Statins Increase Mitochondrial and Peroxisomal Fatty Acid Oxidation in the Liver and Prevent Non-Alcoholic Steatohepatitis in Mice
Article information
Abstract
Background
Non-alcoholic fatty liver disease is the most common form of chronic liver disease in industrialized countries. Recent studies have highlighted the association between peroxisomal dysfunction and hepatic steatosis. Peroxisomes are intracellular organelles that contribute to several crucial metabolic processes, such as facilitation of mitochondrial fatty acid oxidation (FAO) and removal of reactive oxygen species through catalase or plasmalogen synthesis. Statins are known to prevent hepatic steatosis and non-alcoholic steatohepatitis (NASH), but underlying mechanisms of this prevention are largely unknown.
Methods
Seven-week-old C57BL/6J mice were given normal chow or a methionine- and choline-deficient diet (MCDD) with or without various statins, fluvastatin, pravastatin, simvastatin, atorvastatin, and rosuvastatin (15 mg/kg/day), for 6 weeks. Histological lesions were analyzed by grading and staging systems of NASH. We also measured mitochondrial and peroxisomal FAO in the liver.
Results
Statin treatment prevented the development of MCDD-induced NASH. Both steatosis and inflammation or fibrosis grades were significantly improved by statins compared with MCDD-fed mice. Gene expression levels of peroxisomal proliferator-activated receptor α (PPARα) were decreased by MCDD and recovered by statin treatment. MCDD-induced suppression of mitochondrial and peroxisomal FAO was restored by statins. Each statin's effect on increasing FAO and improving NASH was independent on its effect of decreasing cholesterol levels.
Conclusion
Statins prevented NASH and increased mitochondrial and peroxisomal FAO via induction of PPARα. The ability to increase hepatic FAO is likely the major determinant of NASH prevention by statins. Improvement of peroxisomal function by statins may contribute to the prevention of NASH.
INTRODUCTION
Non-alcoholic fatty liver disease (NAFLD) is a clinical spectrum of liver damage, from simple steatosis to more advanced stages, such as non-alcoholic steatohepatitis (NASH), fibrosis, or cirrhosis [12]. Because NAFLD is closely associated with obesity, diabetes, and cardiovascular disease, it is regarded as a representative hepatic phenotype of metabolic syndrome [3]. NAFLD is the most common cause of chronic liver disease in developed countries [4], and the prevalence in the general population ranges from 20% to 30% [5].
Statins competitively inhibit 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase, the rate-limiting enzyme in cholesterol synthesis, and are widely used as cholesterol-lowering drugs. The overall benefits of statins seem to be greater than what might be expected from an alteration in the lipid profile alone, suggesting that statins have cholesterol-independent pleiotropic effects [6]. However, the basic mechanism underlying these pleiotropic effects is largely unknown. Statins prevent hepatic steatosis in animals, and suggested mechanisms include prevention of carbohydrate response element-binding protein activation [7] and the induction of peroxisomal proliferator-activated receptor α (PPARα) [8], the master regulator of fatty acid oxidation (FAO). Statins also attenuate hepatic inflammatory reactions induced by angiotensin II [9] and prevent hepatic fibrosis by inactivating hepatic stellate cells [10]. Accumulation of free cholesterol in the mitochondria is suggested to be a major mechanism for steatohepatitis [11], and lowering of free cholesterol by statins may also be a mechanism of how statins prevent NASH.
It is well known that decreased mitochondrial function contributes to the development of hepatic steatosis [12]. Recent studies have also highlighted the association between peroxisomal dysfunction and hepatic steatosis [1314]. Peroxisomes are ubiquitous, single-membrane-bounded organelles and exist in all eukaryotes [15]. The main metabolic functions of peroxisomes in mammalian cells include degradation of very long chain and branched-chain fatty acids, which cannot be instantly oxidized in mitochondria. Peroxisomes are responsible for the biosynthesis of plasmalogen, a special class of lipids, and docosahexaenoic acid, a final elongation and desaturation product of n-3 polyunsaturated fatty acids [16]. Peroxisomes also play a critical role in maintenance of intracellular redox balance. Production of reactive oxygen species is inevitable in fuel metabolism, and peroxisomes possess several anti-oxidative systems, including catalase, superoxide dismutases, and peroxiredoxins [17]. Despite their importance, less attention has been paid to peroxisomes than other organelles, such as mitochondria, endoplasmic reticulum, and lysosomes.
In this study, we found that treatment with various statins ameliorated hepatic steatosis and steatohepatitis and that this was associated with increased hepatic FAO. In particular, peroxisomal FAO, as well as mitochondrial FAO, was significantly decreased in the liver of NASH animals, and this was recovered by statins.
METHODS
Animals
Seven-week-old male C57BL/6N mice were purchased from Central Lab Animal Inc. (Seoul, Korea) and acclimated for 1 week prior to the experiment. Animals were housed at an ambient temperature (22℃±1℃) on a 12-hour/12-hour light/dark cycle with free access to water and diet. Mice were fed normal chow diet (ND; n=10), methionine- and choline-deficient diet (MCDD; n=10; Dyets Inc., Bethlehem, PA, USA), or MCDD with 15 mg/kg/day of each statin supplementation (n=6 to 10) for 6 weeks. At the end of experiment period, mice were fasted (5 hours) in the morning and then sacrificed. Blood samples were collected and the livers were rapidly harvested, quickly frozen in liquid nitrogen, and stored at –80℃. All animal experiment protocols were approved by the Institutional Animal Care and Use Committee of the Asan Institute for Life Sciences, Seoul, South Korea.
Histological analysis
Liver tissue samples were fixed with 4% paraformaldehyde and embedded for 5 µm serial paraffin sections. The sections were stained with hematoxylin and eosin for evaluation of the steatosis and with the Masson's trichrome (MT) for determination of the fibrosis. The severities of the hepatic histological changes were assessed and scored in a blind manner using the NASH-Clinical Research Network scoring system [18]. Briefly, the steatosis grade was scored according to the degree of parenchymal involvement as follows: 0, <5%; 1, 5% to 33%; 2, 33% to 66%; and 3, >66%. The steatosis location was scored as follows: 0, zone 3 predominant; 1, zone 1 predominant; 2, azonal; and 3, panacinar. The lobular inflammation grade was scored by the numbers of the inflammation foci in the area of ×200 microscopic fields as follows: 0, no foci; 1, <2 foci per ×200 field; 2, 2 to 4 foci per ×200 field; and 3, >4 foci per ×200 field. The fibrosis stage was scored by the location and density of the fibrosis as follows: 0, none; 1, perisinusoidal or periportal fibrosis; 2, perisinusoidal and periportal fibrosis; 3, bridging fibrosis; and 4, cirrhosis.
Plasma and tissue biochemical assays
Plasma and hepatic triglyceride (TG) levels were measured using the GPO-Trinder kit (Sigma-Aldrich, St. Louis, MO, USA), according to the manufacturer's instructions. Plasma free fatty acid levels were determined using an enzymatic assay kit (Wako Chemicals, Richmond, VA, USA). Plasma alanine aminotransferase (ALT) levels were measured using the IDToxTM Alanine Transaminase Endpoint Assay Kit (ID Labs Inc., London, ON, Canada).
Measurement of FAO
The rate of FAO was measured as 14CO2 generation from 14C palmitate (NEN Life Sciences, Boston, MA, USA), as previously described [19]. Peroxisomal FAO was determined in the presence of inhibitors of mitochondrial oxidation, namely, antimycin A and rotenone (final concentrations 100 and 12.5 µM, respectively) [14].
Quantitative real-time polymerase chain reaction analysis
Total RNA was isolated using TRIzol reagent (Invitrogen, Carlsbad, CA, USA); 1 µg of each sample was reverse transcribed with random primers using the Reverse Aid M-MuLV reverse transcription kit (Fermentas, Hanover, MD, USA). Target cDNA levels were quantified by real-time polymerase chain reaction (PCR) using gene-specific primers (Table 1) and the 7500 Fast RT-PCR system (Applied Biosystems, Foster City, CA, USA) containing SYBR green. The data were normalized to the levels of expression of the internal control t-box protein (Tbp) and expressed in arbitrary units.
Western blot analysis
Liver tissues were homogenized in lysis buffer and centrifuged at 13,000 rpm for 30 minutes at 4℃. Samples with equal amounts of protein (20 to 50 µg) were analyzed by Western blotting using antibodies against PPARα (#sc9000; Santa Cruz Biotechnology, Santa Cruz, CA, USA) and α-tubulin (#NB100-690; Novus Biologicals, Littleton, CO, USA).
Measurement of lipid peroxidation
Hepatic lipid peroxidation was assessed by measuring malondialdehyde (MDA) levels using a Bioxytech MDA-586 assay kit (OxisResearch, Portland, OR, USA), according to the manufacturer's instruction. MDA values were corrected to the tissue protein contents.
Statistical analyses
All values are presented as the mean±standard error of the mean. Statistical significance of the differences between experimental groups was determined by the Student t-test or one-way analysis of variance with the Bonferroni correction using SPSS version 18 (SPSS Inc., Chicago, IL, USA). P<0.05 was considered statistically significant.
RESULTS
Administration of statins prevent hepatic lipid accumulation and steatohepatitis in MCDD-fed mice
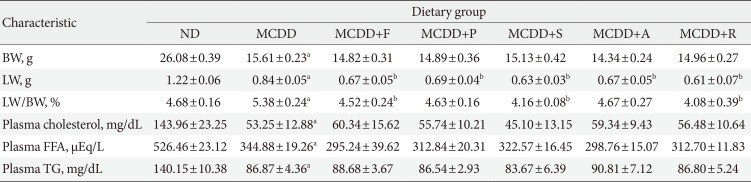
Of the animal models of NAFLD, the MCDD model has been used frequently as a valuable model of NASH. Consistent with previous studies [20], administration of MCDD to C57BL6/N mice for 6 weeks caused NASH and mild hepatic fibrosis (Figs. 1 and 2A). Feeding MCDD significantly increased plasma ALT levels, a specific marker of liver injury, and hepatic TG contents (Fig. 2B). The effects of different types of statins, including fluvastatin, pravastatin, simvastatin, atorvastatin, and rosuvastatin, on MCDD-induced NAFLD were examined. It is well known that the efficacy and potency of lowering plasma cholesterol are different between the types of statins. However, it is not established whether this lipid-lowering effect correlates with pleiotropic effects of the drugs. Because the effective dose of each statin for reducing hepatic steatosis has not been established in rodent models, we treated five statins with the same dose (15 mg/kg/day) [21]. Table 2 shows the changes in body weight, liver weight, and plasma levels of cholesterol, free fatty acid, and TG in each experimental group. Interestingly, administration of various statins with MCDD for 6 weeks failed to further reduce plasma cholesterol levels in the mice fed MCDD alone (Table 2), but most statins reduced hepatic TG levels and plasma ALT levels (Fig. 2B). Among them, fluvastatin showed most prominent effect on reducing hepatic TG levels and plasma ALT levels. The histological analysis of statin-fed mice revealed a significant reduction in hepatic lipid accumulation, as well as inflammation or fibrosis (Figs. 1 and 2).
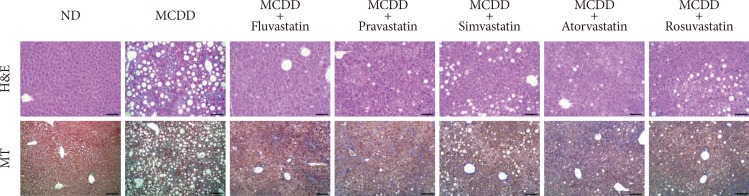
Statin treatment attenuates methionine- and choline-deficient diet (MCDD)-induced hepatic steatosis and steatohepatitis. Representative histological images of each experimental group. H&E (×200; scale bar=100 µm), MT (×100; scale bar=50 µm). ND, normal chow diet; MT, Masson's trichrome.
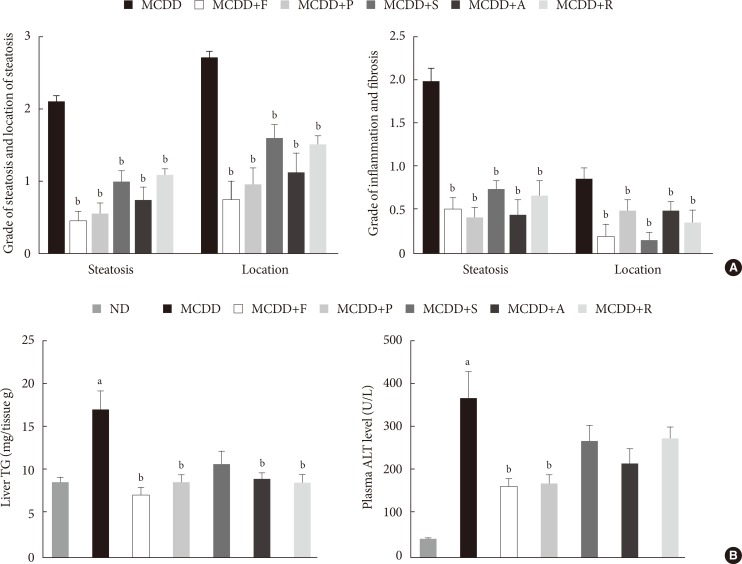
Histologic scores and hepatic triglyceride (TG) content and plasma alanine aminotransferase (ALT) level. (A) Histologic scores for location and severity of steatosis, inflammation, and fibrosis, according to criteria of Kleiner et al. [18]. (B) Hepatic TG content and plasma ALT level in mice fed normal chow diet (ND) and methionine- and choline-deficient diet (MCDD) with or without various statins for 6 weeks. F, fluvastatin; P, pravastatin; S, simvastatin; A, atorvastatin; R, rosuvastatin. aP<0.05 compared with ND, bP<0.05 compared with MCDD.
Gene expression of PPARα and enzymes responsible for hepatic FAO was decreased in MCDD and recovered by statin treatment
PPARα is a master regulator of FAO. After 6 weeks of MCDD feeding, a gene expression level of PPARα was significantly decreased (Fig. 3A). All statins recovered PPARα mRNA levels in the liver compared with MCDD-fed mice. Western blot analysis also showed that MCDD feeding significantly decreased and fluvastatin treatment increased, respectively, protein expression of PPAR (Fig. 3B). In line with this result, the PPARα target genes encoding enzymes involved in mitochondrial FAO (carnitine-palmitoyltransferase-1α and -1β), and peroxisomal FAO (acyl-CoA oxidase-1 and D-bifunctional protein-1) were decreased by MCDD and recovered by most of statins (Fig. 3C). In addition, MCDD significantly decreased and most statins significantly increased the expression of peroxisomal biogenesis factor (Pex)-7, a gene encoding a protein that imports several essential enzymes into peroxisomes (Fig. 3D) [22].
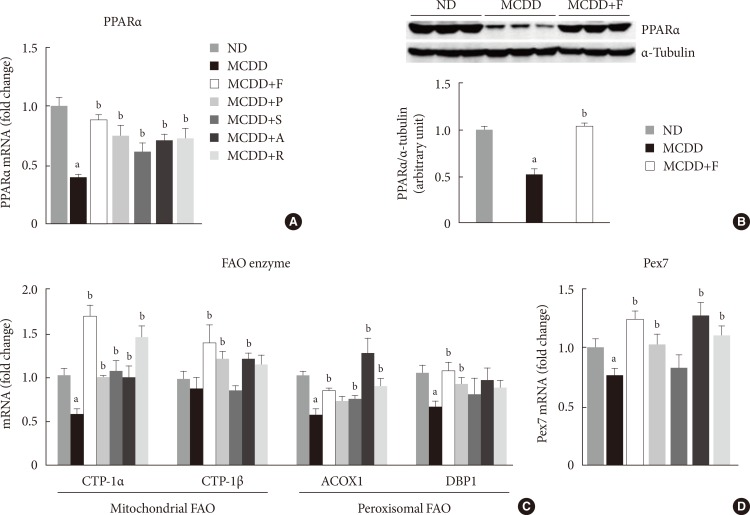
Treatment with statins recovers methionine- and choline-deficient diet (MCDD)-induced suppression of peroxisomal proliferator-activated receptor α (PPARα) and target gene expression levels in the liver. (A) mRNA expression levels and (B) protein expression levels of PPARα. Expression levels of genes involved in mitochondrial and peroxisomal (C) fatty acid oxidation (FAO), and (D) peroxisomal biogenesis factor (Pex) 7. ND, normal chow diet; F, fluvastatin; P, pravastatin; S, simvastatin; A, atorvastatin; R, rosuvastatin; CPT, carnitine palmitoyltransferase; ACOX, acyl CoA oxidase; DBP, D-bifunctional protein. aP<0.05 compared with ND, bP<0.05 compared with MCDD.
Statin restores MCDD-induced suppression of hepatic mitochondrial and peroxisomal FAO
To ensure the effect of MCDD and statins on hepatic FAO rate, we directly measured both mitochondrial and peroxisomal FAO using 14C palmitate oxidation. In agreement with previous studies [23], feeding MCDD significantly suppressed mitochondrial FAO in the liver (Fig. 4A). Treatment with statins significantly increased mitochondrial FAO, except for simvastatin (P=0.21). Feeding MCDD also significantly decreased peroxisomal FAO and fluvastatin, whereas pravastatin and simvastatin supplementation significantly increased it (Fig. 4B).
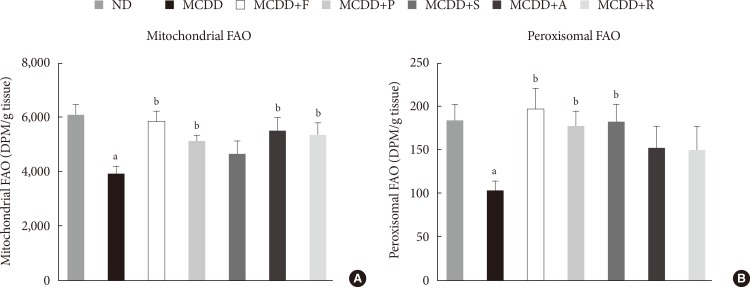
Measurement of hepatic fatty acid oxidation (FAO) in mice fed methionine- and choline-deficient diet (MCDD) with or without statins. (A) Mitochondrial FAO. (B) Peroxisomal FAO. DPM, disintegrations per minute; ND, normal chow diet; F, fluvastatin; P, pravastatin; S, simvastatin; A, atorvastatin; R, rosuvastatin. aP<0.05 compared with ND, bP<0.05 compared with MCDD.
Hepatic gene expression levels of anti-oxidative enzymes was decreased in MCDD-fed mice but not recovered by statins
A recent study showed that MCDD increases oxidative stress through decreased anti-oxidative capacity [24]. It was also shown that statins reduce inflammatory responses [25]. Accordingly, the MDA assay showed that the lipid peroxidation levels in the liver of MCDD-fed mice were more elevated than ND-fed mice, whereas these findings were ameliorated in the fluvastatin supplementation group (Fig. 5A).
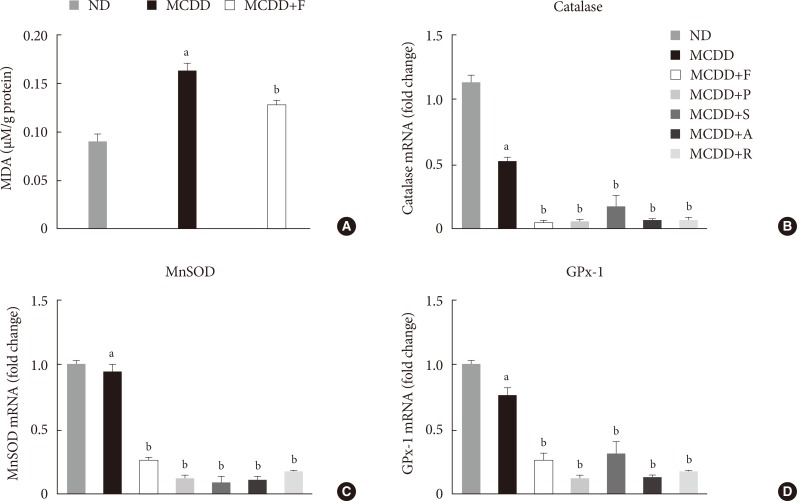
Changes in hepatic lipid peroxidation and gene expression levels of several anti-oxidative enzymes. (A) Hepatic malondialdehyde (MDA) levels in mice fed methionine- and choline-deficient diet (MCDD) and fluvastatin supplementation. (B) mRNA expression levels of catalase, (C) manganese superoxide dismutase (MnSOD), and (D) glutathione peroxidase 1 (GPx-1). ND, normal chow diet; F, fluvastatin; P, pravastatin; S, simvastatin; A, atorvastatin. aP<0.05 compared with ND, bP<0.05 compared with MCDD.
Thus, we examined the possibility that statins may increase the gene expression of anti-oxidant enzymes: catalase, a major peroxisomal anti-oxidant; manganese superoxide dismutase, which exists in both mitochondria and peroxisomes; and glutathione peroxidase (GPx)-1, a cytosolic anti-oxidant. MCDD significantly decreased gene expression levels of catalase and GPx-1. However, contrary to our expectation, statins further decreased mRNA expressions of all of these enzymes (Fig. 5B-D).
DISCUSSION
In the present study, we showed that various statins prevented MCDD-induced hepatic steatosis and NASH and increased hepatic mitochondrial and peroxisomal FAO. Gene expression levels of PPARα and its target genes, which are responsible for mitochondrial and peroxisomal FAO, were increased by statin treatment. Our study is the first to demonstrate that statin treatment increases peroxisomal FAO to prevent NASH development.
Interestingly, the overall preventive effect of statins was not related with its potency to reduce plasma cholesterol levels. In our study, we used same dosages of various statins because there has been no study directly comparing potency of these drugs. Interestingly, fluvastatin, known to have lower potency to reduce plasma cholesterol level than atorvastatin and rosuvastatin, showed most prominent effects on reducing hepatic steatosis and NASH. Fluvastatin was also most effective in increasing mitochondrial and peroxisomal FAO, as well as expression of PPARα and its downstream FAO enzymes. Thus, it is suggested that each statin's effect on increasing FAO is independent of its effect on decreasing cholesterol level and that the ability to increase hepatic FAO is the major determinant of NASH prevention by statins. However, the molecular mechanism by which this kind of discrepancy occurs among various statins is presently unknown.
The two-hit hypothesis is a well-known theory to explain the pathogenesis of NAFLD and its progression from steatosis to NASH [26]. The "first hit" is the accumulation of fatty acids or TGs in the liver that may increase susceptibility of the hepatocellular damage induced by second hits. There are several mechanisms leading to the development of hepatic steatosis: (1) increased hepatic fatty acid uptake, (2) increased de novo lipogenesis in the liver, (3) decreased hepatic FAO, and (4) decreased very low density lipoprotein secretion from the liver. The "second hit" is a combination of inflammatory responses, oxidative stress, and mitochondrial dysfunction, which leads to hepatocellular damage and fibrosis [26].
Among them, FAO occurs mainly in mitochondria, but peroxisomes and microsomes also play a role. Peroxisomal β-oxidation is required for efficient mitochondrial β-oxidation [27]. Peroxisomal dysfunction induces functional abnormalities in mitochondria and consequently compromises cellular ATP production [28]. Especially when the liver is overloaded with fatty acids, the role of peroxisomal β-oxidation becomes more important because dicarboxylic acids are increased through ω-oxidation in endoplasmic reticulum [1429]. In line with this, recent studies have highlighted the association between peroxisomal dysfunction and hepatic steatosis. The upregulation of genes, which regulates peroxisomal biogenesis and FAO in a certain strain of mice, was related with resistance to diet-induced hepatic steatosis [13]. The liver-specific Pex5-/- mice developed hepatic steatosis even though mitochondrial FAO was increased [14].
Mitochondria and peroxisomes are closely related organelles and play a critical role in the cellular energy metabolism. X-linked adrenoleukodystrophy (X-ALD) is an inherited disorder caused by mutation of the ABCD1 gene, which encodes a peroxisomal transporter of very long chain fatty acids. The mouse model of X-ALD showed impaired oxidative phosphorylation of mitochondria and increased oxidative stress [30]. Peroxisomal biogenesis disorder, Zellweger syndrome, is characterized by severe neurologic deficits with multiple organ dysfunctions. Pex5-/- mice, a mouse model for Zellweger syndrome, caused alteration of mitochondrial morphology, changes of mitochondrial respiratory chains, and increased oxidative stress in the liver [31]. In our study, statin treatment increased both peroxisomal and mitochondrial FAO, suggesting that improvement of peroxisomal FAO may underlie improvement of mitochondrial FAO. Taken together, improvement of peroxisomal FAO may be the primary mechanism of NASH prevention by statins. However, it should be noted that each statin showed a different level of effect on mitochondrial or peroxisomal FAO, whereas all statins improved steatosis and NASH. Therefore, there may be additional mechanisms of preventive effect of statins on steatosis and NASH.
Increased oxidative stress and altered anti-oxidative system play an important role in the development of NASH/NAFLD [32]. Because mitochondria and peroxisomes are major sources of free radical generation, resulting in oxidative stress, maintenance of its function is critical to prevent NAFLD. In agreement with previous reports [24], feeding MCDD significantly decreased gene expression levels of peroxisomal anti-oxidative enzymes, including catalase and GPx. A number of studies have demonstrated that statins act as an anti-oxidant in various tissues [33]. In line with this, we demonstrated that the MCDD-induced hepatic lipid peroxidation was suppressed by statin treatment. Previous studies have shown that treatment with various statins increased the activity of anti-oxidant enzymes, such as catalase or SOD [34]. However, in our study, statins failed to increase gene expression levels of anti-oxidant enzymes. The reason for this discrepancy between current study and previous studies is presently unclear. We recently found that fluvastatin treatment significantly increased hepatic level of plasmalogen, which is well known to act as an endogenous anti-oxidant (unpublished data) [3536]. Thus, it can be suggested that changes in anti-oxidant enzymes are not the primary reason of improvement of anti-oxidative defense function by statins.
Statins are known to cause several hepatic adverse effects, ranging from transient elevation of transaminases to acute liver failure [37]. However, recent studies have reported that statin-induced acute liver failure is extremely rare and may be related with idiosyncratic reaction [38]. Indeed, several recent studies showed that statins can be used safely in NASH patients [39]. In addition, a recent meta-analysis showed that statins may improve serum aminotransferase levels and ultrasound findings in NASH patients [40]. Therefore, it is suggested that favorable effects of statins on liver function in animal studies can be extended to humans.
In summary, statin treatment prevented hepatic steatosis and NASH in MCDD-fed mice. Feeding MCDD for 6 weeks caused hepatic steatosis, inflammation, and early fibrosis through decreased hepatic mitochondrial and peroxisomal FAO. Various statins exhibited significant improvement of histological scores and enhanced hepatic FAO via induction of PPARα and target genes. Interestingly, these pleiotropic effects were not correlated with cholesterol-lowering potency of statins. Based on these data, we suggest a new possibility that improvement of peroxisomal function by statins may contribute to the prevention of NASH.
ACKNOWLEDGMENTS
This work was supported by the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2006-2005412, K.U.L.; 2012R1A1A3012626, E.H.K.). This work was also supported by the grants (2009-006, 2013-578, 2014-006) from the Asan Institute for Life Sciences, Seoul, South Korea.
Notes
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.