- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Articles
- Page Path
- HOME > Diabetes Metab J > Volume 40(5); 2016 > Article
-
Original ArticleOthers Repeated Glucose Deprivation/Reperfusion Induced PC-12 Cell Death through the Involvement of FOXO Transcription Factor
-
Na Han1, You Jeong Kim2, Su Min Park2, Seung Man Kim2, Ji Suk Lee3, Hye Sook Jung3, Eun Ju Lee2, Tae Kyoon Kim2, Tae Nyun Kim2, Min Jeong Kwon2, Soon Hee Lee2, Mi-kyung Kim2,3
 , Byoung Doo Rhee2, Jeong Hyun Park2,3
, Byoung Doo Rhee2, Jeong Hyun Park2,3
-
Diabetes & Metabolism Journal 2016;40(5):396-405.
DOI: https://doi.org/10.4093/dmj.2016.40.5.396
Published online: September 1, 2016
1Division of Endocrinology and Metabolism, Department of Internal Medicine, Onhospital, Busan, Korea.
2Division of Endocrinology and Metabolism, Department of Internal Medicine, Inje University College of Medicine, Busan, Korea.
3Paik Institute for Clinical Research, Molecular Therapy Lab, Inje University, Busan, Korea.
- Corresponding author: Mi-kyung Kim. Division of Endocrinology and Metabolism, Department of Internal Medicine, Inje University College of Medicine, 875 Haeun-daero, Haeundae-gu, Busan 48108, Korea. kmkdoc@hanmail.net
- Corresponding author: Jeong Hyun Park. Division of Endocrinology and Metabolism, Department of Internal Medicine, Inje University College of Medicine, 75 Bokji-ro, Busanjin-gu, Busan 47392, Korea. pjhdoc@chol.net
Copyright © 2016 Korean Diabetes Association
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
-
Background
- Cognitive impairment and brain damage in diabetes is suggested to be associated with hypoglycemia. The mechanisms of hypoglycemia-induced neural death and apoptosis are not clear and reperfusion injury may be involved. Recent studies show that glucose deprivation/reperfusion induced more neuronal cell death than glucose deprivation itself. The forkhead box O (FOXO) transcription factors are implicated in the regulation of cell apoptosis and survival, but their role in neuronal cells remains unclear. We examined the role of FOXO transcription factors and the involvement of the phosphatidylinositol 3-kinase (PI3K)/Akt and apoptosis-related signaling pathways in PC-12 cells exposed to repeated glucose deprivation/reperfusion.
-
Methods
- PC-12 cells were exposed to control (Dulbecco's Modified Eagle Medium [DMEM] containing 25 mM glucose) or glucose deprivation/reperfusion (DMEM with 0 mM glucose for 6 hours and then DMEM with 25 mM glucose for 18 hours) for 5 days. MTT assay and Western blot analysis were performed for cell viability, apoptosis, and the expression of survival signaling pathways. FOXO3/4',6-diamidino-2-phenylindole staining was done to ascertain the involvement of FOXO transcription factors in glucose deprivation/reperfusion conditions.
-
Results
- Compared to PC-12 cells not exposed to hypoglycemia, cells exposed to glucose deprivation/reperfusion showed a reduction of cell viability, decreased expression of phosphorylated Akt and Bcl-2, and an increase of cleaved caspase-3 expression. Of note, FOXO3 protein was localized in the nuclei of glucose deprivation/reperfusion cells but not in the control cells.
-
Conclusion
- Repeated glucose deprivation/reperfusion caused the neuronal cell death. Activated FOXO3 via the PI3K/Akt pathway in repeated glucose deprivation/reperfusion was involved in genes related to apoptosis.
- Diabetes mellitus is a progressive disease which can result in complications of multiple organs. In addition to classic chronic diabetic complications, type 2 diabetes mellitus patients are vulnerable to dementia, cognitive impairment, and Alzheimer's disease [1]. Type 2 diabetes mellitus is also associated with cerebral atrophy [2]. Most complications resulting from diabetes are related to hyperglycemia, but several recent studies reported that cognitive impairment and brain damage were associated with severe hypoglycemia in animals and humans [345].
- Dementia is defined as an acquired deterioration in cognitive abilities that impairs the successful performance of activities of daily living, which is associated with loss of memory. A systematic literature review found that diabetes is associated with an increased risk of Alzheimer's dementia (AD), vascular dementia, and overall dementia [6]. The mechanism through which diabetes increases dementia is unclear. It has been proposed that vascular damage, hyperinsulinemia and insulin resistance, β-amyloid, and accumulation of advanced glycation end-products within brain tissue are involved [789]. Recently, several epidemiologic studies demonstrated that chronic recurrent hypoglycemia is associated with dementia [345]. The prolonged and severe hypoglycemia associated with diabetes increased oxidative stress and mitochondrial dysfunction; and discrete neuronal death was observed in the more vulnerable brain areas [1011121314]. Another suggested mechanism of hypoglycemia-induced neuronal cell death and apoptosis is related to reperfusion [15]. Harmful hypoglycemic stress conditions used in previous studies can be divided into hypoglycemia itself and hypoglycemia/reperfusion injury. Some reported that glucose deprivation/reperfusion produced more oxidative stress and more neuronal cell death than glucose deprivation itself. The timing of glucose deprivation/reperfusion also varied among different studies, generating different degrees of damage. The rat pheochromocytoma cell line PC-12 is widely used for AD research [16]. For example, PC-12 cells exposed to hypoglycemia for a prolonged time for such experiments used a 48-hour hypoglycemic condition [17].
- Forkhead box O (FOXO) transcription factors are involved in diverse cellular processes, such as glucose metabolism, cell cycle, and apoptosis [1819]. The FOXO transcription factors are one of the major direct substrates of the protein kinase Akt in cellular response, and the phosphatidylinositol 3-kinase (PI3K)/Akt signaling pathway plays a critical role in mediating survival signals in neuronal cells [20]. One study reported that sustained hypoglycemia inhibited cell proliferation via the PI3K/Akt pathway and induced neuronal apoptosis through caspase-3 activation, and activated FOXO transcription factors in the brain of the silkworm [21]; but, there are few studies that show the relationship between repeated glucose deprivation/reperfusion and FOXO transcription factors in neuronal cell death or damage. Therefore, we examined the role of FOXO transcription factors, and the involvement of the PI3K/Akt and apoptosis-related signaling pathways in PC-12 cells exposed to repeated glucose deprivation/reperfusion injury.
INTRODUCTION
- Cell culture
- PC-12 cells, derived from a pheochromocytoma of the rat adrenal medulla, obtained from Dr. Young Hoon Kim of Inje University Busan Paik Hospital, South Korea, were plated and maintained in Dulbecco's Modified Eagle Medium (DMEM) (Gibco; Thermo Fisher Scientific, Waltham, MA, USA) with 25 mM glucose and 10% fetal bovine serum; and the cells were stabilized for 24 hours. All experiments were incubated in a humidified atmosphere of 5% CO2 and 95% air at 37℃.
- Cell
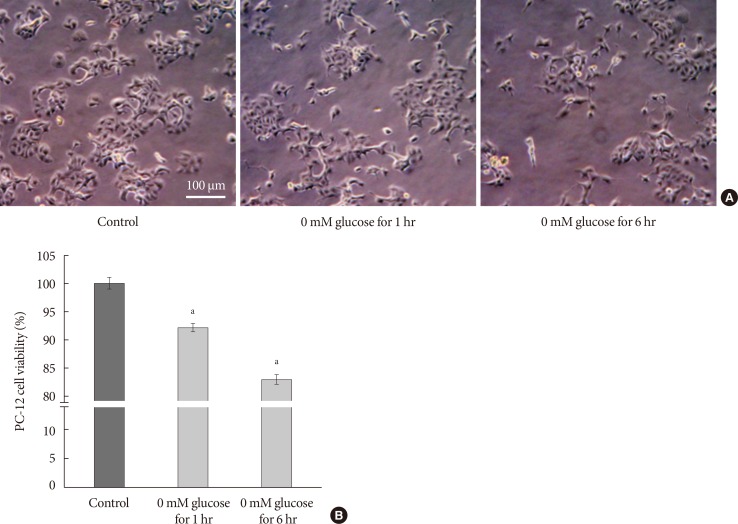
- The PC-12 cells were plated at a density of 3×104 cells/well in a 96-well plate for cell viability assay, in 60 mm diameter culture dishes for Western blot analysis, and in 6-well plates onto cover-slides for FOXO staining. After the cells were stabilized for 24 hours at 37℃, they were cultured for 6 hours a day in culture medium with 0 mM glucose DMEM. Cells were then switched and reperfused for 18 hours in culture medium with 25 mM glucose DMEM. For the controls, the cells were cultured with 25 mM glucose DMEM for 24 hours. This was repeated daily for 5 days. This was a modification of the methods in a previous study using the same PC-12 cells, which used 4-hour oxygen and glucose deprivation, followed by 20 hours reperfusion to study PC-12 cell apoptosis [15]. We used glucose deprivation for 1 hour/reperfusion for 23 hours and glucose deprivation for 6 hours/reperfusion for 18 hours; and continued for 5 days to evaluate the involvement of FOXO in repeated glucose deprivation/reperfusion. These were compared with control cells not exposed to hypoglycemia. The cell growths were decreased in 0 mM glucose DMEM for 1 hour and for 6 hours compared with the controls. Cell growth was decreased in proportion to the time exposed to glucose deprivation (Fig. 1A). The cell viability was decreased by 8% in 0 mM glucose DMEM for 1 hour and by 19% in 0 mM glucose DMEM for 6 hours, compared with the controls not exposed hypoglycemia (Fig. 1B). The nuclear FOXO3 localization showed a 10% increase in 0 mM glucose DMEM for 1 hour and 31% increase in 0 mM glucose DMEM for 6 hours when compared with the controls (data not shown). Both glucose deprivation times showed a decrease in Bcl-2 and phosphorylated Akt (phospho-Akt) and increased cleaved caspase-3 compared with the controls (data not shown). We conducted the glucose deprivation for 6 hours/reperfusion for 18 hours to emphasize the relationship of FOXO in repeated glucose deprivation/reperfusion in PC-12 cells.
- Cell viability assay (MTT assay)
- The PC-12 cells were plated at a density of 3×104 cells/well in 96-well plate and were stabilized for 24 hours. The cells were incubated and treated according to the above experimental schedule for 5 days. The MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) solution was added to the cell samples to make a final concentration of 0.5 mg/mL. The reaction mixture was allowed to incubate at 37℃ for 4 hours. The 96-well plates were wrapped in aluminum foil after removal from the incubator. The reaction mixture was carefully removed, and 100 µL of dimethyl sulfoxide was added to each well. The 96-well plate wrapped in aluminum foil was shaken for 10 minutes at room temperature. The absorbance (optical density) values were measured by spectrophotometry at 570 nm.
- Immunofluorescence microscopy
- The PC-12 cells were plated at a density of 3×104 cells/well in a 6-well plate placed on the cover glass and were stabilized for 24 hours. The cells were incubated and treated according to the experimental schedule for 5 days. The cells were rinsed three times using phosphate-buffered saline (PBS) (1 mL/well) for 5 minutes and were fixed and permeabilized with cytoperm/cytofix (BD, San Jose, CA, USA) for 20 minutes and were rinsed the times using PBS for 5 minutes. The cells were blocked in 100 µL/well of blocking buffer (1×PBS/5% normal goat serum/0.3% Triton X-100) for 60 minutes. After aspirating the blocking solution, the diluted primary Ab (FOXO) was added to each well. The 6-well plate wrapped in aluminum foil was incubated overnight at 4℃. The cells were rinsed three times using PBS for 5 minutes, and incubated with fluorescence-conjugated secondary Ab diluted in Ab dilution buffer for 1 hour at room temperature in aluminum foil. A 300 µL of the diluted 4',6-diamidino-2-phenylindole (DAPI) staining solution was added to the coverslip placed on the 6-well plate. The cells were incubated for 20 minutes at 37℃ and rinsed three times using PBS for 5 minutes. After draining the excess buffer from the coverslip, ProLong Gold Antifade Reagent (P36930; Thermo Fisher Scientific, Waltham, MA, USA) was added onto the coverslips. Cells were observed using a fluoroscene microscope with appropriate filters (Olympus BX-51; Olympus, Tokyo, Japan). Quantitative measurement of FOXO3 staining was calculated by counting the nuclear FOXO3 (+) cells among the total cells. For reducing interpreting bias by investigator, three investigators performed counting independently and it was repeated three times.
- Western blot analysis
- The PC-12 cells were plated at a density of 3×104 cells/well in a 6-well plate placed on cover glass and were stabilized for 24 hours. The cells were incubated and treated according to the experimental schedule for 5 days. PC-12 cells were placed on ice and washed using PBS. The cells were dislodged using a cell scraper and pipetted into microcentrifuge tubes. They were centrifuged at 1,500 rpm for 5 minutes and the supernatant was discarded. Cells were lysed in mammalian tissue lysis/extraction reagent including protease inhibitor. Proteins were quantified using the bicinchoninic acid protein assay kit. Samples were prepared in 1×sodium dodecyl sulfate (SDS) sample buffer (50 mM Tris pH 6.8, 2% SDS, 10% glycerol, 50 mM dithiothreitol, and 0.01% bromophenol blue). Proteins were separated in 12% SDS-PAGE and transferred onto a polyvinylidene fluoride membrane. After blocking the membrane with 5% skim milk in Tris-buffered saline and Tween 20 at room temperature for 60 minutes, the membrane was incubated and immunoblotted with anti-caspase-3 (1:1,000), anti-cleaved caspase-3 (1:1,000), anti-Bcl-2 (1:1,000), anti-Akt (1:1,000), anti-phospho-Akt (Ser473, 1:1,000), and anti-α-tubulin (1:1,000) at 4℃ overnight. The biotinylated anti-mouse or anti-rabbit immunoglobulin G secondary antibodies were conjugated and were applied for 1 hour at room temperature. The membrane was developed by the alkaline phosphatase-conjugated development kit (Bio-Rad, Hercules, CA, USA). Developed protein bands were quantified by Multi Gauge version 2.2 program (Fuji photo film, Tokyo, Japan). α-Tubulin served as an internal control.
- Statistical analysis
- The experimental results were reported as mean±standard error of mean. Student t-test was performed to evaluate the significance of the difference and was presented as P<0.05.
METHODS
- Cell growth and cell viability of PC-12 cells exposed to repeated glucose deprivation/reperfusion
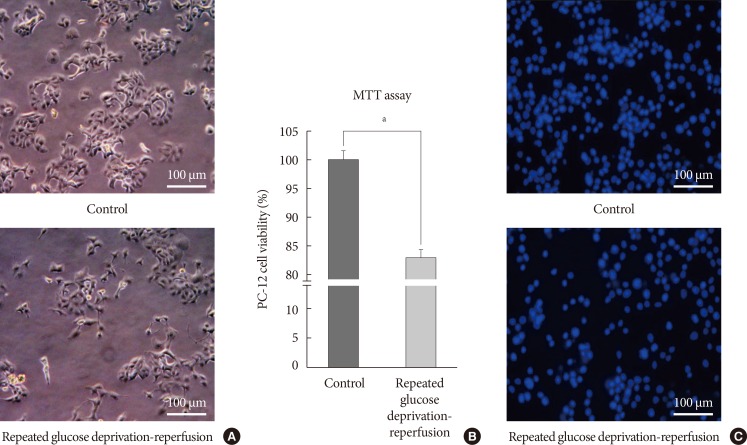
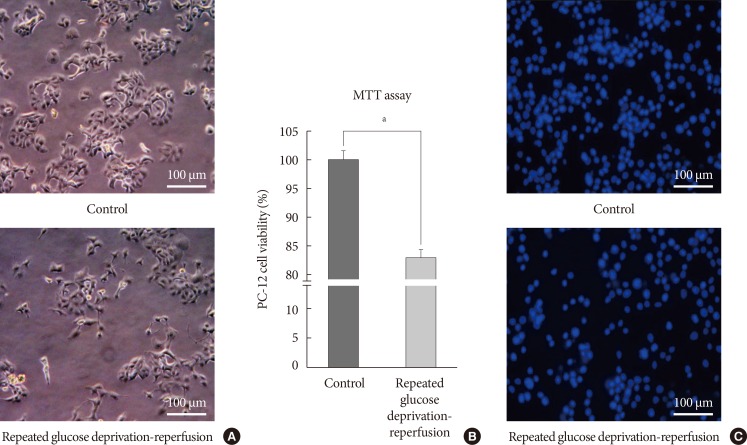
- We checked the cell growth of PC-12 cells cultured in accordance with the experimental schedule. We observed shrinkage of PC-12 cells and vacuolization in the cytoplasm. They were gradually detached from the culture dishes after acute and recurrent glucose deprivation. We took light microscope-slide imaging of PC-12 cells with a digital camera (×400; Canon, Tokyo, Japan). Fig. 2A is an image of PC-12 cells exposed to sustained 25 mM glucose DMEM and PC-12 cells exposed to 0 mM glucose DMEM for 6 hours/25 mM glucose DMEM for 18 hours for 5 days. MTT assay was used to compare cell viability between cells exposed to repeated glucose deprivation/reperfusion condition and the controls. Cell viability in PC-12 cells exposed to repeated glucose deprivation/reperfusion was decreased by 19% compared with that of the controls (P<0.05) (Fig. 2B). We also checked cell viability by DAPI staining (Fig. 2C). Exposure to repeated glucose deprivation/reperfusion caused a significant decrease in cell growth and cell viability of PC-12 cells compared to the controls not exposed to hypoglycemia.
- The nuclear localization of FOXO3 in PC-12 cells exposed to repeated glucose deprivation/reperfusion
- To assess the involvement of FOXO3 in repeated glucose deprivation/reperfusion conditions, we examined double staining (DAPI and FOXO3). Fig. 3A shows an example of FOXO3 staining. FOXO3 staining was expressed in many PC-12 cell nuclei exposed to repeated glucose deprivation/reperfusion (red arrowheads). Nuclear FOXO3 localization was not observed in control PC-12 cells in the representative figure. The percentage of nuclear FOXO3/DAPI in PC-12 cells exposed to repeated glucose deprivation/reperfusion was increased by 31%, compared with the controls not exposed to hypoglycemia (P<0.05 vs. control) (Fig. 3B).
- Protein expressions of phospho-Akt, Bcl-2, caspase-3, and cleaved caspase-3 in PC-12 cells exposed to repeated glucose deprivation/reperfusion
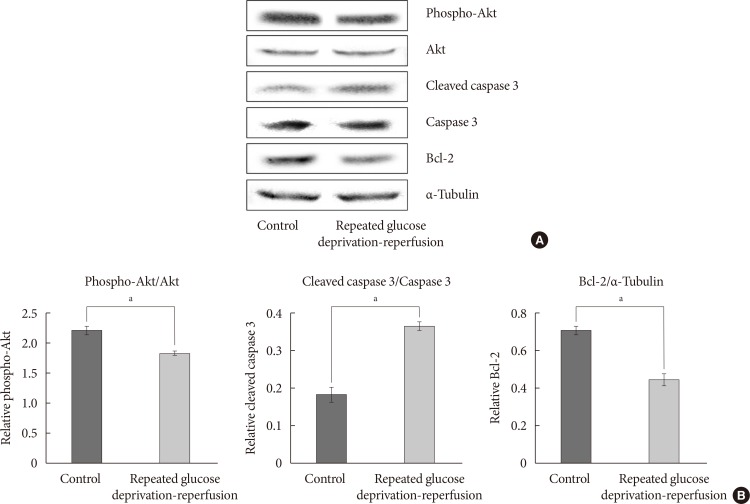
- To evaluate whether the PI3K/Akt pathway regulates cell apoptosis in repeated glucose deprivation/reperfusion conditions, the phospho-Akt and Akt protein levels were measured using Western blot analysis in treated PC-12 cells. As shown in Fig. 4A, phospho-Akt expression was decreased in PC-12 cells exposed to repeated glucose deprivation/reperfusion conditions more than the controls (P<0.05). To evaluate the expression of proteins related to the apoptosis of neuronal cells in repeated glucose deprivation/reperfusion, we measured the change in the protein expressions of anti-apoptotic gene Bcl-2 and pro-apoptotic genes, i.e., caspase-3 and cleaved caspase-3, using Western blot analysis. In Fig. 4, Bcl-2 protein levels were significantly reduced and cleaved caspase-3 protein was significantly increased in PC-12 cells exposed to repeated glucose deprivation/reperfusion compared with the controls not exposed to hypoglycemia (both P<0.05).
RESULTS
- In our study, cell growth and viability of PC-12 cells were decreased in repeated glucose deprivation/reperfusion. That stimulus localized FOXO3 to the nucleus when compared to the controls. The expressions of phospho-Akt and Bcl-2 proteins were decreased and cleaved caspase-3 expression was increased in repeated glucose deprivation/reperfusion. This study has demonstrated that PC-12 cell death due to repeated glucose deprivation/reperfusion was related to FOXO transcription factors, which induced changes in expression of PI3K/Akt, Bcl-2, caspase-3 and neuronal cell apoptosis.
- The neuronal death resulting from hypoglycemia is not only a result of energy failure, but also the sequence of events initiated by hypoglycemia. Suggested mechanisms, through previous studies, were depletion of adenosine triphosphate/depolarization [22], glutamine release [23], calcium release [2224], and reactive oxygen species production [2526]. Amyloid β-peptide-caused Alzheimer's disease inhibits glucose transport, which in turn triggers the glucose deprivation apoptotic cascade in the cortex and hippocampus [2728]. Another suggested mechanism of hypoglycemia-induced neuronal cell death and apoptosis is related to reperfusion. Hypoglycemia itself needs severe and prolonged exposure time to induce neuronal injury, but reperfusion/hypoglycemia caused neuronal damage within a relatively shorter hypoglycemic period. One study showed superoxide production occurred mainly during the period of glucose reperfusion, rather than during hypoglycemia itself [29]. Global cerebral ischemia results in subsequent reperfusion injury in the vulnerable brain, including the hippocampus, which is related to dementia [30]. Several studies have used experimental models, so called 'ischemia induced oxygen-glucose deprivation.' PC-12 cells were exposed to culture medium without glucose and placed in a controlled atmosphere chamber for 4 to 10 hours in that model. They reported that reperfusion after oxygen-glucose deprivation caused neuronal cell apoptosis [313233]. We modified the experimental conditions from previous reports for the reperfusion/hypoglycemic condition. The previous studies used oxygen-glucose deprivation for 4 hours/reperfusion for 20 hours [15]; serum and glucose deprivation for 6 and 18 hours [3435]; and 2.5 hours oxygen-glucose deprivation, followed by a 24-hour reoxygenation period [36] to study PC-12 cell apoptosis. We used glucose deprivation for 1 hour/reperfusion for 23 hours and glucose deprivation for 6 hours/reperfusion for 18 hours, and continued for 5 days to evaluate the involvement of FOXO in repeated glucose deprivation/reperfusion, and compared them with the controls not exposed to hypoglycemia.
- FOXO transcription factors are activated upon inhibition of PI3K/Akt signaling and involved in diverse cellular processes such as glucose metabolism, cell cycle, and apoptosis [1819]. On inhibition of the PI3K/Akt pathway, FOXO transcription factors are localized in the nucleus, which cause cell cycle arrest, stress resistance, and cell death. The activated PI3K/Akt pathway relocates FOXO proteins from the nucleus to the cytoplasm, and inhibits FOXO-dependent transcription; which causes cell proliferation, stress sensitivity, and cell survival [37]. FOXO proteins are expressed to varying degrees in all tissues in mammals. FOXO1 mRNA is predominantly expressed in adipose tissues, FOXO3 mRNA in the brain, FOXO4 mRNA in the heart, and FOXO6 mRNA in the developing brain [38]. Sustained hypoglycemia-induced apoptosis through involvement of FOXO transcription factors and caspase-3 activation was found in the brain of the silkworm Bombyx mori [21]. We experimented with FOXO3 on neuronal cells and observed FOXO3 nuclear localization against repeated glucose deprivation/reperfusion conditions. FOXO3 translocation was related to PC-12 cell viability and apoptotic pathways in our study.
- One of limitations of this study is that we did not apply FOXO inhibitor or PI3K inhibitor to evaluate the causal relationship between FOXO-related transcription factors and neuronal cell death. But, previous studies already showed that FOXO3 is closely related to the PI3K/Akt pathway, apoptotic signals, and cell death. The second limitation is that we did not compare glucose deprivation/reperfusion stimuli to hypoglycemia only, or high glucose treatment in PC-12 cells. Our major interest was to determine if FOXO3 and related proteins could change under reperfusion/hypoglycemia stimuli in neuronal cells. A third limitation is that we calculated nuclear FOXO3 localization ratio only by imaging, and did not perform Western blots on FOXO3 with the cytosolic fraction or the nuclear fraction of cell extracts. However, we repeated counting the nuclear FOXO3 staining by three different investigators to reduce the bias of interpreters. Finally, our fourth limitation is that we did not evaluate the relationship between FOXO and oxidative stress under reperfusion/hypoglycemia stimuli. The previous studies already showed that superoxide is produced at the time of glucose reperfusion after hypoglycemia, and the degree of superoxide production and neuronal death increased with increasing glucose concentration during the reperfusion period [29].
- In conclusion, FOXO3 nuclear localization was observed in PC-12 cells against repeated glucose deprivation/reperfusion, and activated FOXO proteins were related to a decrease of Bcl-2 and an increase of cleaved caspase-3 expression. We showed that FOXO transcription factors may be involved in repeated glucose deprivation/reperfusion-induced neuronal cell death.
DISCUSSION
-
Acknowledgements
- Rat PC-12 cells were provided by Young Hoon Kim MD, PhD of Paik Institute for Neuroscience Research, Inje University, Busan, South Korea.
ACKNOWLEDGMENTS
-
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.
NOTES
- 1. Cukierman-Yaffe T, Gerstein HC, Williamson JD, Lazar RM, Lovato L, Miller ME, Coker LH, Murray A, Sullivan MD, Marcovina SM, Launer LJ. Action to Control Cardiovascular Risk in Diabetes-Memory in Diabetes (ACCORD-MIND) Investigators. Relationship between baseline glycemic control and cognitive function in individuals with type 2 diabetes and other cardiovascular risk factors: the action to control cardiovascular risk in diabetes-memory in diabetes (ACCORD-MIND) trial. Diabetes Care 2009;32:221-226. ArticlePubMedPMCPDF
- 2. Bruehl H, Wolf OT, Sweat V, Tirsi A, Richardson S, Convit A. Modifiers of cognitive function and brain structure in middle-aged and elderly individuals with type 2 diabetes mellitus. Brain Res 2009;1280:186-194. ArticlePubMedPMC
- 3. Ahtiluoto S, Polvikoski T, Peltonen M, Solomon A, Tuomilehto J, Winblad B, Sulkava R, Kivipelto M. Diabetes, Alzheimer disease, and vascular dementia: a population-based neuropathologic study. Neurology 2010;75:1195-1202. ArticlePubMed
- 4. Whitmer RA, Karter AJ, Yaffe K, Quesenberry CP Jr, Selby JV. Hypoglycemic episodes and risk of dementia in older patients with type 2 diabetes mellitus. JAMA 2009;301:1565-1572. ArticlePubMedPMC
- 5. Auer RN. Hypoglycemic brain damage. Metab Brain Dis 2004;19:169-175. ArticlePubMed
- 6. Biessels GJ, Staekenborg S, Brunner E, Brayne C, Scheltens P. Risk of dementia in diabetes mellitus: a systematic review. Lancet Neurol 2006;5:64-74. ArticlePubMed
- 7. Fishel MA, Watson GS, Montine TJ, Wang Q, Green PS, Kulstad JJ, Cook DG, Peskind ER, Baker LD, Goldgaber D, Nie W, Asthana S, Plymate SR, Schwartz MW, Craft S. Hyperinsulinemia provokes synchronous increases in central inflammation and beta-amyloid in normal adults. Arch Neurol 2005;62:1539-1544. PubMed
- 8. Matsuzaki T, Sasaki K, Tanizaki Y, Hata J, Fujimi K, Matsui Y, Sekita A, Suzuki SO, Kanba S, Kiyohara Y, Iwaki T. Insulin resistance is associated with the pathology of Alzheimer disease: the Hisayama study. Neurology 2010;75:764-770. ArticlePubMed
- 9. Yaffe K, Lindquist K, Schwartz AV, Vitartas C, Vittinghoff E, Satterfield S, Simonsick EM, Launer L, Rosano C, Cauley JA, Harris T. Advanced glycation end product level, diabetes, and accelerated cognitive aging. Neurology 2011;77:1351-1356. ArticlePubMedPMC
- 10. Yu S, Liu M, Gu X, Ding F. Neuroprotective effects of salidroside in the PC12 cell model exposed to hypoglycemia and serum limitation. Cell Mol Neurobiol 2008;28:1067-1078. ArticlePubMedPDF
- 11. Silverstein JM, Musikantow D, Puente EC, Daphna-Iken D, Bree AJ, Fisher SJ. Pharmacologic amelioration of severe hypoglycemia-induced neuronal damage. Neurosci Lett 2011;492:23-28. ArticlePubMedPMC
- 12. Yousefzade G, Nakhaee A. Insulin-induced hypoglycemia and stress oxidative state in healthy people. Acta Diabetol 2012;49(Suppl 1):S81-S85. ArticlePubMedPDF
- 13. Wang J, Alexanian A, Ying R, Kizhakekuttu TJ, Dharmashankar K, Vasquez-Vivar J, Gutterman DD, Widlansky ME. Acute exposure to low glucose rapidly induces endothelial dysfunction and mitochondrial oxidative stress: role for AMP kinase. Arterioscler Thromb Vasc Biol 2012;32:712-720. PubMed
- 14. Languren G, Montiel T, Julio-Amilpas A, Massieu L. Neuronal damage and cognitive impairment associated with hypoglycemia: an integrated view. Neurochem Int 2013;63:331-343. ArticlePubMed
- 15. Fan L, Dang X, Shi Z, Zhang C, Wang K. Hydroxysafflor yellow A protects PC12 cells against the apoptosis induced by oxygen and glucose deprivation. Cell Mol Neurobiol 2011;31:1187-1194. ArticlePubMedPDF
- 16. Dong H, Mao S, Wei J, Liu B, Zhang Z, Zhang Q, Yan M. Tanshinone IIA protects PC12 cells from beta-amyloid(25-35)-induced apoptosis via PI3K/Akt signaling pathway. Mol Biol Rep 2012;39:6495-6503. ArticlePubMedPDF
- 17. Mao W, Iwai C, Keng PC, Vulapalli R, Liang CS. Norepinephrine-induced oxidative stress causes PC-12 cell apoptosis by both endoplasmic reticulum stress and mitochondrial intrinsic pathway: inhibition of phosphatidylinositol 3-kinase survival pathway. Am J Physiol Cell Physiol 2006;290:C1373-C1384. ArticlePubMed
- 18. Myatt SS, Lam EW. The emerging roles of forkhead box (Fox) proteins in cancer. Nat Rev Cancer 2007;7:847-859. ArticlePubMedPDF
- 19. Ho KK, Myatt SS, Lam EW. Many forks in the path: cycling with FoxO. Oncogene 2008;27:2300-2311. ArticlePubMedPDF
- 20. Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 1999;96:857-868. ArticlePubMed
- 21. Kim JH, Choi JS, Lee BH. PI3K/Akt and MAPK pathways evoke activation of FoxO transcription factor to undergo neuronal apoptosis in brain of the silkworm Bombyx mori (Lepidoptera: Bombycidae). Cell Mol Biol (Noisy-le-grand) 2012;(Suppl.58):OL1780-OL1785. PubMed
- 22. Harris RJ, Wieloch T, Symon L, Siesjo BK. Cerebral extracellular calcium activity in severe hypoglycemia: relation to extracellular potassium and energy state. J Cereb Blood Flow Metab 1984;4:187-193. ArticlePubMedPDF
- 23. Sandberg M, Butcher SP, Hagberg H. Extracellular overflow of neuroactive amino acids during severe insulin-induced hypoglycemia: in vivo dialysis of the rat hippocampus. J Neurochem 1986;47:178-184. ArticlePubMed
- 24. Ferrand-Drake M, Friberg H, Wieloch T. Mitochondrial permeability transition induced DNA-fragmentation in the rat hippocampus following hypoglycemia. Neuroscience 1999;90:1325-1338. ArticlePubMed
- 25. McGowan JE, Chen L, Gao D, Trush M, Wei C. Increased mitochondrial reactive oxygen species production in newborn brain during hypoglycemia. Neurosci Lett 2006;399:111-114. ArticlePubMed
- 26. Paramo B, Hernandez-Fonseca K, Estrada-Sanchez AM, Jimenez N, Hernandez-Cruz A, Massieu L. Pathways involved in the generation of reactive oxygen and nitrogen species during glucose deprivation and its role on the death of cultured hippocampal neurons. Neuroscience 2010;167:1057-1069. ArticlePubMed
- 27. Mark RJ, Pang Z, Geddes JW, Uchida K, Mattson MP. Amyloid beta-peptide impairs glucose transport in hippocampal and cortical neurons: involvement of membrane lipid peroxidation. J Neurosci 1997;17:1046-1054. ArticlePubMedPMC
- 28. Keller JN, Pang Z, Geddes JW, Begley JG, Germeyer A, Waeg G, Mattson MP. Impairment of glucose and glutamate transport and induction of mitochondrial oxidative stress and dysfunction in synaptosomes by amyloid beta-peptide: role of the lipid peroxidation product 4-hydroxynonenal. J Neurochem 1997;69:273-284. PubMed
- 29. Suh SW, Gum ET, Hamby AM, Chan PH, Swanson RA. Hypoglycemic neuronal death is triggered by glucose reperfusion and activation of neuronal NADPH oxidase. J Clin Invest 2007;117:910-918. ArticlePubMedPMC
- 30. Kirino T, Sano K. Selective vulnerability in the gerbil hippocampus following transient ischemia. Acta Neuropathol 1984;62:201-208. ArticlePubMedPDF
- 31. Lin HC, Narasimhan P, Liu SY, Chan PH, Lai IR. Postconditioning mitigates cell death following oxygen and glucose deprivation in PC12 cells and forebrain reperfusion injury in rats. J Neurosci Res 2015;93:140-148. ArticlePubMed
- 32. Nakamura T, Minamisawa H, Katayama Y, Ueda M, Terashi A, Nakamura K, Kudo Y. Increased intracellular Ca2+ concentration in the hippocampal CA1 area during global ischemia and reperfusion in the rat: a possible cause of delayed neuronal death. Neuroscience 1999;88:57-67. ArticlePubMed
- 33. Frantseva MV, Carlen PL, Perez Velazquez JL. Dynamics of intracellular calcium and free radical production during ischemia in pyramidal neurons. Free Radic Biol Med 2001;31:1216-1227. ArticlePubMed
- 34. Shafaei-Bajestani N, Emami SA, Asili J, Tayarani-Najaran Z. Anti-apoptotic effect of taxodione on serum/glucose deprivation-induced PC12 cells death. Cell Mol Neurobiol 2014;34:1103-1109. ArticlePubMedPDF
- 35. Mousavi SH, Tayarani-Najaran Z, Asghari M, Sadeghnia HR. Protective effect of Nigella sativa extract and thymoquinone on serum/glucose deprivation-induced PC12 cells death. Cell Mol Neurobiol 2010;30:591-598. ArticlePubMedPDF
- 36. Zhao J, Bai Y, Zhang C, Zhang X, Zhang YX, Chen J, Xiong L, Shi M, Zhao G. Cinepazide maleate protects PC12 cells against oxygen-glucose deprivation-induced injury. Neurol Sci 2014;35:875-881. ArticlePubMedPDF
- 37. Greer EL, Brunet A. FOXO transcription factors at the interface between longevity and tumor suppression. Oncogene 2005;24:7410-7425. ArticlePubMedPDF
- 38. Jacobs FM, van der Heide LP, Wijchers PJ, Burbach JP, Hoekman MF, Smidt MP. FoxO6, a novel member of the FoxO class of transcription factors with distinct shuttling dynamics. J Biol Chem 2003;278:35959-35967. ArticlePubMed
REFERENCES
Cell growth and cell viability in glucose deprivation for 1 hour/reperfusion for 23 hours and glucose deprivation for 6 hours/reperfusion for 18 hours for 5 days. (A) Representative light microscopic imaging (×400) of PC-12 cells not exposed to hypoglycemia, 0 mM glucose Dulbecco's Modified Eagle Medium (DMEM) for 1 hour, and 0 mM glucose DMEM for 6 hours for 5 days. (B) Quantification of cell viability used MTT assay in PC-12 cells exposed to 0 mM glucose DMEM for 1 hour and for 6 hours compared with the controls, not exposed to hypoglycemia. Scale bars represent 100 µm. Data are mean±standard error of mean. Statistical significance was tested using a Student t-test. The data shown represents three independent experiments. aRepresents P<0.05 vs. control.

Cell growth and cell viability in PC-12 cells exposed to glucose deprivation/reperfusion. (A) Representative light microscopic imaging (×400) of PC-12 cells not exposed to hypoglycemia and exposed to repeated glucose deprivation/reperfusion for 5 days. (B) Quantification of cell viability in PC-12 cells exposed to repeated glucose deprivation/reperfusion and the controls by using MTT assay. The intact nuclei were counted on a haemocytometer. Scale bars represent 100 µm. Data are mean±standard error of mean. Statistical significance was tested using a Student t-test. (C) Representative 4',6-diamidino-2-phenylindole counterstaining of PC-12 cells exposed to repeated glucose deprivation-reperfusion for 5 days and control. The data shown represent three independent experiments. aRepresents P<0.05 vs. control.
The nuclear localization of forkhead box O 3 (FOXO3) in PC-12 cells. (A) Representative FOXO3 staining in PC-12 cells. FOXO3 is localized to the nuclei (red arrowheads) in PC-12 cells exposed to repeated glucose deprivation/reperfusion while FOXO3 is localized to the cytoplasm in the controls. (B) Quantification of nuclear localization of FOXO3 in PC-12 cells exposed to repeated glucose deprivation/reperfusion and the controls. Scale bars represent 200 µm. Data are mean±standard error of mean. Statistical significance was tested using an unpaired, two-tailed Student t-test. The data shown represent three independent experiments. DAPI, 4',6-diamidino-2-phenylindole. aP<0.05 compared with control.

Protein expressions of Akt, phosphorylated Akt (phospho-Akt), caspase-3, cleaved caspase-3, and Bcl-2 in PC-12 cells exposed to repeated glucose deprivation/reperfusion condition. (A) The expressions of phospho-Akt, Akt, cleaved caspase-3, and Bcl-2 were assessed by Western blot. (B) Quantification of phospho-Akt/Akt, cleaved caspase-3/caspase-3 and Bcl-2 expressions. Data are mean±standard error of mean from three independent experiments. Statistical significance was tested using an unpaired, two-tailed Student t-test. aRepresents P<0.05.
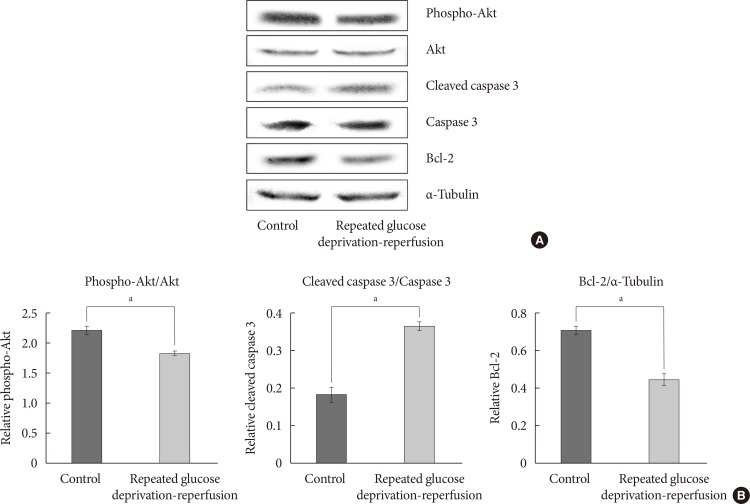
Figure & Data
References
Citations

- Predictive factors for the development of diabetes in cancer patients treated with phosphatidylinositol 3-kinase inhibitors
Gyuri Kim, Myungeun Yoo, Min Hee Hong, Byung-Wan Lee, Eun Seok Kang, Bong-Soo Cha, Hye Ryun Kim, Yong-ho Lee, Byoung Chul Cho
Cancer Chemotherapy and Pharmacology.2019; 84(2): 405. CrossRef

 KDA
KDA PubReader
PubReader Cite
Cite