Baseline-Corrected QT (QTc) Interval Is Associated with Prolongation of QTc during Severe Hypoglycemia in Patients with Type 2 Diabetes Mellitus
Article information
Abstract
Background
We investigated an association between baseline heart rate-corrected QT (QTc) interval before severe hypoglycemia (SH) and prolongation of QTc interval during SH in patients with type 2 diabetes mellitus (T2DM).
Methods
Between January 2004 and June 2014, 208 patients with T2DM, who visited the emergency department because of SH and underwent standard 12-lead electrocardiography within the 6-month period before SH were consecutively enrolled. The QTc interval was analyzed during the incidence of SH, and 6 months before and after SH. QTc intervals of 450 ms or longer in men and 460 ms or longer in women were considered abnormally prolonged.
Results
The mean age and diabetes duration were 68.1±12.1 and 14.1±10.1 years, respectively. The mean QTc intervals at baseline and SH episodes were 433±33 and 460±33 ms, respectively (P<0.001). One hundred and fourteen patients (54.8%) had a prolonged QTc interval during SH. There was a significant decrease in the prolonged QTc interval within 6 months after SH (QTc interval prolongation during SH vs. after recovery, 54.8% vs. 33.8%, P<0.001). The prolonged QTc interval was significantly associated with baseline QTc interval prolongation (odds ratio, 2.92; 95% confidence interval, 1.22 to 6.96; P=0.016) after adjusting for multiple confounders.
Conclusion
A prolonged QTc interval at baseline was significantly associated with prolongation of the QTc interval during SH in patients with T2DM, suggesting the necessity of QTc interval monitoring and attention to those with a prolonged QTc interval to prevent SH.
INTRODUCTION
Because large epidemiologic studies have reported that a chronic uncontrolled glycemic status was related to the development of macrovascular and microvascular complications [1234], intensive glycemic control has been emphasized for preventing long-term vascular complications in patients with type 1 diabetes mellitus (T1DM) or type 2 diabetes mellitus (T2DM) [5]. Previously reported large randomized controlled studies, such as Action to Control Cardiovascular Risk in Diabetes (ACCORD), Action in Diabetes and Vascular Disease (ADVANCE), and Veterans Affairs Diabetes Trial (VADT), showed that intensive glycemic control reduces microvascular complications in patients with T2DM [678]. However, in spite of achieving a glycemic target, the trials did not demonstrate beneficial effects on cardiovascular events [678]. Weight gain and significantly increased hypoglycemia episodes have been thought to contribute to this unfavorable outcome in the intensive treatment group. There were 1.86- to 3-fold more severe hypoglycemia (SH) events in the intensive control group than in the standard control group in the ACCORD and ADVANCE studies [67].
The incidence rate of SH events has been reported as 0.4 to 16.6 events per 100 patients per year in patients with T2DM [67]. In Korea, the incidence of SH events has also recently increased to 2.11 to 2.71 events per 100 patients per year in patients with T2DM in 2009, which is higher than in 2004 (0.46 to 2.19 events per 100 patients per year) [9]. Therefore, to achieve a glycemic target for preventing chronic diabetic complications, we should consider and manage the accompanying risk of hypoglycemia in patients with T2DM.
SH is associated with cognitive dysfunction, seizure, coma, and major cardiovascular events, such as myocardial infarction, stroke, and arrhythmia, as well as all-cause mortality in T2DM [510]. Nocturnal hypoglycemia or SH is considered to be associated with sudden unexplained death in T1DM, named "dead in bed syndrome," and cardiovascular autonomic neuropathy with abnormal cardiac repolarization, as evidenced by corrected QT (QTc) interval prolongation, is suggested to play a role in cardiac arrhythmia [11]. In addition, the QTc interval has been reported to predict cardiac death in several diseases, including T1DM, advanced heart failure, systemic hypertension, and peripheral vascular disease in patients with diabetes [1213]. Recent studies have found an association between SH and prolonged QTc in T2DM [14]. However, few studies have evaluated the relationship between QTc prolongation at baseline and during an SH episode in subjects with T2DM [14151617].
The aim of this study was to evaluate the relationship among baseline QTc, QTc during SH, and the changes in the QTc interval after SH recovery. In addition, we also sought to identify the risk factors that could predict QTc interval prolongation during SH.
METHODS
By retrospective medical record review, we screened patients with T2DM who were 25 years of age or older and visited the emergency department because of SH between January 2004 and June 2014. Among them, the subjects who had both undergone electrocardiography (ECG) that was conducted within 3 to 6 months of experiencing SH (baseline ECG) and had no evidence of cardiovascular events during that period were consecutively enrolled.
The patients with previously diagnosed coronary artery disease, atrial fibrillation, T1DM, or any critical illness, such as liver cirrhosis, sepsis, malignancy, end stage renal disease, and those fitted with a pacemaker were excluded. In addition, patients with T1DM were excluded, which was defined as participants who were 25 years old and younger or had a history of diabetic ketoacidosis or had fasting serum C-peptide levels lower than 0.6 ng/mL (0.20 nmol/L) [18].
Patients taking drugs that influence the QT interval, such as β-blockers or antiarrhythmic, anti-psychiatric or antihistamine agents, and who were given glucose solution before arriving at the emergency department were also excluded from the study. The patients were defined as alcohol drinkers if their average consumption was 1 to 2 drinks per day [19]. The patients were also classified as to their current smoking status at the time of enrollment. The Catholic Medical Center Ethics Committee approved this study (IRB No. VC12EISI0103). Written informed consent was obtained from all participants.
Hypertension was defined as systolic blood pressure ≥140 mm Hg, diastolic blood pressure ≥90 mm Hg, or the use of antihypertensive medications. The plasma glucose levels were assayed using an enzymatic method, and the glycosylated hemoglobin (HbA1c) level was assayed by high-performance liquid chromatography (Bio-Rad, Montreal, QC, Canada). The total cholesterol, triglyceride level, and high density lipoprotein cholesterol were enzymatically assayed using an automatic analyzer (model 736-40; Hitachi, Tokyo, Japan) after at least 8 hours of fasting. To determine chronic kidney disease (CKD), the estimated glomerular filtration rate (eGFR) using the four-component Modification of Diet in Renal Disease equation was calculated during SH [20]. CKD was defined as a status with a decreased eGFR less than 60 mL/min/1.73 m2, or presence of microalbuminuria (30 to 300 mg/day) or macroalbuminuria (≥300 mg/day) for more than 3 months [2122]. The urinary albumin excretion rate was assayed from a 24-hour urine collection using immunoturbidimetry (Eiken, Tokyo, Japan).
Evaluation of QTc interval prolongation
SH was defined as a hypoglycemic event requiring the assistance of another person to administer carbohydrates and other resuscitative actions, or to provide hospitalization or medical care in an emergency department [20]. The QTc interval was measured using a standard resting 12-lead ECG during the SH event, immediately on arrival using an ECG device (PI-19E; Fukuda Denshi, Tokyo, Japan). The ECG device recorded all leads simultaneously, which facilitated accurate assessment of the QT interval, which was defined as the interval from the onset of the QRS complex to the end of the T-wave in the cardiac electric cycle. The QT and RR intervals were measured by two experienced physicians, blinded to diagnosis, using five consecutive beats on the lead II. The QT interval was corrected for heart rate using Bazett's formula (QTc interval=QT/RR1/2) [23]. QTc measurements ≥450 ms in men and ≥460 ms in women were considered abnormally prolonged [24]. The interobserver variability of QT interval assessed by coefficient of variation was 3.1%. In addition to the baseline ECG, we also collected the standard 12-lead ECG between 3 and 6 months after the SH episode to evaluate the change in the QTc interval after SH recovery.
Statistical analyses
Clinical characteristics were described as the mean±standard deviation for continuous variables and frequency (percentage) for categorical variables. The clinical characteristics were compared between the patients with prolonged QTc and those without prolonged QTc interval stratified by sex. Chi-square tests or Fisher exact tests were used for the categorical variables, and independent Student t-test was used for continuous variables. Analyses of changes in the QTc interval from the baseline QTc to QTc interval after recovery were estimated using a repeated measures analysis of variance. Non-normally distributed data were log transformed. We used Pearson correlation to estimate the relationship between clinical variables and the QTc interval during SH. In addition, we used logistic regression analysis to test the associations between the prolongation of the QT interval at baseline which was conducted within 3 to 6 months before SH without any cardiovascular events, and during the SH episode. The relationships were explored after adjusting for the following factors: age, sex, duration of diabetes, body mass index (BMI), the habit of smoking, presence of hypertension, CKD, history of SH, and the use of diabetic medication such as insulin or sulfonylurea. All statistical analyses were performed using SAS version 9.2 (SAS Institute Inc., Cary, NC, USA). P<0.05 was considered significant. The results are reported as the odds ratios (ORs) with 95% confidence intervals (CIs).
RESULTS
Clinical characteristics
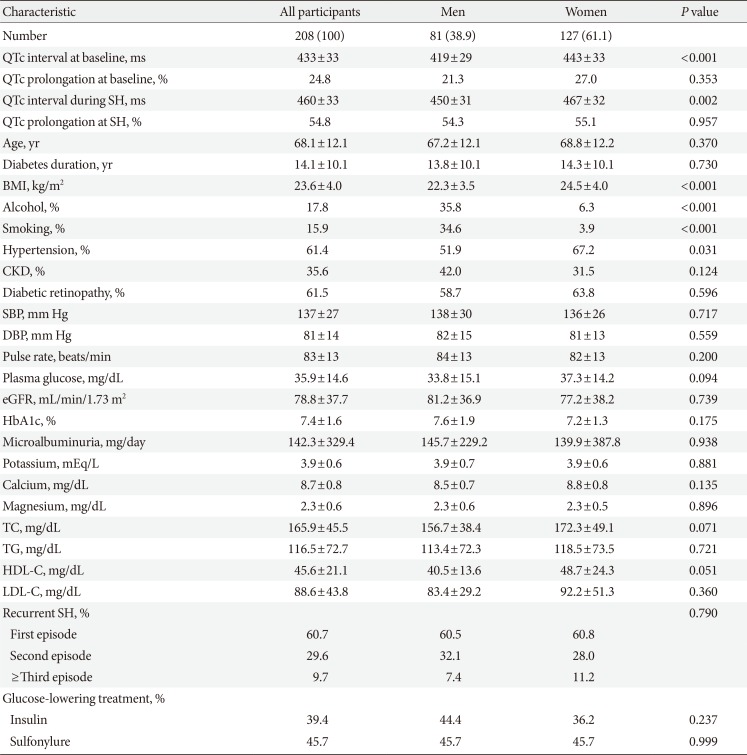
During the study period, 208 patients were enrolled. The mean age and diabetes duration of participants were 68.1±12.1 and 14.1±10.1 years, respectively. One hundred and twenty-seven patients (61.1%) were women. On arrival at the emergency room following an episode of SH, the mean plasma glucose and HbA1c level were 35.9±14.6 mg/dL (1.9±0.8 mmol/L) and 7.4%±1.6%, respectively. Seventy-four subjects (35.6%) had CKD (Table 1). Female subjects had a higher BMI (24.5±4.0 kg/m2 vs. 22.3±3.5 kg/m2, P<0.001), had hypertension (67.2% vs. 51.9%, P=0.031), drank less alcohol (6.3% vs. 35.8%, P<0.001) and had a lower proportion of current smokers (3.9% vs. 34.6%, P<0.001), compared to those of male subjects (Table 1).
QTc interval prolongation during SH episodes
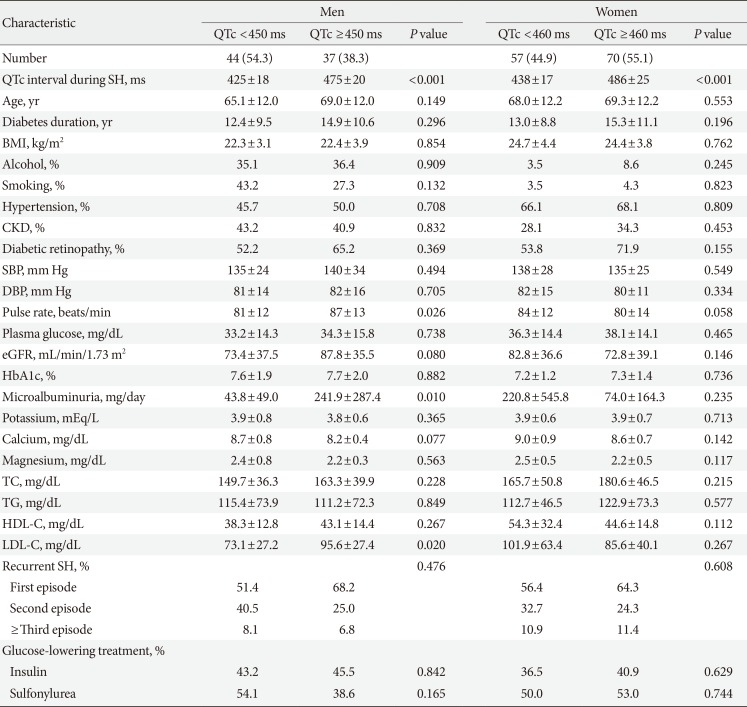
The mean QTc interval during the SH episode was 460±33 ms (males, 450±31 ms; females, 467±32 ms), and 114 patients (54.8%; males, 54.3%; females, 55.1%) had a prolonged QT interval during SH. As shown in Table 2, male patients with a prolonged QTc interval were more likely to have a faster pulse rate (87±13 beats per minute vs. 81±12 beats per minute, P=0.026), higher microalbuminuria level (241.9±287.4 mg/day vs. 43.8±49.0 mg/day, P=0.010), and higher low density lipoprotein cholesterol level (95.6±27.4 mg/dL vs. 73.1±27.2 mg/dL, P=0.020). There were no significant differences in the electrolyte disturbance during SH, including hypokalemia (potassium <3.5 mEq/L, P=0.523), hypocalcemia (calcium <8.2 mg/dL, P=0.131), and hypomagnesemia (magnesium <1.8 mg/dL, P=0.322) and the use of diabetic medications such as insulin (males, P=0.842; females, P=0.629) or sulfonylurea (males, P=0.165; females, P=0.744) (Table 2).

Comparison of clinical characteristics between patients with prolonged QTc and patients without prolonged QTc during the SH episode
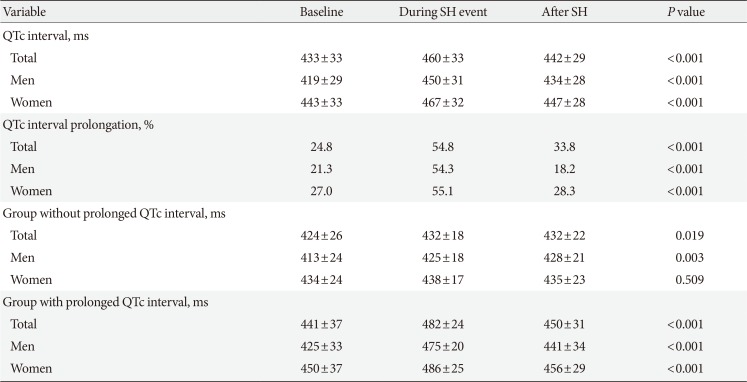
As shown in Table 3, the QTc interval was significantly prolonged during SH and improved after SH recovery (baseline vs. during SH vs. after SH recovery, 433±33 ms vs. 460±33 ms vs. 442±29 ms, P<0.001). Compared with the group without a prolonged QTc at baseline, male and female patients with a prolonged QTc at baseline had significant QTc interval prolongation during SH (P<0.001) and showed recovery after SH (P<0.001).
We assessed the correlations between clinical variables and QTc interval during SH. There were no significant correlations between QTc interval (ms) and diabetic duration (r=0.036, P=0.621), plasma glucose level (r=0.108, P=0.120), or HbA1c level (r=−0.098, P=0.251) during SH (Fig. 1A-C). However, baseline QTc interval before SH was significantly correlated with QTc interval during SH (r=0.333, P<0.001) (Fig. 1D).
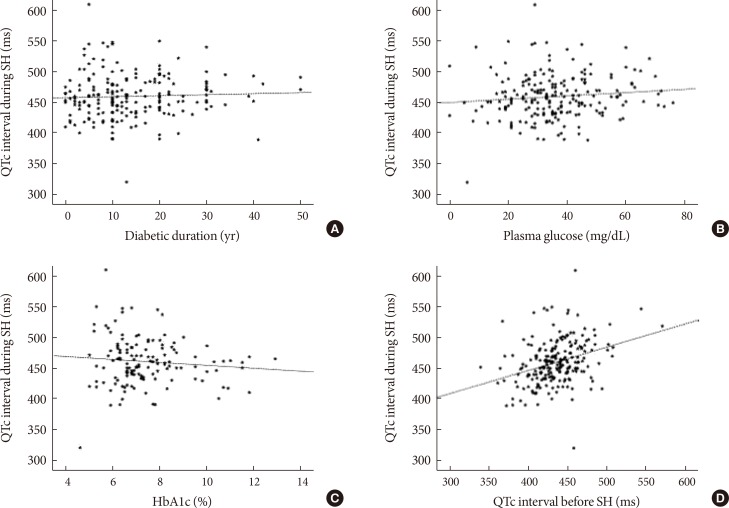
Correlations between clinical variables and heart rate-corrected QT (QTc) interval during severe hypoglycemia (SH) episode. (A) Duration of diabetes (years; r=0.036, P=0.621). (B) Plasma glucose (mg/dL; r=0.108, P=0.120). (C) Glycosylated hemoglobin (HbA1c) level (%; r=−0.098, P=0.251). (D) QTc interval before SH (ms; r=0.333, P<0.001).
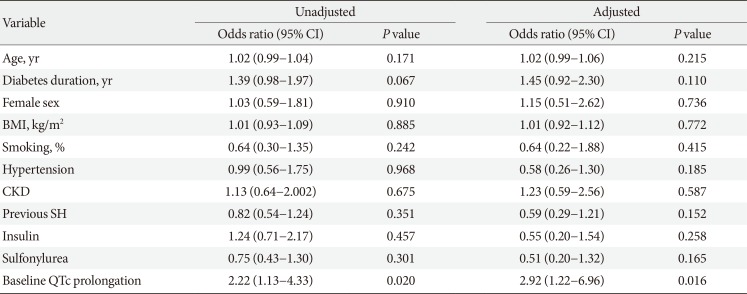
Logistic regression analysis showed that QTc prolongation at baseline was significantly associated with QTc prolongation during SH in univariable analysis. In multivariable analysis, independent of the patient's age, sex, diabetes duration, BMI, habit of smoking, presence of hypertension and CKD, previous SH, and use of insulin or sulfonylurea, the prolongation of the QTc interval at baseline was strongly associated with a prolonged QTc interval during SH (OR, 2.92; 95% CI, 1.22 to 6.96; P=0.016) (Table 4).
Cardiovascular outcomes during follow-up period
During 6 months follow-up after SH, one episode of acute myocardial infarction developed within 3 months after SH, and five patients were diagnosed with atrial fibrillation during SH episodes. Among five patients with newly diagnosed atrial fibrillation, four patients had prolonged QTc interval during SH episodes. In addition, six patients have been diagnosed with a stroke within 3 months after SH, which was confirmed by radiological examination.
We also found that two patients died because of ventricular tachycardia or ventricular fibrillation 1 month after an episode of SH, and one patient died of sudden death 6 months after SH, showing pulseless electrical activity on arrival at the emergency department. In addition, one patient died because of acute myocardial infarction 6 months after SH.
DISCUSSION
We investigated the changes in the QTc interval at baseline, during SH, and recovery after SH and associated factors with prolonged QTc interval in patients with T2DM. We demonstrated that baseline prolongation in the QTc interval was an independent predictor of a prolonged QTc interval during SH in patients with T2DM after adjusting for age, sex, diabetes duration, BMI, the habit of smoking, presence of hypertension, CKD, previous SH, and the use of insulin or sulfonylurea.
Hypoglycemia is a clinically important obstacle for glycemic control in patients with T1DM and T2DM, especially with intensive glucose-lowering treatment [292526]. Hypoglycemia causes acute problems in the brain, immediately affecting cognition, mood, and the level of consciousness [227]. Recent episodes of hypoglycemia cause a vicious cycle of recurrent hypoglycemia, which is known as hypoglycemia-associated autonomic failure [28]. Additionally, hypoglycemia can have fatal outcomes with an estimated mortality of 3.6% to 5.1% per year in patients with T2DM [5]. The link between hypoglycemia or SH and mortality has been studied in previous work. Tattersall and Gill [29] reported unexpected deaths in patients with T1DM who lacked a history of complications. This has become known as "dead-in-bed syndrome," and it was suggested that the deaths were due to hypoglycemia [29].
According to the ACCORD and ADVANCE trials, participants with T2DM during SH have a 2.28- to 4.86-fold higher incidence of mortality: ACCORD intensive treatment group (hazard ratio [HR], 1.28; 95% CI, 1.19 to 2.47), standard treatment group (HR, 2.87; 95% CI, 1.73 to 4.76); ADVANCE (HR, 4.86; 95% CI, 3.60 to 6.57; P<0.001) [530]. Zoungas et al. [5] reported that SH was associated with all-cause, cardiovascular, and non-cardiovascular mortality. Postulated mechanisms of cardiovascular mortality included sympathoadrenal activation, abnormal cardiac repolarization, leukocyte activation, increased thrombogenesis, inflammation, and vasoconstriction [1531].
Because the QT interval reflects the duration of myocardial depolarization and repolarization, a prolonged QTc interval is associated with an increased risk of ventricular arrhythmia, which could lead to sudden cardiac death [32]. Bazett's formula, which is most commonly used to correct heart rates, overcorrects at faster heart rates and undercorrects at slower heart rates [2432]. The QT interval overcorrects heart rates; therefore, it is essential to correct the QT interval for heart rate. Tsujimoto et al. [14] reported that SH was associated with QTc prolongation, severe hypertension, and newly developed atrial fibrillation in patients with T1DM and T2DM. Robinson et al. [15] also reported that the QTc interval during experimental hypoglycemia, using a hyperinsulinemic clamp, increased (67 ms, P<0.001). Therefore, sudden death or ventricular arrhythmia that develops during an SH occurrence might be predicted by the QTc interval prolongation in T1DM and T2DM.
We also found that two patients died because of ventricular tachycardia or ventricular fibrillation 1 month after SH, and one patient died of sudden death 6 months after SH. Prolonged QTc interval after SH might affect depolarization and repolarization of the myocardium and cause death, but correlation and a causal relationship between the development of SH and death could not be investigated because of short-term follow-up and a small number of subjects. Further studies are needed to evaluate clinical outcomes of patients with SH and QTc prolongation.
In this study, we demonstrated that 54.8% of patients with T2DM experienced QTc interval prolongation during SH and that the prolongation was significantly associated with the baseline QTc interval. In addition to the baseline QTc interval, only the female sex was associated with QTc prolongation during SH. Many studies have reported that young and middle-aged females have a longer QT interval than males and diabetes patients [2433]. The relationship between female sex and QTc interval in patients with T2DM was consistent with previous studies. For these reasons, we used 450 ms as the cut-off of QTc interval prolongation in men and 460 ms in women, respectively [24]. In addition, we could not identify any correlation between the QTc interval during an SH episode and diabetic duration or glycemic control status.
To the best of our knowledge, no previously published observational studies have analyzed the serial relationship of the QTc interval before and after an SH episode in T2DM. This study suggests that we should pay more scrupulous attention to the QTc interval and the presence of arrhythmia during SH as well as educating patients to avoid SH because of these prolonged QTc intervals, which are associated with an increased risk of a life-threatening cardiac arrhythmia. In addition, we suggest checking a 12-lead ECG to evaluate the QTc interval during the annual check-up of patients with diabetic complications.
The limitations of this study are as follows. First, this study was conducted retrospectively with a relatively small number of participants. Prospective studies are needed to estimate the causal relationship between fatality or higher mortality and QTc prolongation during SH. Second, we could not definitively explain the mechanism of QTc prolongation in SH. Third, we analyzed a single QT interval at the time of SH episodes and 3 to 6 months after SH; however, serial short-term follow-up of the QTc interval after SH would improve our understanding of the changes in the QTc prolongation and clinical outcomes.
In conclusion, the presence of a prolonged baseline QTc interval was an important predictive marker of QTc prolongation during an SH event in patients with T2DM. Therefore, we recommend that QTc interval monitoring be performed with screening for typical diabetic complications, especially in patients with a high risk of developing SH, such as patients with T2DM.
Notes
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.