Glycated Albumin Is a More Useful Glycation Index than HbA1c for Reflecting Renal Tubulopathy in Subjects with Early Diabetic Kidney Disease
Article information
Abstract
Background
The aim of this study was to investigate which glycemic parameters better reflect urinary N-acetyl-β-D-glucosaminidase (uNAG) abnormality, a marker for renal tubulopathy, in subjects with type 2 diabetes mellitus (T2DM) subjects with normoalbuminuria and a normal estimated glomerular filtration rate (eGFR).
Methods
We classified 1,061 participants with T2DM into two groups according to uNAG level—normal vs. high (>5.8 U/g creatinine)—and measured their biochemical parameters.
Results
Subjects with high uNAG level had significantly higher levels of fasting and stimulated glucose, glycated albumin (GA), and glycosylated hemoglobin (HbA1c) and lower levels of homeostasis model assessment of β-cell compared with subjects with normal uNAG level. Multiple linear regression analyses showed that uNAG was significantly associated with GA (standardized β coefficient [β]=0.213, P=0.016), but not with HbA1c (β=−0.137, P=0.096) or stimulated glucose (β=0.095, P=0.140) after adjusting confounding factors. In receiver operating characteristic analysis, the value of the area under the curve (AUC) for renal tubular injury of GA was significantly higher (AUC=0.634; 95% confidence interval [CI], 0.646 to 0.899) than those for HbA1c (AUC=0.598; 95% CI, 0.553 to 0.640), stimulated glucose (AUC=0.594; 95% CI, 0.552 to 0.636), or fasting glucose (AUC=0.558; 95% CI, 0.515 to 0.600). The optimal GA cutoff point for renal tubular damage was 17.55% (sensitivity 59%, specificity 62%).
Conclusion
GA is a more useful glycation index than HbA1c for reflecting renal tubulopathy in subjects with T2DM with normoalbuminuria and normal eGFR.
INTRODUCTION
Diabetic kidney disease (DKD) is the leading cause of chronic kidney disease (CKD), which results in end-stage renal disease in many countries. DKD is also a main cause of diabetes-related morbidity and mortality. Therefore, earlier adoption of methods to estimate CKD risks along with a more accurate glycemic index for patients with type 2 diabetes mellitus (T2DM) is highly desirable and could guide physicians in limiting development and progression of DKD. Among the known CKD and DKD parameters, albuminuria and estimated glomerular filtration rate (eGFR) have traditionally been considered the best markers of renal glomerular injury and function, respectively. However, many patients with diabetes and low eGFR do not secrete significant amounts of albuminuria, and an eGFR decrease frequently precedes development of microalbuminuria. This indicates that change in the glomerulus might be neither the initial step in DKD development nor the major determinant of renal prognosis in subjects with T2DM. It is now increasingly recognized that tubules play an important role in DKD pathogenesis [1]. One of the widely used tubular injury markers is N-acetyl-β-D-glucosaminidase (NAG), a lysosomal enzyme of renal proximal tubular epithelial cells. Previous studies have demonstrated that urinary NAG (uNAG) excretion is also elevated in patients with diabetes who still have normoalbuminuria and normal eGFR, which is consistent with the view that proximal tubular injury might be a measurable component of early DKD [23]. Furthermore, we previously demonstrated that stimulated glucose and glycated albumin (GA), an early Amadori glycated protein of the nonenzymatic glycation reaction between glucose and serum albumin, might be associated with diabetic renal tubulopathy, as assessed by uNAG [4]. GA is a well-established glycemic index for reflecting glycemic excursions and postprandial hyperglycemia compared with glycosylated hemoglobin (HbA1c) [56]. Furthermore, GA is known to contribute to increased oxidative stress in patients with diabetes since glycation of albumin impairs albumin's antioxidant activities [7]. Considering the crucial effects of both glycemic excursion and oxidative stress on renal damage, we hypothesize that GA is a better reflector of early DKD than HbA1c. However, few studies have investigated the associations between glycemic parameters and renal tubular damage in subjects with early-stage DKD.
Herein, we investigated the associations between various glycemic control indices and DKD markers, including the tubular index of uNAG, in T2DM subjects with normoalbuminuria and normal eGFR. Additionally, we compared GA with other surrogate markers of glycemic parameters, such as HbA1c, fasting plasma glucose, and stimulated glucose in terms of their ability to predict early tubular dysfunction.
METHODS
Study subjects
The present study was a retrospective investigation of patients with T2DM who visited Severance Hospital Diabetes Center between March 2015 and November 2016, Wonju Severance Christian Hospital Diabetes Center between October 2016 and April 2017, or Samsung Medical Center Diabetes Center between February 2016 and November 2016. All patients were tested for serum GA, HbA1c, and uNAG. They all underwent a standardized liquid-meal test. Participants who met the following criteria were excluded: <20 years of age, having type 1 diabetes mellitus (T1DM), taking a sodium-glucose cotransporter 2 inhibitor, pregnancy, liver cirrhosis, urine albumin-to-creatinine ratio (ACR) ≥30 mg/g, or eGFR <60 mL/min/1.73 m2. A total of 1,061 participants with T2DM were finally recruited. The Institutional Review Board at Severance Hospital approved this study protocol (4-2017-0667). Written informed consent was waived because the database was accessed only for analysis purposes and personal information was not used.
Measurement of blood glucometabolic parameters
After the subjects fasted overnight, blood samples were collected before (0 minute, designated as basal) and after (90 minutes, designated as stimulated) participants ingested two cans (total 400 mL, 400 kcal, 18 g fat, 44 g carbohydrate, and 20 g protein) of a standardized mixed meal (Mediwell Diabetic Meal; Meail Dairies Co., Yeongdong, Korea) to measure glucose and insulin/C-peptide levels and to perform other chemistry tests. The homeostasis model assessment of β-cell (HOMA-β) was calculated as 20×fasting insulin level (mIU/mL)/[fasting plasma glucose (mg/dL)−3.5]. The homeostasis model assessment of insulin resistance (HOMA-IR) was calculated as fasting plasma glucose (mg/dL)×fasting insulin (mIU/mL)/22.5 [8]. The eGFR was derived from the Chronic Kidney Disease Epidemiology Collaboration equation [9]. HbA1c was measured by immunoassay using an Integra 800 CTS (Roche, Hercules, CA, USA). Serum GA level was determined by an enzymatic method (Lucica GA-L; Asahi Kasei Pharma Co., Tokyo, Japan) using a Hitachi 7600 autoanalyzer (Hitachi Ltd., Tokyo, Japan). Serum glucose and creatinine were also measured using the Hitachi 7600 analyzer. For serum creatinine, a compensated kinetic Jaffe method (Clinimate CRE; Sekisui Medical Co. Ltd., Tokyo, Japan) was used in which the creatinine level was standardized by isotope dilution mass spectrometry. Serum insulin and C-peptide were measured with an electrochemiluminescence immunoassay (Cobas® e601 analyzer; Roche Diagnostics, Basel, Switzerland).
Measurement of urinary glomerular and tubular damage markers
Urinary NAG, albumin, glucose, and creatinine levels were measured from the fasting morning spot urine sample obtained from each participant. Urinary NAG, albumin, and glucose levels were expressed as uNAG-to-creatinine ratio and ACR to minimize the influence of variance in kidney function. Urinary NAG activity was considered abnormal when >5.8 U/g creatinine [10]. Urinary NAG was measured using a reagent from Nittobo Medial Co. Ltd. (Tokyo, Japan) and a JCA-BM 6010/c automated chemistry analyzer (JEOL Ltd., Tokyo, Japan). Urine-albumin level was measured via the immunoturbidimetric method using an AU680 automated chemistry analyzer (Beckman Coulter Inc., Brea, CA, USA). Urine-creatinine level was also measured with an AU680 analyzer using the kinetic Jaffe method.
Statistical analysis
A normality test was performed on all continuous variables. The data are presented as mean±standard deviation for normally distributed continuous variables and median (interquartile range) for non-normally distributed continuous variables. Categorical data are expressed as number and percentage. The characteristics of the study participants were analyzed according to uNAG level using the two-sample Student t-test for continuous variables and the chi-square test for categorical variables. Correlations between uNAG and other parameters were analyzed with Pearson correlation analysis. Stepwise multiple linear regression analysis was performed on logarithm-transformed uNAG values to model the relationships between uNAG and glycemic parameters. To correct for skewed distributions, non-normally distributed continuous variables, such as urinary ACR, were logarithmically transformed before statistical analysis. Receiver operating characteristic (ROC) curve analysis was conducted to evaluate the capacity to discriminate between glycemic parameters, such as GA, HbA1c, stimulated glucose, and fasting glucose, to predict the presence of renal tubular dysfunction. The area under the curve (AUC) was calculated using binary logistic regression, and statistical comparisons of the AUCs among glycemic parameters followed the DeLong method [11]. All data analyses were performed using SPSS version 20.0 (IBM Co., Armonk, NY, USA). P values <0.05 were considered significant.
RESULTS
Characteristics of the study participants
Mean age of the study population was 60.14±11.37 years, and 55.6% were men. Due to our inclusion criteria (ACR <30 mg/g and eGFR ≥60 mL/min/1.73 m2), mean eGFR and ACR were 91.17±15.33 mL/min/1.73 m2 and 7.59 mg/g (interquartile range [IQR], 4.54 to 12.47 mg/g), respectively. Based on a previous finding of Japanese nondiabetic subjects that states the normal reference range for uNAG as 1.6 to 5.8 U/g Cr (creatinine) [10], we arbitrary classified subjects into a normal uNAG group and a high group (normal vs. high [>5.8 U/g Cr]) (Table 1). The median uNAG values among participants with normal and high uNAG levels were 3.99 U/g Cr (IQR, 2.82 to 4.96 U/g Cr) and 9.48 U/g Cr (IQR, 7.38 to 13.51 U/g Cr), respectively. Participants with high uNAG level were significantly less likely to be obese or more likely to be older, had longer duration of diabetes, and higher level of low density lipoprotein cholesterol (LDL-C) compared with normal uNAG subjects. Blood pressure did not differ between the two groups. Regarding glucometabolic parameters, subjects with high uNAG level had significantly higher levels of fasting glucose (133.15±32.96 vs. 141.20±45.31, P=0.001), stimulated plasma glucose (181.10±50.78 vs. 202.87±64.85, P<0.001), δ glucose (49.80±39.96 vs. 60.44±43.64, P=0.001), GA (17.42%±4.18% vs. 19.65%±5.99%, P<0.001), and HbA1c (7.04%±1.05% vs. 7.41%±1.38%, P<0.001) than subjects with normal uNAG level. Participants with high uNAG level had lower levels of stimulated C-peptide (5.52±2.39 vs. 4.95±2.21, P=0.002), δ C-peptide (3.13±1.94 vs. 2.67±1.71, P=0.001), and HOMA-β (48.09 [IQR, 31.71 to 74.74] vs. 39.09 [IQR, 23.74 to 63.93], P<0.001) than participants with normal uNAG level, whereas HOMA-IR levels were not different between the two groups.
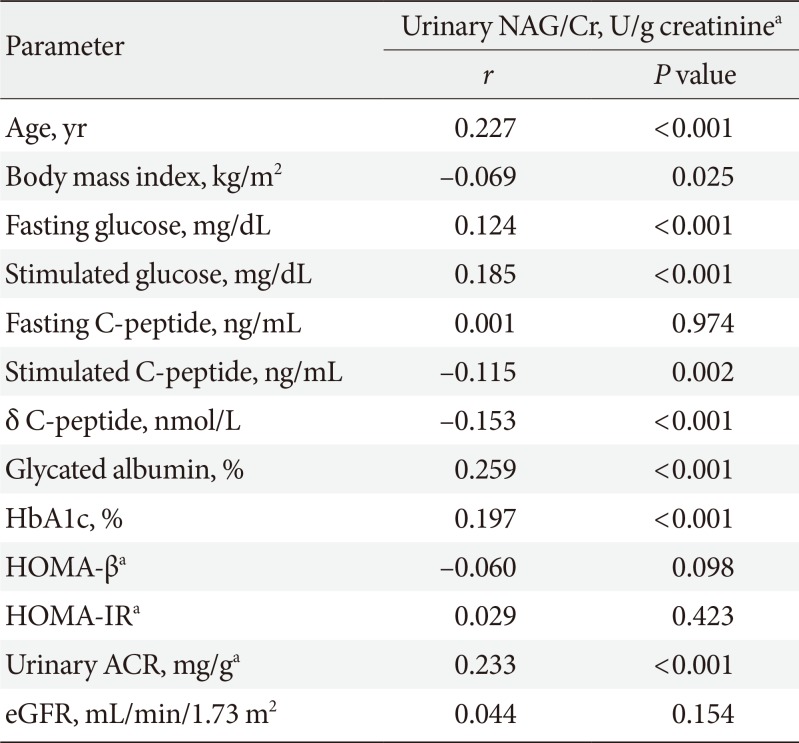
Correlations between uNAG and glucose parameters
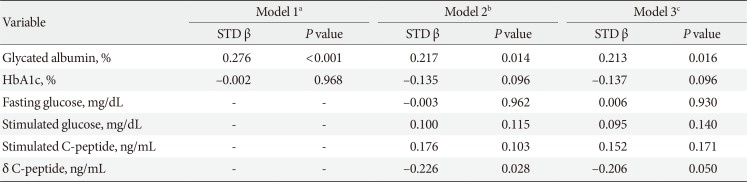
In Pearson correlation analysis, uNAG was positively significantly correlated with ACR (r=0.233, P<0.001), but not with eGFR (r=0.044, P=0.154). Urinary NAG was also positively associated with fasting and stimulated glucose, stimulated C-peptide, δ C-peptide, GA, and HbA1c. Among the various glucometabolic variables, GA had a stronger relationship with uNAG than HbA1c (r=0.259, P<0.001 vs. r=0.197, P<0.001) (Table 2). We also evaluated the independent associations between glucose parameters and uNAG as a renal tubulopathy marker, for which we performed multiple linear regression analyses (Table 3). In model 1, after adjusting for confounding factors, such as age, sex, body mass index, diabetes duration, and current smoking, GA was independently associated with uNAG level (standardized β=0.276, P<0.001), whereas HbA1c was not (standardized β=−0.033, P=0.539). After further adjustment for other confounding variables, including fasting glucose, stimulated glucose, stimulated C-peptide, δ C-peptide, eGFR, LDL-C, albumin, and log-transformed urinary ACR (model 2), GA was still significantly associated with uNAG level (standardized β=0.217, P=0.014), whereas HbA1c and stimulated glucose were not. This trend remained statistically significant even after additional adjustments for sulfonylurea use, insulin use, angiotensin converting enzyme inhibit or, angiotensin receptor blocker use, and diuretic use (model 3).
GA for predicting renal tubular damage in T2DM
The ability of glycemic parameters, such as serum GA, HbA1c, stimulated glucose, and fasting glucose, to discriminate the presence of renal tubular damage in patients with T2DM who have normoalbuminuria and normal eGFR was assessed by comparing the AUCs from ROC analyses (Fig. 1). The AUC values for all four glycemic parameters were statistically significant. The AUC for renal tubular damage of GA was significantly higher (0.634; 95% confidence interval [CI], 0.646 to 0.899; P<0.001) than the AUC of HbA1c (0.598; 95% CI, 0.553 to 0.640; P<0.001), stimulated glucose (0.594; 95% CI, 0.552 to 0.636; P<0.001), and fasting glucose (0.558; 95% CI, 0.515 to 0.600; P=0.01). The optimal GA cutoff point for renal tubular damage was 17.55%. The sensitivity and specificity at this level were 59% and 62%, respectively.
DISCUSSION
Accumulating evidence indicates that GA has greater clinical relevance than HbA1c for evaluating glucose fluctuation and for predicting vascular complications in patients with T1DM and T2DM [1213]. However, the optimal interpretation of GA in the context of early diabetic nephropathy, especially renal tubular damage, has not been fully evaluated. Based on previous findings that uNAG might be a more sensitive urinary biomarker than urinary ACR for early DKD detection, as well as its association with postprandial glucose and GA in patients with T2DM, we hypothesized that GA, which more sensitively reflects increases in blood glucose excursions than HbA1c, especially postprandial glucose, might signal renal tubular damage earlier in the subclinical stage of DKD. To test this hypothesis, we investigated various glucometabolic parameters and kidney-damage markers in patients with T2DM with normoalbuminuria (ACR <30 mg/g) and normal eGFR (≥60 mL/min/1.73 m2) who were recruited from three hospital databases. We focused on the association between GA and uNAG excretion and observed that GA was significantly associated with uNAG excretion independent of other confounding factors, but that HbA1c was not. Furthermore, we observed that GA can better discriminate early renal tubular dysfunction using AUC comparison analysis. These findings suggest that GA can be a more reliable marker than HbA1c or fasting plasma glucose for detecting early renal tubular damage in patients with T2DM without overt DKD.
DKD not only occurs in 20% to 40% of all patients with diabetes, but it is also one of the major end-organ complications of diabetes and continues to be the most common cause of end-stage renal disease [14].
Regarding DKD, growing evidence suggests that some patients with diabetic nephropathy display neither substantial glomerular pathology nor proteinuria and that kidney function commonly assessed by eGFR declines well before traditional indicators of kidney disease, such as albuminuria. This might indicate that changes in the glomerulus are neither the initial events of DKD development nor the major determinant of renal prognosis in subjects with T2DM. Furthermore, it is now increasingly recognized that tubules play an important role in DKD pathogenesis. In the context of DKD, it is well known that uNAG is markedly elevated, even in patients with normal to mildly increased albuminuria, notwithstanding that it is also higher in patients with more aggravated albuminuria [4]. Thus, to examine the associations between various glycemic parameters and early renal damage in patients with T2DM, we adopted uNAG as an early tubulopathy marker.
Many previous studies have demonstrated that intensive glycemic control can delay the onset and progression of DKD in patients with T2DM [1516]. Measurement of HbA1c is generally considered the gold standard index for glycemic control. Because periodic monitoring of HbA1c level can be informative for evaluating the degree of glycemic control in patients with diabetes, it is possible to assess the relationship between glycemic control and development of diabetes-related complications using these measures. However, GA is now gaining popularity as an index of glycemic control during intensive treatment. GA is not affected by hemoglobin level and is superior to HbA1c as a representation of short-term glycemic control. Furthermore, recent studies have reported that GA is a better reflection of glycemic excursions and postprandial hyperglycemia compared with HbA1c in various clinical settings [5171819]. From this background, some studies have demonstrated that GA as well as the GA-to-HbA1c ratio, but not HbA1c alone, were associated with carotid intima media thickness or plaque and severity of coronary atherosclerosis [2021]. In terms of DKD, several studies have concluded that mean GA level, rather than mean HbA1c level, is more closely associated with DKD progression [1222]. However, these studies included individuals with a broad range of renal impairment (normal to advanced CKD) and a broad range of albuminuria (normal to macroalbuminuria). Although cumulative evidence supports GA's predictive role for microvascular complications, the relationship between GA and early renal tubular dysfunction in patients with T2DM has not yet been evaluated. Thus, to investigate the predictive ability of various glycemic parameters for early renal tubular dysfunction among patients with T2DM without overt DKD, we included subjects who had normal eGFR (≥60 mL/min/1.73 m2) and normoalbuminuria (urine ACR ≤30 mg/g).
The major interest of this study was the association between GA and renal tubular marker independently of HbA1c and other confounding factors. We restricted our subjects to those without overt CKD to minimize the possibility of confounding with CKD characteristics, such as albumin excretion or hematologic alteration. Consequently, our results suggest that GA can be a strong independent predictor of early renal tubular damage, beyond its role as a surrogate marker of glucose control. Because our study did not directly measure oxidative stress markers, degree of glucose excursion, or inflammation, the mechanisms underlying the close relationship between GA and early renal tubular dysfunction remain unclear. One possibility is that, since glycation of albumin impairs the antioxidant activities of albumin, GA can contribute to increased oxidative stress in patients with diabetes [7] and, in turn, induces tubular injury. Furthermore, because GA has not only been shown to be a marker of postprandial glycemic excursion, but also has been postulated to promote atherosclerosis, GA can independently influence diabetes microvascular complications [23].
With respect to glucometabolic parameters, recent studies have shown that impaired insulin secretory function, which might be complexly influenced by T2DM duration in cases in which β-cell function gradually decreases over time, is more sensitively reflected by GA than by HbA1c. Additionally, GA was statistically more strongly associated with diabetes duration than HbA1c [24]. Consistent with previous findings, our data demonstrated that uNAG was significantly associated with insulin secretory function as presented as C-peptide values (Table 3, Model 2, 3). This suggests that impaired insulin secretory function might have a role in early renal tubular dysfunction of DKD. These findings could explain GA's strong involvement in the pathogenesis of major diabetic complications and the identification of people at risk for microvascular complications.
In terms of GA's value for predicting renal tubular dysfunction, we concluded that the optimal cutoff point was 17.55%. Although several studies have reported that GA is associated with a higher risk of diabetes complications, few studies have investigated GA cutoff values for predicting chronic complication in participants with diabetes. One recent study demonstrated that the GA cutoff value for predicting overall survival in subjects with diabetes undergoing hemodialysis was 18.6% (sensitivity 73%, specificity 67%) [25]. Selvin et al. [26] reported that GA >23% was significantly associated with diabetic retinopathy in the ARIC (Atherosclerosis Risk in Communities) study. The optimal GA cutoff point for predicting renal tubulopathy in our study was lower. Our lower cutoff point can be explained by the fact that we excluded subjects with overt DKD. Considering that GA has been considered a good indicator of diabetes complications, further large-scale prospective studies might be warranted to determine the GA cutoff value for predicting early renal tubular dysfunction in subjects without overt CKD.
This study has several limitations. First, our analysis included only cross-sectional data; we were therefore unable to determine a causal relationship between GA level and either initiation or progression of diabetic tubulopathy. Second, we could not collect complete data about medicinal intake that could affect kidney function, such as nonsteroidal anti-inflammatory drugs. Finally, we did not assess the associations between glycemic indices and renal tubulopathy using other renal tubular damage markers, like kidney injury molecule 1 or liver fatty-acid binding protein [27].
Collectively, this study suggests that GA level might be a good predictor of renal tubulopathy in patients with T2DM without overt DKD, regardless of HbA1c level or other conventional risk factors. Moreover, we demonstrated that GA has better discriminatory capacity for renal tubular dysfunction than HbA1c. These findings indicate that GA monitoring, rather than HbA1c monitoring, is most useful for detecting higher risk of diabetes-induced tubulopathy as an early disease event that contributes to CKD development in patients with T2DM. Further large-scale prospective studies might be warranted to generalize the relationship between GA and renal tubular damage in patients with T2DM with overt albuminuria or impaired renal function.
Notes
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.