- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Articles
- Page Path
- HOME > Diabetes Metab J > Volume 35(4); 2011 > Article
-
Original ArticlePostprandial Triglyceride Is Associated with Fasting Triglyceride and HOMA-IR in Korean Subjects with Type 2 Diabetes
- Seo Hee Lee, Byung-Wan Lee, Hee Kwan Won, Jae Hoon Moon, Kwang Joon Kim, Eun Seok Kang, Bong Soo Cha, Hyun Chul Lee
-
Diabetes & Metabolism Journal 2011;35(4):404-410.
DOI: https://doi.org/10.4093/dmj.2011.35.4.404
Published online: August 31, 2011
Division of Endocrinology and Metabolism, Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea.
- Corresponding authors: Byung-Wan Lee, Hyun Chul Lee. Department of Internal Medicine, Yonsei University College of Medicine, 250 Seongsan-ro, Seodaemoon-gu, Seoul 120-752, Korea. bwanlee@yuhs.ac, endohclee@yuhs.ac
Copyright © 2011 Korean Diabetes Association
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
-
Background
- Recent studies indicate postprandial triglyceride (TG) had a better association with cardiovascular events and metabolic syndrome than fasting TG. The authors of the present study investigated the metabolic and clinical relevance of postprandial TG.
-
Methods
- In a cross-sectional retrospective study, the authors of the present study compared fasting and postprandial TG and analyzed the relationship between postprandial TG and various demographic and metabolic parameters in 639 Korean subjects with type 2 diabetes (T2D, group I, n=539) and impaired fasting glucose (IFG, group II, n=100) after ingestion of a standardized liquid meal (total 500 kcal, 17.5 g fat, 68.5 g carbohydrate, and 17.5 g protein).
-
Results
- Fasting and postprandial TG were significantly correlated (r=0.973, r=0.937, P<0.001) in group I and II, respectively. Of the variables, total cholesterol, waist circumference and body mass index were significantly correlated with fasting and postprandial TG in both groups. Only postprandial TG showed a significant correlation with glucose metabolic parameters (e.g., postprandial glucose, homeostatic model assessment of insulin resistance [HOMA-IR], and fasting C-peptide) in subjects with T2D. Multiple regression analysis showed fasting TG and HOMA-IR could be predictable variables for postprandial TG in subjects with T2D.
-
Conclusion
- Postprandial TG was very strongly correlated with fasting TG. The authors of the present study suggest insulin resistance may be more associated with postprandial TG than fasting TG in Korean T2D patients on a low-fat diet.
- Well-known characteristics of diabetic dyslipidemia include elevated levels of fasting triglyceride (TG), decreased levels of high density lipoprotein cholesterol (HDL-C), and increased portion of small dense low density lipoprotein cholesterol (LDL-C) particles in LDL cholesterol [1]. Similar to the abrupt surge in postprandial glucose after an oral glucose tolerance test in type 2 (T2) diabetics, a rise in circulating TG is a common occurrence after ingestion of a high-fat meal. Although elevated postprandial TG is expected after consumption of a fat-containing meal, not all subjects present hypertriglyceridemia requiring TG-lowering therapy. The levels of postprandial or non-fasting TG are mainly influenced by not only the diet contents [2] but also the time of sampling for non-fasting TG [3]. Postprandial TG level differs according to the fat amount in a meal. Several studies reported a very low (5 g) or low (<15 g) amount of dietary fat did not significantly increase postprandial TG, a moderate (30 to 50 g) amount of fat increased postprandial TG dose dependently, and a very high (>80 g) amount of fat exaggeratedly increased postprandial TG without dose-dependence [2,4].
- Postprandial TG has been suggested as an independent risk factor for cardiovascular disease (CVD) in healthy subjects [5-7]. Several studies support the hypothesis postprandial TG is correlated with carotid IMT and waist circumference (WC), and furthermore superior to fasting TG in association with metabolic syndrome [8-10]. However, the potential effects of postprandial hypertriglyceridemia on the development of atherosclerosis are still controversial in subjects with type 2 diabetes (T2D) [11]. In addition, the metabolic associations of postprandial TG in Korean patients with T2D are not well elucidated, and the clinical and laboratory parameters affecting the levels of non-fasting TG also remain unclear. Thus, the authors of the present study investigated the metabolic relation and clinical implications of postprandial TG after a particular low-fat diet in Korean T2 diabetic patients who used to have a balanced low-fat diet.
INTRODUCTION
- Patients and research design
- In a clinic-based, retrospective cross-sectional study, patients who satisfied specific criteria based on their medical records were analyzed. Inclusion criteria were patients enrolled in the diabetes registry of Severance Diabetes Center between July 2009 and August 2010. The subjects were registered first-time visitors to the center, patients who performed a standardized mixed-meal stimulation test (Ensure; total 500 kcal, 17.5 g fat, 68.5 g carbohydrate, and 17.5 g protein; Meiji Dairies Co., Tokyo, Japan) or patients who had diabetes complication work-up performed. In the registry protocol, blood samples were collected at 0 and 90 minutes (basal and stimulated level, respectively) for glucose, TG, insulin, and C-peptide analyses after injection of a standardized mixed-meal stimulation test. Exclusion criteria included insulin users, severe liver or kidney disease, thyroid disorders, pregnancy, steroid therapy, heavy alcoholics, and hematologic as well as malignant diseases. The study protocol was approved by the Ethics Committee of the Yonsei University College of Medicine. The subjects were classified into 2 groups based on the American Diabetes Association (ADA) 2011 guidelines: group I, subjects with T2D; group II, subjects with impaired fasting glucose subjects (IFG; increased risk for diabetes).
- Anthropometric measurements of individuals wearing light clothing and no shoes were conducted. WC was measured with the tape measure placed horizontally at the level of the umbilicus while the participant gently exhaled. The body mass index (BMI) was calculated as weight in kilograms divided by a square of height in meters.
- Laboratory measurements
- Plasma TG, total cholesterol, HDL-C, blood urea nitrogen, creatinine, AST, and ALT, were assayed by routine Hitachi 7600 autoanalyzer (Hitachi Instruments Service, Tokyo, Japan). LDL-C was calculated using the Friedewald equation. Plasma glucose was measured using the glucose oxidase method, and A1C was measured by high-performance liquid chromatography (HPLC) using Variant II Turbo (Bio-Rad Laboratories, Hercules, CA, USA). Serum insulin and C-peptide levels were measured in duplicate by using an immunoradiometric assay (IRMA) method (Beckman Coulter, Fullerton, CA, USA). Samples for laboratory measurements were simultaneously collected during a standardized mixed-meal stimulation test.
- Assessment of the β-cell functional status
- Pancreatic β-cell function and insulin sensitivity were obtained by homeostasis model assessment (HOMA) using HOMA of pancreatic β-cell function (HOMA-β) calculated as basal insulin (µIU/mL)×20/basal glucose (mmol/L)-3.5, and HOMA of insulin resistance (HOMA-IR) calculated as basal insulin (µIU/mL)×glucose (mmol/L)/22.5. Pancreatic β-cell secretary function or insulin reserve were also determined from a mixed-meal stimulation test calculated by dividing the increase in insulin level by the increase in glucose level over the same period (insulinogenic index; IGI=[insulin (90')-insulin (0')]/[glucose (90')-glucose (0')]).
- Statistical analyses
- Data are given as means±standard deviation (SD) for normally distributed variables, and otherwise as medians (25th and 75th percentile values). Mean values and median value were compared between diabetes and IFG patients using unpaired Student's t-test and Mann-Whitney U-test as appropriate. Pearson's correlation coefficient was used to determine the correlation between continuous parameters. Multiple linear regression analyses were performed using postprandial TG as a dependent factor. Several clinical and laboratory factors (e.g., age, sex, WC, glucose AC, HDL-C, and HOMA-IR) were entered as independent factors. An alpha level of 0.05 was accepted as significant for all statistical procedures. All statistical analyses were conducted using SPSS for Windows software version 18.0 (SPSS Inc., Chicago, IL, USA).
METHODS
- Baseline characteristics of the patients
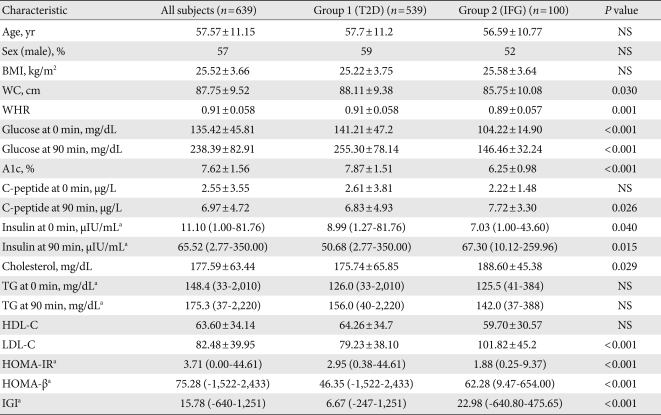
- A total of 639 patients satisfied the criteria, and were classified into 2 groups depending on their glucose status: group I, T2D patients (n=539); group II, IFG patients (n=100). The patients' demographic and laboratory data are summarized in Table 1 and Supplemental Table 1. The mean age was 57.5 years, and gender (364 men and 275 women) was relatively evenly distributed. The mean age was 57.5 years, and gender (364 men and 275 women) was relatively evenly distributed.
- There were no significant differences in age, sex and BMI in both groups. The level of fasting and postprandial glucose, and A1c were significantly higher in group I (141.21±47.2 mg/dL, 255.30±78.14 mg/dL, 7.87±1.51 mg/dL, respectively) than in group II (104.22±14.90 mg/dL, 146.46±32.24 mg/dL, 6.25±0.98 mg/dL) (P<0.001 for all). Postprandial insulin, C-peptide and HOMA-β were statistically higher in group II (67.30 [10.12 to 259.96] µIU/mL, 7.72±3.30 µg/L, 62.28 [9.47 to 654.00], respectively) than group I (50.68 [2.77 to 350.00] µIU/mL, 6.83±4.93 µg/L, 46.35 (-1,522 to 2,433), respectively) (P=0.015, P=0.026, P<0.001). WC, waist/hip ratio (WHR) and HOMA-IR were significantly higher in group I (88.11±9.38 cm, 0.91±0.058, 2.95 [0.38 to 44.61], respectively) than in group II (85.75±10.08 cm, 0.89±0.057, 1.88 (0.25 to 9.37), respectively) (P=0.030, P=0.001, P<0.001). However, fasting TG (126 [33 to 2,010] vs. 125.5 [41 to 384]; group I vs. group II, P=0.432) and postprandial TG (156 [40 to 2,220] vs. 142 [37 to 388]; group I vs. group II, P=0.052) were not significantly different between the 2 groups.
- Independent correlation between plasma TG levels (fasting and postprandial) and clinical and metabolic parameters
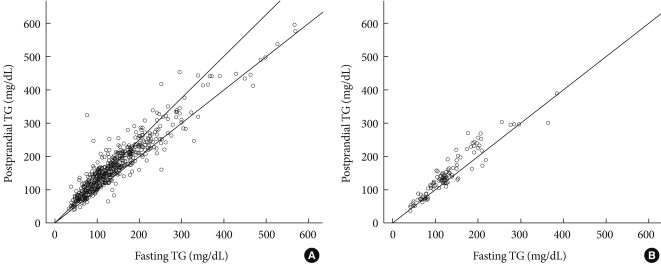
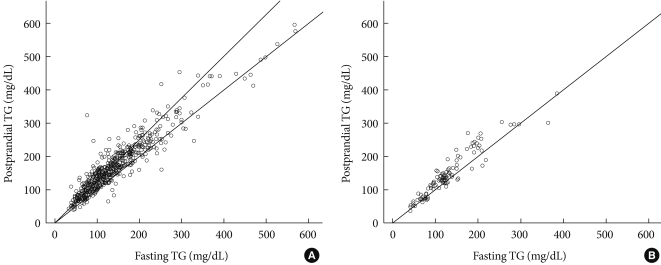
- A very strong (at least 0.9) linear relationship was observed between fasting and postprandial TG in T2D and IFG groups (r=0.973, r=0.937, respectively, P<0.001 for both; Fig. 1). In group I, fasting TG and postprandial TG were statistically correlated with total cholesterol (r=0.309, r=0.305, respectively), fasting glucose (r=0.151, r=0.162), A1c (r=0.143, r=0.162), WC (r=0.138, r=0.123), and BMI (r=0.118, r=0.108). Only postprandial TG was correlated with postprandial glucose (r=0.114, P=0.009), HOMA-IR (r=0.091, P=0.037), and fasting C-peptide (r=0.089, P=0.042).
- In group II, fasting TG and postprandial TG were statistically correlated with postprandial insulin (r=0.328, r=0.330, respectively), postprandial C-peptide (r=0.312, r=0.320), WC (r=0.303, r=0.246), BMI (r=0.275, r=0.246), total cholesterol (r=0.238, r=0.246), and HOMA-IR (r=0.226, r=0.228, Table 2).
- Multiple linear regression analysis (postprandial TG as a dependent variable)
- Of the several clinical and laboratory variables, only age, sex, HOMA-IR, WC, fasting and postprandial TG, HDL, and A1c showed a multicollinearity referring to a situation in which 2 or more explanatory variables were highly linearly related during a multiple regression model. In multiple regression analysis, the postprandial TG was entered as the dependent variable. Independent variables were age, sex, fasting TG, HOMA-IR, HDL-C, glucose AC and WC. As shown in Table 3 the variables independently associated with postprandial TG were fasting TG in both groups (β=0.957, P<0.001 in the T2D group and β=0.906, P<0.001 in the IFG group) and HOMA-IR in the T2D group (β=0.827, P=0.024). In this regard, fasting TG and HOMA-IR could be a predictable variable for postprandial TG in subjects with T2D.
RESULTS
- The gold standard measurement for hypertriglyceridemia is the fasting TG. Although controversial, fasting TG is known to be an independent risk factor for CVD [5,12]. However, 15 to 18 hours a day are spent in the postprandial state and postprandial TG significantly contributes to overall TG exposure. In this regard, the use of fasting TG as a predictor for CVD does not provide information on diurnal TG profiles and individual metabolism [9]. Accordingly, postprandial or non-fasting TG levels are increasingly used as a predictor for CVD [13].
- While traditional Korean meals are usually not high in fat, the Korean diet has become more westernized. The increase of postprandial TG is known as mainly influenced by diet composition [2,4,14]. Additionally, patient's characteristics such as the use of lipid-lowering agents [15], WC [16,17], glycemic status [18,19], menopausal status and age [9] also affect the non-fasting TG profiles. Based on previous reports, the authors of the present study questioned the metabolic associations and clinical implications of postprandial TG in Korean patients with IFG and T2D. To answer these questions, the results of a standardized mixed-meal stimulation test were retrospectively analyzed in order to first identify the close correlation between fasting and postprandial TG and second, the clinical and laboratory relevance of postprandial TG. In the present analysis, the mixed-meal stimulation test (Ensure; total 500 kcal, 17.5 g fat, 68.5 g carbohydrate, and 17.5 g protein) allowed for standardization of the calories, fat contents, and the times for blood sampling (basal and 90 minutes after meal loading). In addition, the influential effects of glucose status and insulin secretion or resistance on both fasting and non-fasting TG levels could be analyzed.
- The present study shows 3 main results. First, fasting and postprandial TG demonstrated a very strong (at least 0.9) linear relationship in all subjects regardless of glucose status. In addition, the TG increment after a mixed-meal load was not contrasting. The likely explanation for these results is the type of low calorie and fat content diet (500 kcal, 17.5 g fat liquid loading) and a 30-minute earlier check of peak TG level. Specifically, 2 to 4 hours after a high-fat diet loading was the necessary time point for postprandial TG to reach the highest level [3,9,12-14,20]. Only 1 well-designed study that enrolled Korean young women showed postprandial TG reached the peak time at 120 minutes after a high-fat meal ingestion [3]. However, no increase in postprandial TG after eating a high carbohydrate meal was found [3]. Second, although both fasting and postprandial TG showed a similar correlation with other demographic and laboratory parameters in subjects with IFG, only postprandial TG showed a significant correlation with glucose metabolic parameters such as postprandial glucose, HOMA-IR and fasting C-peptide in subjects with T2D. In both T2D and IFG groups, fasting and postprandial TG were statistically correlated with total cholesterol and central obesity parameters such as WC and BMI. Third, fasting TG and HOMA-IR could be predictable variables for postprandial TG in subjects with T2D. In subjects with impaired fasting glucose, only fasting TG level predicted the level of postprandial TG.
- Regarding the characteristics of enrolled subjects, patients with T2D showed the typical pathophysiologic features with increased insulin resistance (WC, WHR, and HOMA-IR) and decreased insulin secretory function (HOMA-β, insulin and C-peptide levels at 90 minutes) resulting in increased glucose and A1c values. However, levels of total cholesterol and LDL-C were significantly lower in subjects with T2D than with IFG (Table 1). The likely explanation involves the 50% and 27% usage of lipid-lowering drugs in T2D and IFG subjects, respectively, which may affect the lower levels of cholesterols.
- There are several limitations of the present study. First, the study is a retrospective cross-sectional study, and the measurements were taken after a single liquid meal. Second, subjects were diabetic patients who are usually taking some type of drug which were not individually analyzed in the present study. However, the study protocol using the low-fat mixed-meal test and measuring postprandial TG at 90 minutes is appropriate to analyze the influential effects of glucose status and insulin secretion or resistance on both fasting and non-fasting TG levels in Korean subjects who traditionally do not eat a high-fat diet. This is the first, well-designed and relatively large scale study on clinical implication of postprandial TG on Korean T2 diabetics. Further studies are essential to investigate the postprandial TG in all drug-naïve pre-diabetic and diabetic patients.
- In conclusion, postprandial TG was strongly correlated with fasting TG. In addition, the present study shows a low-fat meal may have largely similar dietary effects on fasting and postprandial TG in Koreans with T2D. Especially, insulin resistance may be more associated with postprandial TG than fasting TG in Korean diabetic patients on a low-fat diet.
DISCUSSION
Supplementary Material
- 1. Taskinen MR. Diabetic dyslipidemia. Atheroscler Suppl 2002;3:47-51. ArticlePubMed
- 2. Dubois C, Beaumier G, Juhel C, Armand M, Portugal H, Pauli AM, Borel P, Latge C, Lairon D. Effects of graded amounts (0-50 g) of dietary fat on postprandial lipemia and lipoproteins in normolipidemic adults. Am J Clin Nutr 1998;67:31-38. ArticlePubMed
- 3. Shin Y, Park S, Choue R. Comparison of time course changes in blood glucose, insulin and lipids between high carbohydrate and high fat meals in healthy young women. Nutr Res Pract 2009;3:128-133. ArticlePubMedPMC
- 4. Lopez-Miranda J, Williams C, Lairon D. Dietary, physiological, genetic and pathological influences on postprandial lipid metabolism. Br J Nutr 2007;98:458-473. ArticlePubMed
- 5. Stalenhoef AF, de Graaf J. Association of fasting and nonfasting serum triglycerides with cardiovascular disease and the role of remnant-like lipoproteins and small dense LDL. Curr Opin Lipidol 2008;19:355-361. ArticlePubMed
- 6. Bansal S, Buring JE, Rifai N, Mora S, Sacks FM, Ridker PM. Fasting compared with nonfasting triglycerides and risk of cardiovascular events in women. JAMA 2007;298:309-316. ArticlePubMed
- 7. Nordestgaard BG, Benn M, Schnohr P, Tybjaerg-Hansen A. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA 2007;298:299-308. ArticlePubMed
- 8. Ryu JE, Howard G, Craven TE, Bond MG, Hagaman AP, Crouse JR 3rd. Postprandial triglyceridemia and carotid atherosclerosis in middle-aged subjects. Stroke 1992;23:823-828. ArticlePubMed
- 9. Oka R, Kobayashi J, Miura K, Nagasawa S, Moriuchi T, Hifumi S, Miyamoto S, Kawashiri MA, Nohara A, Inazu A, Takeda Y, Mabuchi H, Yagi K, Yamagishi M. Difference between fasting and nonfasting triglyceridemia: the influence of waist circumference. J Atheroscler Thromb 2009;16:633-640. ArticlePubMed
- 10. Rector RS, Linden MA, Zhang JQ, Warner SO, Altena TS, Smith BK, Ziogas GG, Liu Y, Thomas TR. Predicting postprandial lipemia in healthy adults and in at-risk individuals with components of the cardiometabolic syndrome. J Clin Hypertens (Greenwich) 2009;11:663-671. ArticlePubMedPMC
- 11. Enkhmaa B, Ozturk Z, Anuurad E, Berglund L. Postprandial lipoproteins and cardiovascular disease risk in diabetes mellitus. Curr Diab Rep 2010;10:61-69. ArticlePubMedPMC
- 12. Patsch JR, Miesenbock G, Hopferwieser T, Muhlberger V, Knapp E, Dunn JK, Gotto AM Jr, Patsch W. Relation of triglyceride metabolism and coronary artery disease: studies in the postprandial state. Arterioscler Thromb 1992;12:1336-1345. ArticlePubMed
- 13. Uiterwaal CS, Grobbee DE, Witteman JC, van Stiphout WA, Krauss XH, Havekes LM, de Bruijn AM, van Tol A, Hofman A. Postprandial triglyceride response in young adult men and familial risk for coronary atherosclerosis. Ann Intern Med 1994;121:576-583. ArticlePubMed
- 14. Karpe F, de Faire U, Mercuri M, Bond MG, Hellenius ML, Hamsten A. Magnitude of alimentary lipemia is related to intima-media thickness of the common carotid artery in middle-aged men. Atherosclerosis 1998;141:307-314. ArticlePubMed
- 15. Weiss R, Harder M, Rowe J. The relationship between nonfasting and fasting lipid measurements in patients with or without type 2 diabetes mellitus receiving treatment with 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors. Clin Ther 2003;25:1490-1497. ArticlePubMed
- 16. Couillard C, Bergeron N, Prud'homme D, Bergeron J, Tremblay A, Bouchard C, Mauriege P, Despres JP. Gender difference in postprandial lipemia: importance of visceral adipose tissue accumulation. Arterioscler Thromb Vasc Biol 1999;19:2448-2455. PubMed
- 17. Blackburn P, Lamarche B, Couillard C, Pascot A, Tremblay A, Bergeron J, Lemieux I, Despres JP. Contribution of visceral adiposity to the exaggerated postprandial lipemia of men with impaired glucose tolerance. Diabetes Care 2003;26:3303-3309. ArticlePubMedPDF
- 18. Madhu SV, Kant S, Srivastava S, Kant R, Sharma SB, Bhadoria DP. Postprandial lipaemia in patients with impaired fasting glucose, impaired glucose tolerance and diabetes mellitus. Diabetes Res Clin Pract 2008;80:380-385. ArticlePubMed
- 19. Oka R, Yagi K, Hifumi S, Miyamoto S, Mabuchi H, Yamagishi M, Takeda Y, Inazu A, Nohara A, Kawashiri M, Kobayashi J. Postprandial triglyceridaemia in men with impaired fasting glucose, impaired glucose tolerance and diabetes. Diabet Med 2008;25:1008-1010. Article
- 20. Hamsten A, Silveira A, Boquist S, Tang R, Bond MG, de Faire U, Bjorkegren J. The apolipoprotein CI content of triglyceride-rich lipoproteins independently predicts early atherosclerosis in healthy middle-aged men. J Am Coll Cardiol 2005;45:1013-1017. ArticlePubMed
REFERENCES
T2D, type 2 diabetes; IFG, impaired fasting glucose; NS, not significant; BMI, body mass index; WC, waist circumference; WHR, waist/hip ratio; TG, triglyceride; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol; HOMA-IR, homeostatic model assessment of insulin resistance; HOMA-β, homeostatic model assessment of pancreatic β-cell function; IGI, insulinogenic index.
aDifferences between the 2 groups were analyzed by Mann-Whitney U-test. Data were presented as the median and 25th and 75th percentile values.
Figure & Data
References
Citations

- Impaired ketogenesis is associated with metabolic-associated fatty liver disease in subjects with type 2 diabetes
Sejeong Lee, Jaehyun Bae, Doo Ri Jo, Minyoung Lee, Yong-ho Lee, Eun Seok Kang, Bong-Soo Cha, Byung-Wan Lee
Frontiers in Endocrinology.2023;[Epub] CrossRef - Postprandial lipaemia following consumption of a meal enriched with medium chain saturated and/or long chain omega-3 polyunsaturated fatty acids. A randomised cross-over study
Grace Austin, Jessica JA. Ferguson, Rohith N. Thota, Harjinder Singh, Tracy Burrows, Manohar L. Garg
Clinical Nutrition.2021; 40(2): 420. CrossRef - Effects of fatty acids composition in a breakfast meal on the postprandial lipid responses: a systematic review and meta-analysis of randomised controlled trials
Yuanhang Yao, Sheri Xueqi Pek, Darel Wee Kiat Toh, Xuejuan Xia, Jung Eun Kim
International Journal of Food Sciences and Nutrition.2020; 71(7): 793. CrossRef - The Forgotten Lipids: Triglycerides, Remnant Cholesterol, and Atherosclerotic Cardiovascular Disease Risk
Pratik B Sandesara, Salim S Virani, Sergio Fazio, Michael D Shapiro
Endocrine Reviews.2019; 40(2): 537. CrossRef - Determinant of postprandial triglyceride levels in healthy young adults
Tri J.E. Tarigan, Anandhara I. Khumaedi, Syahidatul Wafa, Michael Johan, Murdani Abdullah, Ingrid S. Surono, Dicky L. Tahapary
Diabetes & Metabolic Syndrome: Clinical Research & Reviews.2019; 13(3): 1917. CrossRef - Impact of the triglyceride level on coronary plaque components in female patients with coronary artery disease treated with statins
Motoki Yamashita, Atsushi Iwata, Yuta Kato, Makito Futami, Satoshi Imaizumi, Takashi Kuwano, Amane Ike, Makoto Sugihara, Hiroaki Nishikawa, Bo Zhang, Shin’ichiro Yasunaga, Keijiro Saku, Shin-ichiro Miura
Heart and Vessels.2018; 33(10): 1175. CrossRef - Biomarker potential of C-peptide for screening of insulin resistance in diabetic and non-diabetic individuals
Haseeb A. Khan, Samia H. Sobki, Aishah Ekhzaimy, Isra Khan, Mona A. Almusawi
Saudi Journal of Biological Sciences.2018; 25(8): 1729. CrossRef - Postprandial C‐peptide to glucose ratio as a predictor of β‐cell function and its usefulness for staged management of type 2 diabetes
Eun Young Lee, Sena Hwang, Seo Hee Lee, Yong‐ho Lee, A Ra Choi, Youngki Lee, Byung‐Wan Lee, Eun Seok Kang, Chul Woo Ahn, Bong Soo Cha, Hyun Chul Lee
Journal of Diabetes Investigation.2014; 5(5): 517. CrossRef - Genetics and Causality of Triglyceride-Rich Lipoproteins in Atherosclerotic Cardiovascular Disease
Robert S. Rosenson, Michael H. Davidson, Benjamin J. Hirsh, Sekar Kathiresan, Daniel Gaudet
Journal of the American College of Cardiology.2014; 64(23): 2525. CrossRef - The effect of insulin resistance on postprandial triglycerides in Korean type 2 diabetic patients
Kyeong Hye Park, Kwang Joon Kim, Byung-Wan Lee, Eun Seok Kang, Bong Soo Cha, Hyun Chul Lee
Acta Diabetologica.2014; 51(1): 15. CrossRef - Acute effects of an oral supplement of (−)-epicatechin on postprandial fat and carbohydrate metabolism in normal and overweight subjects
Gabriela Gutiérrez-Salmeán, Pilar Ortiz-Vilchis, Claudia M. Vacaseydel, Ivan Rubio-Gayosso, Eduardo Meaney, Francisco Villarreal, Israel Ramírez-Sánchez, Guillermo Ceballos
Food & Function.2014; 5(3): 521. CrossRef - A comparative study of broccoli sprouts powder and standard triple therapy on cardiovascular risk factors following H.pylori eradication: a randomized clinical trial in patients with type 2 diabetes
Parvin Mirmiran, Zahra Bahadoran, Mahdieh Golzarand, Homayoun Zojaji, Fereidoun Azizi
Journal of Diabetes & Metabolic Disorders.2014;[Epub] CrossRef - Association of higher resistin levels with inflammatory activation and endothelial dysfunction in patients with essential hypertension
Chang FANG, Juan LEI, Shu-xian ZHOU, Yu-ling ZHANG, Gui-yi YUAN, Jing-feng WANG
Chinese Medical Journal.2013; 126(4): 646. CrossRef - Epicardial adipose tissue thickness is an indicator for coronary artery stenosis in asymptomatic type 2 diabetic patients: its assessment by cardiac magnetic resonance
Hyun Kim, Kwang Kim, Hye-Jeong Lee, Hee Yu, Jae Moon, Eun Kang, Bong Cha, Hyun Lee, Byung-Wan Lee, Young Kim
Cardiovascular Diabetology.2012; 11(1): 83. CrossRef

 KDA
KDA

 PubReader
PubReader Cite
Cite