had increased by 61.6% (117/190; at baseline, 44.2% [84/190]). The stage of CKD was aggravated in one-third of patients (31.6%, 60/190). Furthermore, in patients with eGFR ≥60 mL/min/1.73 m2, the progression rate to more than CKD stage 3 was 39.6% (42/106) at the end of follow-up. In addition, 30.2% (29/96) of older diabetic patients had progressed to albuminuria from normoalbuminuria (Table 4). The progression rate to macroalbuminuria was 4.7% (7/148) in older patients with T2DM at the end of follow-up.
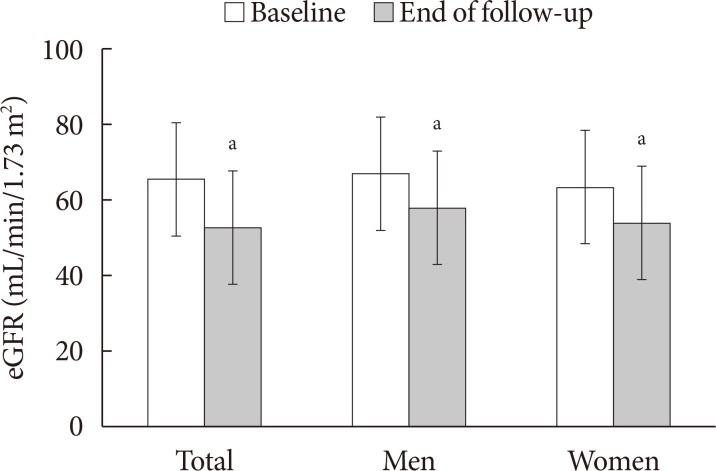
Comparison of estimated glomerular filtration rate (eGFR) from baseline to the end of follow-up. aP<0.05.

Transitions for stage of chronic kidney disease from baseline to the end of follow-up in older patients with type 2 diabetes mellitus

Transitions for albuminuria categories from baseline to the end of follow-up in older patients with type 2 diabetes mellitus
Worsening nephropathy presented in 39.5% (75/190) of older diabetic patients; death from renal disease (n=11); the initiation of renal replacement therapy (n=4); progression to macroalbuminuria (n=7) or a doubling of the serum creatinine level, accompanied by an eGFR of <45 mL/min/1.73 m2 (n=9); worsening of CKD stage at the end of follow-up than at baseline (n=60) all also occurred. Kaplan-Meier analysis showed that the time interval to worsening nephropathy was significantly shorter in older patients with diabetes duration ≥10 years than in those with diabetes duration <5 years (diabetes duration <5 years vs. ≥10 years, P=0.018) (Fig. 2). Sex, the presence of obesity or hypertension, and HbA1c level were no different for cumulative renal survival among older diabetic patients.
DISCUSSION
Increased life expectancy and a higher T2DM incidence lead to an increase in the number of older diabetic patients with CKD [123]. In patients with T2DM, CKD significantly increases cardiovascular morbidity and mortality, and it is the main reason for renal replacement therapy [45678910]. Despite the high number of older diabetic patients with CKD, few studies have investigated the prevalence of CKD and progression rate to CKD in this population. In this study, we show that CKD was commonly observed in older patients with T2DM, and the progression rate to CKD was also high. Furthermore, older diabetic patients with diabetes duration ≥10 years had a high risk for worsening nephropathy.
The prevalence and incidence of CKD in patients with T2DM have increased over the past two decades. The prevalence of CKD was 19.8% in participants aged 65 years or older, and it is very high compared with 0.7% in those aged 35 to 44 years in urban Korea [18]. In Taiwan, an observational cohort study reported that 39.4% of older patients had CKD, stage 3 to 5 [24]. The mean age was 75.7 years, and only 30.8% of them had hyperglycemia. We could presume that older patients with diabetes develop higher CKD prevalence than those without diabetes. Comparatively, CKD prevalence was 30.3% in diabetic patients aged 30 years or more and 39.0% in those aged 65 years or more in Korea [25]. According to the data from NHANES, the prevalence of CKD in older diabetic patients (aged ≥65 years) increased from 27.3% between 1988 and 1994 to 40.6% between 2009 and 2014 [14]. In this data, the prevalence of patients who had diabetes duration ≥10 years also increased from 23.6% between 1988 and 1994 to 37.7% between 2009 and 2014 even though this prevalence was calculated in all age groups. In present study, the prevalence of CKD was 59.5% in older patients with T2DM (mean age, 70.4 years). The reason a higher CKD prevalence was observed than in previous studies might be that this study included many older diabetic patients who already had diabetes longer than 10 years at baseline. Although our study sample was not large and representative of the general Korean population, we could know that CKD was commonly observed in older patients with T2DM.
In general, the progression rate to CKD is slow, although there is a high prevalence of CKD in older patients [2627]. In an observational study including 4,562 patients older than 65 years at US Veterans Affairs health care facilities, only 44% of the patients progressed at a rate of 1 to 4 mL/min/1.73 m2 per year [28]. In another study, the annual rate of GFR decline was only 0.8 to 1.4 mL/min/1.73 m2 in nondiabetic patients older than 65 years [29]. However, there are few studies of the progression rate to CKD in older patients with T2DM. In this study of older diabetic patients, eGFR declined at a rate of 2.42 mL/min/1.73 m2 per year among all participants, and the stage of CKD was aggravated in one-third of patients (31.6%, 60/190). In addition, in patients with eGFR ≥60 mL/min/1.73 m2, the progression rate to more than CKD stage 3 was 39.6% (42/106) at the end of follow-up; 30.2% (29/96) of older diabetic patients progressed to albuminuria from normoalbuminuria. Compared to the slow progression to CKD in nondiabetic older patients, the faster decline of renal function would be due to T2DM. This is important because when we manage older patients with T2DM, we should pay attention to their renal function.
Old age, obesity, hypertension, and diabetes mellitus are traditional risk factors for CKD [16171819]. In this study, longer diabetes duration was an independent factor related to the presence of CKD in older diabetic patients. Furthermore, diabetes duration ≥10 years was still associated with worsening nephropathy at the end of follow-up. No similar study was conducted with older diabetic patients, but cumulative incidence of kidney complications such as macroalbuminuria and end-stage renal disease increased with longer duration of diabetes during a 25-year follow-up of patients with T1DM [30]. In addition, being diagnosed with T1DM before 6 years of age carries a lower risk of kidney disease development, but this relationship diminished with longer diabetes duration. It is not certain, but we could assume that longer duration of diabetes accumulates renal damage regardless of glucose level.
Interestingly, HbA1c is not associated with CKD at baseline and worsening nephropathy at the end of follow-up. In the UK Prospective Diabetes Study, a modest decrease in HbA1c over 10 years from 7.9% to 7.0% lowered the risk of microvascular endpoints, reducing the onset of microalbuminuria by 25% [31]. However, the study population was younger patients with newly diagnosed T2DM (mean age, 54 years). Until now, there have been no long-term randomized studies that show the benefits of intensive blood glucose control in the older population. Because the benefit of controlling blood glucose for CKD and its progression would be greater in those younger and recently diagnosed with T2DM than in older patients with T2DM [10], HbA1c difference would not have influenced CKD and its progression in this study (mean age, 70.4 years; mean diabetes duration, 10.6 years at baseline). Old age and T2DM itself are strong risk factors for CKD, so the difference might not appear to depend on HbA1c.
In addition, hypertension is also not associated with CKD at baseline and worsening nephropathy at the end of follow-up. One possible explanation is that angiotensin-converting enzyme inhibitor (ACEI) or angiotensin II receptor blocker (ARB) has been used more than in other studies. Compared to 24.4% of older diabetic patients who have used ACEI or ARB between 1988 and 1994 and 56.2% between 2009 and 2014 according to the data from NHANES [14], 61.6% of patients in this study have used ACEI or ARB. Another possible reason would be that over 70% of patients already had hypertension, so it would not have influenced CKD and its progression.
Renal blood flow and GFR would be diminished by time [32]. Pathologically, the aging kidney may be associated with changes in basement membrane thickening and mesangial expansion [3]. In addition, because they commonly have age-related, coexisting conditions such as vasculitis, amyloidosis, and atherosclerotic disease, older persons tend to high risk for CKD [9]. The mechanism of the progression to CKD in patients with diabetes is still controversial. Hyperactivity of the renin-angiotensin-aldosterone (RAS) system, osmotic sodium retention, endothelial dysfunction, dyslipidemia, RAS/RAF/extracellular-signal-regulated kinase pathway, modification of the purinergic system, phosphatidylinositol 3-kinase (PI 3-kinase)–dependent signaling pathways, and inflammation were possible pathways that have been identified so far [33]. Without doubt, however, aging and T2DM are strong risk factors for deterioration of renal function.
The therapeutic strategy for T2DM in older patients should consider CKD, which is a major prognostic factor [34]. It is well known that morbidity and mortality in older patients with T2DM could be reduced by preventing the progression to CKD [456]. However, it is not easy to prevent the progression to CKD in such patients because they are exposed to polypharmacy to control their comorbidities. The problems of polypharmacy are overdosing caused by drug-drug interaction or reduced clearance of drugs. In this study, so many older patients with T2DM had CKD; we should pay attention to their renal function. It is very important for physicians not only to monitor the renal function regularly and adjust the dose of drugs but also to prevent worsening of renal function when they manage older diabetic patients with CKD, especially in those who had diabetes longer than 10 years.
Our study has some limitations. First, the study sample was not large and representative of the Korean population. Therefore, the results should be interpreted with caution. Second, the MDRD equation has not been validated in patients >70 years old, and no coefficient of the MDRD equation has yet been published for Koreans. This limitation might render CKD prevalence results imprecise. Third, we did not compare older patients with younger patients because we could not find a matched young-patient group. Nevertheless, this study might be valuable in presenting the finding that CKD was commonly observed in older patients with T2DM and that the progression rate to CKD was also high compared to the general population. Fourth, because this study included only the Korean population, the results cannot be applied directly to other ethnic populations. Finally, we could not analyze and reflect HbA1c variability and medication change during the follow-up period due to missing data. Large-scale prospective study would be warranted. However, this study might be meaningful because it evaluated renal function in older patients with T2DM and monitored them for up to 6 years.
Because CKD was commonly observed in older patients with T2DM, and progression rate to CKD was also high, it is important to identify and manage CKD as early as possible in older patients with T2DM, especially in those whose diabetes duration was longer than 10 years.
ACKNOWLEDGMENTS
We thank all the study participants for their contribution to this study.
Notes
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.