- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Articles
- Page Path
- HOME > Diabetes Metab J > Volume 39(2); 2015 > Article
-
ReviewOthers Current Antiplatelet Treatment Strategy in Patients with Diabetes Mellitus
- Jung Hwa Jung1, Udaya S. Tantry2, Paul A. Gurbel2, Young-Hoon Jeong3
-
Diabetes & Metabolism Journal 2015;39(2):95-113.
DOI: https://doi.org/10.4093/dmj.2015.39.2.95
Published online: April 20, 2015
1Division of Endocrinology, Department of Internal Medicine, Gyeongsang National University Hospital, Gyeongsang National University School of Medicine, Jinju, Korea.
2Sinai Center for Thrombosis Research, Baltimore, MD, USA.
3Division of Cardiology, Department of Internal Medicine, Gyeongsang National University Hospital, Gyeongsang National University School of Medicine, Jinju, Korea.
- Corresponding author: Young-Hoon Jeong. Division of Cardiology, Department of Internal Medicine, Gyeongsang National University Hospital, Gyeongsang National University School of Medicine, 79 Gangnam-ro, Jinju 660-702, Korea. goodoctor@naver.com
Copyright © 2015 Korean Diabetes Association
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
- Patients with diabetes mellitus (DM) have accelerated atherosclerosis with an increased risk for atherothrombotic cardiovascular complications. A state of high platelet reactivity and activation, hypercoagulability (prothrombotic state) and a subdued response to standard antiplatelet agents may explain high rate of adverse cardiovascular events in patients with DM. Several antithrombotic treatment strategies have been developed to control the prothrombotic state in patients with DM: dose modification of commonly used agents; use of potent agents; and addition of a third antithrombotic drug (triple therapy) to commonly prescribed dual antiplatelet therapy of aspirin and a P2Y12 inhibitor. The present review aims to provide an overview of the current knowledge on platelet abnormalities in patients with DM, focusing on the challenges and perspectives of antiplatelet treatment strategies in this population.
- Cardiovascular disease (CVD) including stroke and coronary artery disease (CAD) is the global leading cause of morbidity and mortality [1]. Diabetes mellitus (DM) is associated with accelerated atherothrombosis; consequently, DM patients have shown a 2- to 4-fold greater risk of CAD and cerebrovascular disease than non-DM patients [2]. Of note, diabetic subjects without a history of CAD have shown a similar risk of future CAD events similar to nondiabetic subjects with a history of myocardial infarction (MI) [3]. Following the first manifestation of CVD, DM patients also have a higher risk of recurrent cardiovascular complications than non-DM patients despite standard medical treatment.
- Because the global prevalence of DM is increasing rapidly (e.g., 165% between 2000 and 2050), there is an unmet need to reduce the incremental burden of atherothrombotic events in these DM patients [4]. Heightened cardiovascular risk in diabetic patients despite controlling traditional risk factors such as hypertension, smoking, hypercholesterolemia, and physical inactivity suggests that prothrombotic state may be the more important factor in these patients. Moreover, a subdued response to standard antiplatelet agents reported in diabetic patients may also explain heightened cardiovascular risk. Therefore, a better understanding of the pathophysiology of atherothrombosis in DM patients may improve the benefits of current pharmacological therapy (e.g., antiplatelet therapy) by maximizing its clinical efficacy and safety.
- The purpose of this article is to review the current status of biologic knowledge on platelet hyperreactivity, to evaluate the clinical benefits and limitations of currently available antiplatelet agents, and to suggest future directions to overcome these limitations by new agents and treatment strategies.
INTRODUCTION
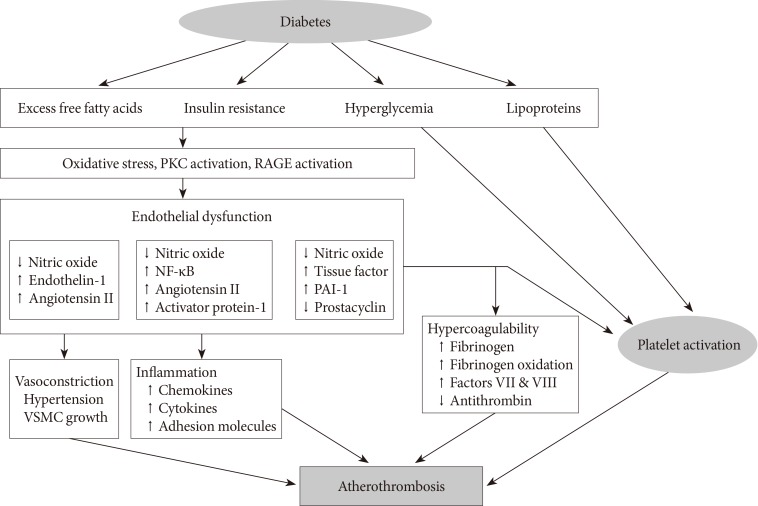
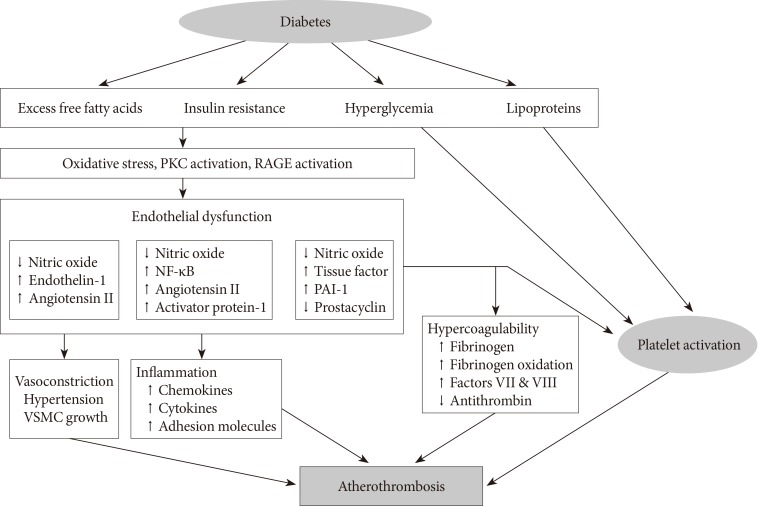
- Diabetes is a "prothrombotic state" often characterized by hyperglycemia, oxidative stress, endothelial dysfunction, platelet activation, hypercoagulability with dysfunctional coagulation pathways and fibrinolysis, and inflammation (Fig. 1) [4,5,6]. Platelets activation and aggregation at the site of plaque rupture is pivotal for the subsequent atherothrombotic complications of arterial systems. Platelets in DM patients appear to be hyperreactive with intensified adhesion, activation, and aggregation [6]. Moreover, platelets influence diverse endothelial and inflammatory responses during the initiation and progression of atherosclerosis.
- Several mechanisms are suggested to explain the platelet dysfunction in DM patients [6]: hyperglycemia enhances platelet aggregation by increasing P-selectin expression, by osmotic effects, by activating protein kinase C, and by glycating platelet surface proteins with a consequent decrease in membrane fluidity. In addition, insulin resistance or deficient action in diabetic patients are associated with impaired responses to antithrombotic molecules (such as prostacyclin and nitric oxide) and insulin receptor substrate-dependent effects are associated with an increase in the intraplatelet calcium concentration and subsequent enhanced degranulation. Metabolic conditions associated with DM (i.e., obesity, dyslipidemia, and systemic inflammation) may also have a role in this process. Finally, upregulation of glycoprotein (GP) IIb/IIIa expression and P2Y12 signaling, increased platelet turnover, and excessive oxidative stress further contribute to the platelet dysfunction in these patients. Furthermore, different cutoff points of high platelet reactivity (HPR) for adverse events in DM patients compared with the overall population following percutaneous coronary intervention (PCI) have been reported [7,8]. Therefore, diabetic subjects need a personalized antiplatelet therapy strategy to reduce atherothrombotic events associated with hyperreactive platelets.
PROTHROMBOTIC STATE IN DIABETES MELLITUS
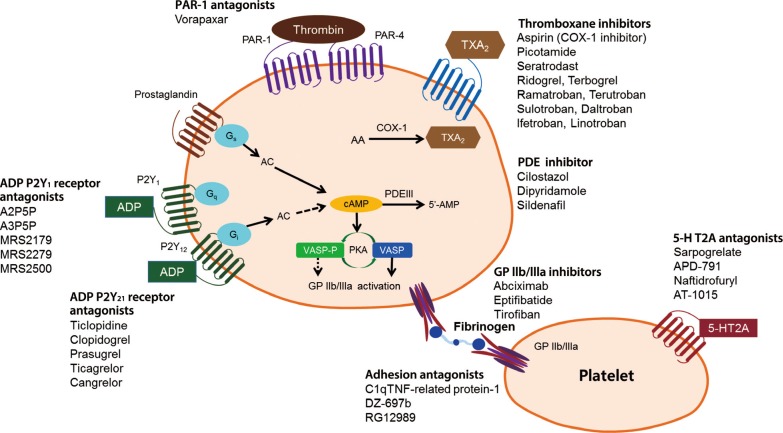
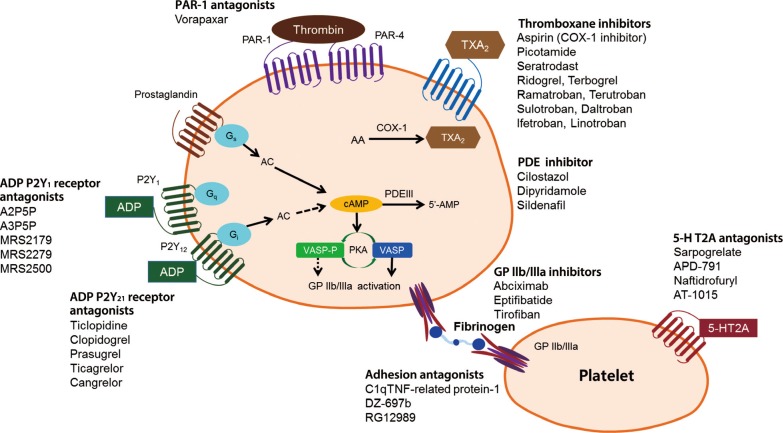
- There are multiple targets for antiplatelet therapy (Fig. 2) [9,10]. Atherosclerotic plaque rupture, erosion or fissure exposes the subendothelial matrix and release prothrombotic factors during CVD or PCIs. These processes result in localized platelet adhesion and subsequent platelet activation results in the release of soluble agonists such as thromboxane A2 (TXA2), adenosine diphosphate (ADP), and generation of thrombin on the activated platelet surface by coagulation. TXA2 is produced from arachidonic acid and binds to TX receptors; ADP is secreted from dense granules and binds to platelet P2Y1 and P2Y12 receptors. These agonists, through an autocrine and paracrine fashion, produce sustained activation of GPIIb/IIIa receptors leading to stable platelet-rich thrombus generation. Platelet activation also results in the exposure of phosphatidyl serine, providing binding sites for coagulation factors. The coagulation process results in the generation of thrombin and subsequent platelet-fibrin clot formation. Endogenous phosphodiesterase (PDE) activity affects intraplatelet cyclic adenosine monophosphate (cAMP) levels and modulates platelet function. Finally, isoprostanes derived from membrane arachidonic acid through peroxidation have been shown to induce platelet aggregation by activating the receptor for TXA2.
- Importantly, the relative contribution of each pathway (ADP-platelet, TXA2-platelet, thrombin-platelet, coagulation, and PDE activity) to the development of thrombus formation is unknown at this time and can be different depending on the disease entity and activity. Therefore, determination of the optimal combination of antiplatelet agents remains an elusive goal. Occurrences of recurrent ischemic events and bleeding events during contemporary antiplatelet therapy may be related in part to the nonselective "one-size-fits-all" dosing that ignores the inherent variability in thrombogenecity and antiplatelet responsiveness.
- Aspirin
- Aspirin selectively and irreversibly acetylates cyclooxygenase-1 (COX-1), thereby blocking platelet TXA2 formation and diminishing platelet aggregation mediated by TXA2 (Fig. 2). This effect is irreversible because platelets are enucleate and unable to resynthesize COX-1. In healthy subjects, even low doses of aspirin (~40 mg daily) cause an almost complete suppression of TXA2 formation and platelet aggregation throughout the entire platelet lifespan [11]. However, aspirin therapy in DM patients has a high prevalence of hyporesponsiveness or "aspirin resistance" [12] leading to concerns regarding its effectiveness in the primary prevention of CVD. Because clinical studies used different assays, agonists, cutoff values, and cohorts, interpretation of the data and generalization in clinical practice may be difficult.
- Primary prevention
- In patients without prior CVD (primary prevention), indication for antiplatelet therapy remains unclear [13]. In this population, aspirin, the only antithrombotic drug studied in a sufficiently large cohort, shows a statistically significant reduction in the risk for a first MI attack at the expense of increased risk of both gastrointestinal (GI) bleeding and hemorrhagic stroke. However, the clinical benefit of aspirin on MI protection can be different according to concomitant use of standard regimen (e.g., angiotensin-converting enzyme inhibitor and statin). In a recent analysis, the clinical benefit of aspirin was not observed in randomized clinical trials (RCTs) published after 2000 (risk ratio [RR], 0.98; 95% confidence interval [CI], 0.84 to 1.14), in contrast to those published before 2000 (RR, 0.67; 95% CI, 0.56 to 0.81; Pinteraction<0.001) [14]. In the meta-analysis by the Antithrombotic Trialists' (ATT) collaboration, aspirin therapy increased major GI and other extracranial bleeds (defined as "a bleed requiring transfusion or resulting in death") (0.10%/year vs. 0.07%/year; RR, 1.54; 95% CI, 1.30 to 1.82; P<0.0001) compared with placebo [15]. When treated with aspirin, the high-risk population would experience 22 more bleeds per 1,000 persons versus 4 more bleeds per 1,000 persons in the low-risk population [16]. A meta-analysis of 16 placebo-controlled RCTs (n=55,462) showed that treatment with aspirin was associated with an increased risk of hemorrhagic stroke by 1.84-fold (P<0.001) [17]. In absolute terms, one could predict 12 incident cases of hemorrhagic stroke per 10,000 patients during chronic aspirin treatment.
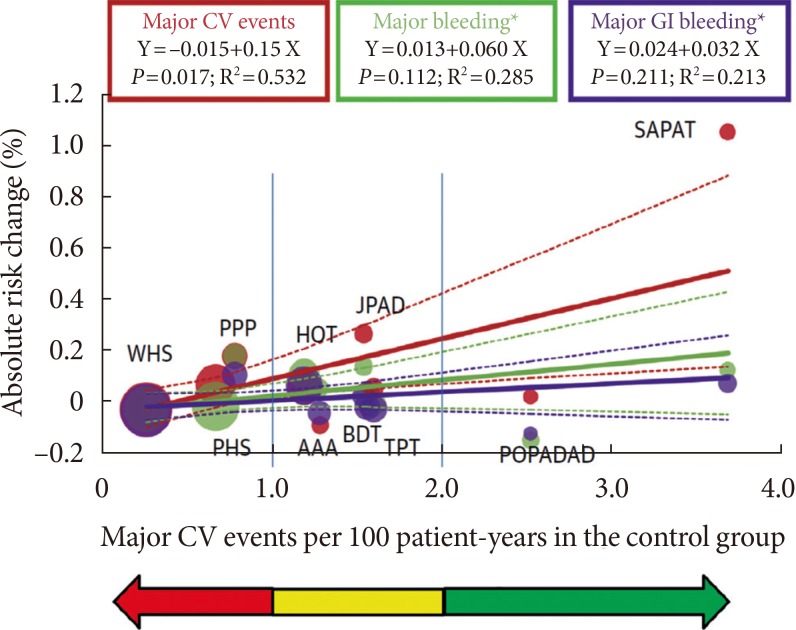
- During primary CVD prevention that includes subjects with a low risk of developing atherothrombotic events, it is essential to estimate the individual risk-benefit ratio profile, in this case bleeding and hemorrhagic risk [13]. Cardiovascular risk can increase proportionally across primary prevention in young healthy individuals to high-risk individuals and then to secondary prevention (Fig. 3). Aspirin can be recommended for primary cardiovascular prevention based on a threshold risk level, defined as major cardiovascular events (death, MI, or stroke) ≥2 per 100 person-years [13]. An "uncertainty area" at risk levels between 1 and 2 per 100 patient-years should be considered in which the decision to prescribe aspirin is left to the physician's discretion and to the patient's preferences. Moreover, recently the Food and Drug Administration (FDA) has reviewed the available data and does not believe that the current evidence supports the general use of aspirin for primary prevention of a heart attack or stroke. The FDA suggested that it should not be routinely used for primary prevention due to serious risks including increased risk of cerebral and GI bleedings.
- Three RCTs conducted specifically in patients with diabetes and six RCTs in which DM patients were subgroups (1% to 22%) failed to show definitive results on the benefit of aspirin in primary CVD prevention (Table 1). A meta-analysis of these nine RCTs found that aspirin therapy was associated with numeric reductions in CAD events (-9%) and cerebrovascular events (-11%) [18]. Based on the overall negative results of these RCTs, it was considered that standard aspirin therapy may be less effective in patients with diabetes than in individuals without diabetes [13]. As such, the current evidence suggests that diabetes should be considered as a unique high-risk entity.
- A position statement by the American Diabetes Association (ADA), the American Heart Association, and the American College of Cardiology Foundation recommended that low-dose aspirin (75 to 162 mg daily) for primary prevention is reasonable for DM adults without a previous history of vascular disease who are at increased CVD risk (10-year CVD risk over 10%) without an increased risk for bleeding. This generally includes men over 50 years of age and women over 60 years of age who also have at least one of the following major risk factors: smoking, hypertension, dyslipidemia, family history of premature CVD, and albuminuria [18]. Furthermore, aspirin is no longer recommended for those at low CVD risk (women under 60 years of age and men under 50 years of age with no major CVD risk factors; 10-year CVD risk under 5%). Clinical judgment should be applied for those at intermediate CVD risk (younger patients with one or more risk factors or older patients with no risk factors; those with a 10-year CVD risk of 5% to 10%) until further research is available.
- Secondary prevention
- The clinical benefit of aspirin therapy is clearly superior to the risk of major bleeding in the setting of secondary CVD prevention. Aspirin is still the bedrock of antiplatelet therapy for secondary prevention of recurrent ischemic events in patients with atherothrombotic disease, including those with DM [19]. The recommended dose of aspirin for secondary prevention in DM patients with atherosclerotic disease is 75 to 162 mg daily. Low-dose aspirin usage is supported mainly by two large meta-analyses of secondary prevention trials performed by the ATT' collaboration involving 212,000 high-risk patients (with acute or previous vascular disease or some other predisposing condition implying an increased risk of occlusive vascular disease) [15,20]. The results of these meta-analyses showed oral antiplatelet agents, mainly aspirin, to be protective for vascular events in high-risk patients. In particular, the incidence of vascular events was reduced from 22.3% to 18.5% in DM patients (P<0.002) and from 16.4% to 12.8% (P<0.00001) in non-DM patients. Although the overall incidence of vascular events was much higher in DM patients, the benefit of antiplatelet therapy was consistent regardless of DM status [20]. In these trials, low-dose aspirin (75 to 150 mg daily) was found to be at least as effective as higher daily doses, and bleeding complications were reduced with lower doses. The first large-scale RCT comparing high- (300 to 325 mg daily) versus low-dose (75 to 100 mg daily) aspirin therapy was the Clopidogrel Optimal Loading Dose Usage to Reduce Recurrent EveNTs-Optimal Antiplatelet Strategy for InterventionS 7 (CURRENT-OASIS 7) trial that included ACS patients scheduled to undergo early coronary angiography [21,22]. The rate of 30-day ischemic events did not differ between high-dose versus low-dose aspirin. However, a trend toward higher rates of GI bleeds was observed in the high-dose versus low-dose group (0.38% vs. 0.24%, P=0.051).
- P2Y12 receptor antagonists
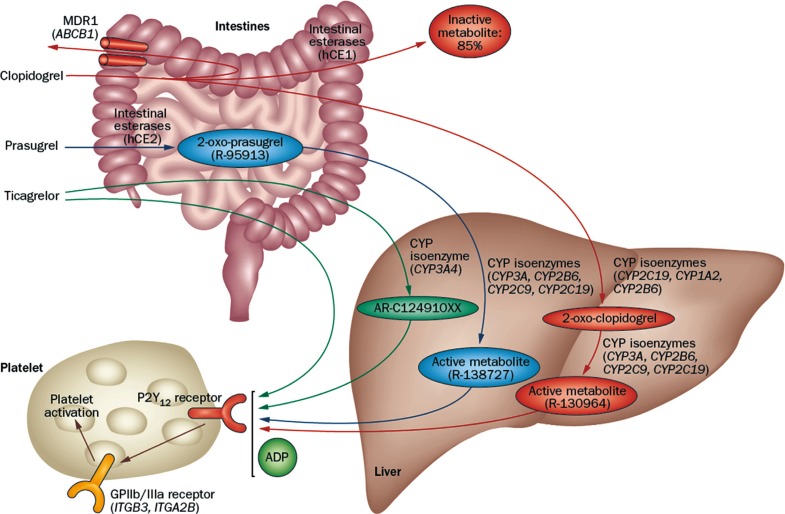
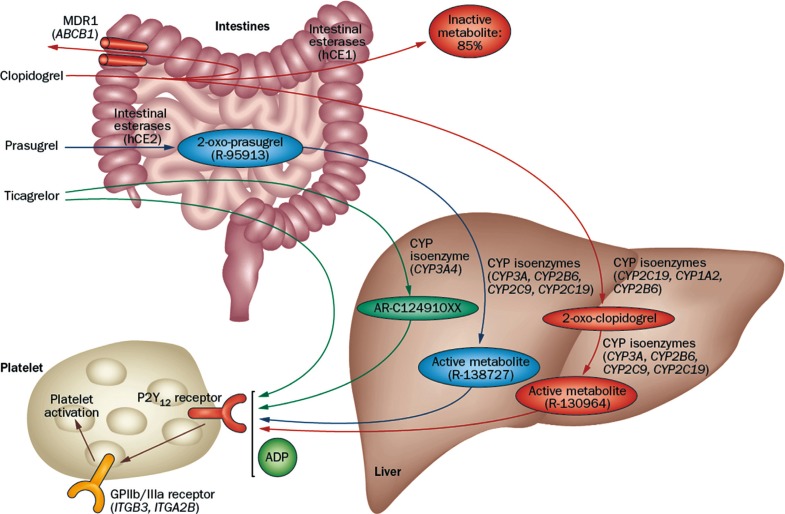
- Thienopyridines (ticlopidine, clopidogrel, and prasugrel) are nondirect irreversible antagonists of the P2Y12 receptor. Clopidogrel is currently the most commonly prescribed antiplatelet agent. It has similar efficacy and better safety profile compared to ticlopidine. Clopidogrel is a prodrug and needs two-step hepatic conversion to become an active metabolite (Fig. 4) [23]. Numerous data have demonstrated a close relationship between low response to clopidogrel or "clopidogrel resistance" and atherothrombotic events in high-risk patients with acute coronary syndrome (ACS) or those treated with coronary stenting [24]. Because DM itself is an important determinant for clopidogrel responsiveness, an intensified antiplatelet regimen may reduce the risk of "clopidogrel resistance" and consequently the rate of ischemic event occurrence for secondary prevention. Compared with the standard dose of clopidogrel (300 mg loading or 75 mg daily maintenance), high-dose clopidogrel (600 mg loading or 150 mg daily maintenance) strategy is associated with enhanced platelet inhibition and reduced risk for HPR [25], but the high dose strategy can't efficiently overcome the risk of HPR to ADP.
- Prasugrel is a third-generation thienopyridine and a prodrug that requires one-step hepatic conversion to its active metabolite to irreversibly inhibit the P2Y12 receptor (Fig. 4). Prasugrel has a more rapid onset of action than clopidogrel and provides greater platelet inhibition because of a more effective conversion into its active metabolite [24]. The Optimizing Antiplatelet Therapy in Diabetes Mellitus-3 (OPTIMUS-3) trial showed that prasugrel (60 mg loading followed by 10 mg maintenance) achieved significantly greater platelet inhibition compared with double-dose clopidogrel (600 mg loading dose followed by 150 mg maintenance) in CAD patients with DM on long-term aspirin treatment, using multiple pharmacodynamics measures [26].
- Ticagrelor is a non-thienopyridine, direct-acting, oral antagonist that binds reversibly to the P2Y12 receptor (Fig. 4). The major metabolite of ticagrelor (AR C124910XX), formed by metabolism via the hepatic cytochrome (CYP) 3A4, is as potent as the parent compound ticagrelor. Compared with clopidogrel, ticagrelor results in faster and greater platelet inhibition, with less patient-to-patient variation. In a crossover study including ACS patients with DM (n=30), ticagrelor treatment (90 mg twice daily for 15 days) showed significantly greater platelet inhibition compared with prasugrel treatment (10 mg daily for 15 days) (45.2 vs. 80.8 P2Y12 reaction units measured by the Verify Now P2Y12 assay; P=0.001) [27].
- Primary prevention
- Currently, the ADA recommends the use of clopidogrel in very high-risk DM patients or as an alternative therapy in patients intolerant to aspirin [19]. However, the use of dual antiplatelet therapy (DAPT) with aspirin and clopidogrel in DM patients without overt atherosclerotic disease has not been supported by clinical evidence.
- The Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance (CHARISMA) trial has compared clopidogrel (75 mg daily)+low-dose aspirin (75 to 162 mg daily) to placebo+low-dose aspirin in high-risk patients (n=15,603, a median follow-up of 28 months), for primary as well as secondary prevention [28]. In this trial, the rates of major vascular events were not significantly different between the two groups. There was a trend towards higher risk of severe bleeding in the primary prevention group compared with the secondary prevention group. In the primary prevention subgroup with multiple risk factors (n=3,284, 80.8% were diabetics), the rate of the primary endpoint was 6.6% with clopidogrel+aspirin versus 5.5% with placebo+aspirin (P=0.20). In addition, there was a significant increase in cardiovascular death (3.9% in the clopidogrel group vs. 2.2% in the placebo group, P=0.01) and also all-cause mortality in the clopidogrel group (5.4% vs. 3.8, P=0.04) [29]. In addition, the rates of severe and moderate bleedings were 2.0% and 2.2% in the clopidogrel group, and 1.2% and 1.4% in the placebo group, respectively (P=0.07 and P=0.08). There is evidence to suggest that atherosclerotic plaques in DM patients are characterized by increased neovascularization of the vasa vasorum [30], which may be associated with a higher risk of intraplaque hemorrhage with consequent rupture or thrombosis.
- Secondary prevention
- The Clopidogrel versus Aspirin in Patients at Risk of Ischemic Events (CAPRIE) trial evaluated the clinical benefits of clopidogrel (75 mg daily) versus high-dose aspirin (325 mg daily) in a secondary prevention population including approximately 20% of DM patients (n=3,866) [31]. The results showed a significantly lower annual risk of the composite endpoint (vascular death, MI, or ischemic stroke) with clopidogrel (5.32% vs. 5.83%, P=0.043). The benefit of clopidogrel therapy was higher in the DM subgroup (15.6% vs. 17.7%, P=0.042), leading to 21 vascular events prevented for every 1,000 DM patients treated [32].
- Among patients with documented prior MI, ischemic stroke, or symptomatic peripheral artery disease in the CHARISMA trial (n=9,478, ~30% were diabetics), the rate of cardiovascular death, MI, or stroke was significantly lower in the clopidogrel group than in the placebo group: 7.3% vs. 8.8% (hazard ratio [HR], 0.83; 95% CI, 0.72 to 0.96; P=0.01) [33]; this benefit was more prominent in patients with prior MI or ischemic stroke than symptomatic peripheral artery disease (HR, 0.774 vs. 0.780 vs. 0.869). There was no significant difference in the rate of severe bleeding (1.7% vs. 1.5%; HR, 1.12; 95% CI, 0.81 to 1.53; P=0.50). Therefore, the antiplatelet effect of DAPT may reduce the risk of ischemic event occurrence in selected patients with overt CVD outside ACS.
- CAD patients with ACS or treated with PCI have a high thrombotic risk and a low responsiveness to aspirin, especially in DM patients; hence, the rationale for combination antiplatelet strategies involves pathways different from TXA2. Multiple placebo-controlled RCTs have demonstrated the clinical benefits of adjunctive clopidogrel combined with aspirin therapy during short- and long-term follow-up (Table 2) [34,35,36]. Although ischemic events were reduced with clopidogrel both in nondiabetic and diabetic patients, diabetic patients showed higher rate of ischemic event occurrences and diminished benefit from adjunctive clopidogrel compared with nondiabetic patients. Thus, patients with DM receive fewer benefits from standard-dose clopidogrel in the setting of ACS or PCI. Intensified inhibition of the platelet ADP-P2Y12 pathway may guarantee more clinical benefits in these patients.
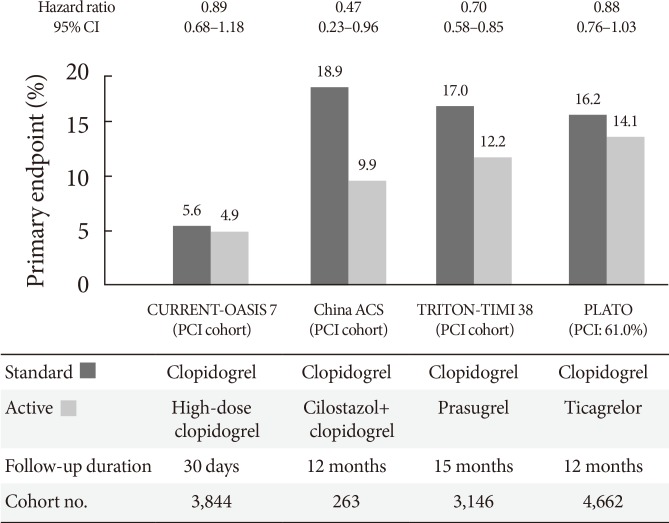
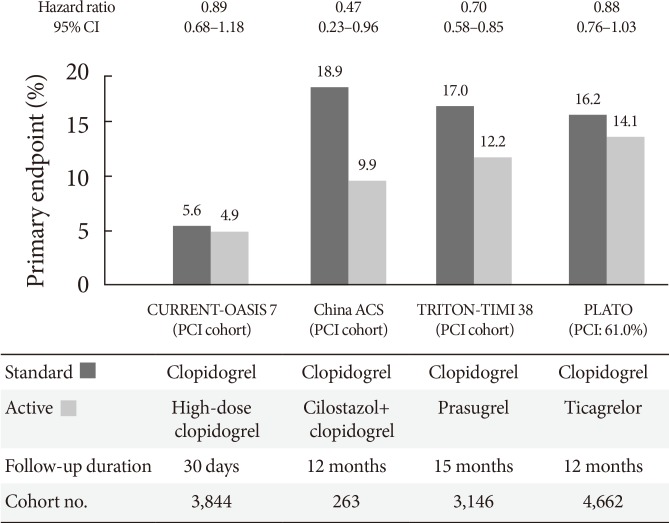
- The CURRENT-OASIS 7 trial evaluated the 30-day clinical benefit of high-dose (600 mg loading followed by 150 mg daily for 1 week) versus standard-dose clopidogrel (300 mg loading followed by 75 mg daily) in ACS patients [21,22]; the subgroup undergoing PCI suggested a clinical benefit in the high-dose group, with a significant reduction in the ischemic event rate (3.9% vs. 4.5%, P=0.039) and stent thrombosis (0.7% vs. 1.3%, P=0.0001) at the expense of major bleeding (1.6% vs. 1.1%, P=0.009) (Fig. 5). Reduction in ischemic events by high-dose clopidogrel was similar in patients with versus without DM (Table 2).
- The Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel-Thrombolysis in Myocardial Infarction 38 (TRITON-TIMI 38) evaluated the efficacy and safety of prasugrel (60 mg loading followed by 10 mg daily maintenance) versus standard-dose clopidogrel (300 mg loading followed by 75 mg daily maintenance) in moderate- to high-risk ACS patients undergoing PCI (n=13,608) [37]. Prasugrel treatment showed a significant reduction in the rates of the primary endpoint (cardiovascular death, nonfatal MI, or nonfatal stroke) compared with clopidogrel treatment over a follow-up period of 15 months (9.9% vs. 12.1%; HR, 0.81; P<0.001), as well as a reduction in the rates of stent thrombosis at the expense of an increased risk of major bleeding in the prasugrel group (Table 2, Fig. 5). No net clinical benefit was observed in elderly patients (≥75 years) and in those weighing <60 kg; a net harm was found in patients with a history of stroke or transient ischemic attack. Compared with non-DM patients, DM patients tended to have a greater reduction in ischemic events (30% vs. 14% reduction; Pinteraction=0.09) without an observed increase in major bleeding rates [38]. This benefit was consistent in patients with (14.3% vs. 22.2%; HR, 0.63; P=0.009) and without insulin treatment (11.5% vs. 15.3%; HR, 0.74; P=0.009). Importantly, although major bleeding was higher in DM patients, there was no difference in major bleeding among DM patients treated with prasugrel versus clopidogrel (2.6% vs. 2.5%; HR, 1.06; P=0.81).
- The PLATelet inhibition and patient Outcomes (PLATO) trial explored the issue of whether upstream administration of ticagrelor improves clinical outcome versus clopidogrel in patients with ST-segment-elevation myocardial infarction (STEMI) or NSTE-ACS (n=18,624) [39]. The PLATO trial demonstrated that ticagrelor, when compared to clopidogrel, reduced ischemic events in ACS patients irrespective of diabetes status and glycemic control, without an increase in major bleeding. In PLATO, reduction of the primary endpoint at 1 year (composite of cardiovascular death, MI, or stroke) by ticagrelor was significant and similar both in patients with and without DM (12% vs. 17% relative risk reduction; Pinteraction=0.49). Among patients planned for an invasive strategy, the benefit of ticagrelor was also observed irrespective of diabetic status (HR, 0.88 in diabetic patients and 0.83 in nondiabetic patients; Pinteraction= 0.72). Importantly, ticagrelor was not associated with an increase in protocol-defined major bleeding, although a higher rate of major bleeding not related to coronary artery bypass grafting was observed (4.5% vs. 3.8%; HR, 1.19; P=0.03).
- Cangrelor is an intravenous, direct, reversible, and potent P2Y12 inhibitor. Platelet inhibition is immediate after bolus infusion, the antiplatelet effect is maintained during a continuous infusion and platelet function is restored within 1 hour after discontinuation. Among clopidogrel-naïve CAD patients on aspirin therapy, cangrelor provided dose-dependent blockade of platelet P2Y12 receptors measured by platelet function testing, without different effects according to diabetic status [40]. In a patient-level pooled analysis from the three randomized A Clinical Trial Comparing Cangrelor to Clopidogrel Standard Therapy (CHAMPION) trials including PCI patients (n=24,910) [41], cangrelor versus control (clopidogrel or placebo) significantly reduced the risks of primary endpoint (composite of death, MI, ischemia-driven revascularization, or stent thrombosis at 48 hours) (3.8% vs. 4.7%; OR, 0.81; 95% CI, 0.71 to 0.91; P=0.0007), without differences in GUSTO severe or life-threatening bleeding at 48 hours (0.2% in both groups): no specific interaction between diabetic status and cangrelor efficacy was found.
- Adjunctive use of third agent
- Despite improved clinical efficacy of DAPT with COX-1 inhibitor aspirin and a potent P2Y12 receptor inhibitor such as prasugrel and ticagrelor, recurrent ischemic event (~10%/year) and increased risk of bleeding episode observed in a significant percentage of ACS [37,39] suggests a ceiling effect of the current DAPT in attenuating ischemic events and some atherothrombotic events are mediated by other pathway(s). Several drugs with different mechanisms have been proposed for use as an adjunctive treatment to DAPT. Agents that have the potential of this "triple therapy" strategies include GP IIb/IIIa inhibitor, PDE inhibitor, protease-activated receptor-1 (PAR-1) antagonists, and new oral anticoagulants (Fig. 4).
- Glycoprotein IIb/IIIa inhibitor
- GP IIb/IIIa inhibitors are intravenous antiplatelet agents showing the highest benefit in high-risk patients with ACS undergoing PCI, but questionable efficacy in low- to moderate-risk ACS patients or in those treated with a conservative approach [42].
- The benefit of GP IIb/III inhibitor pretreatment during clopidogrel therapy appears more pronounced in high-risk ACS patients, including those with DM undergoing PCI. The Intracoronary Stenting and Antithrombotic Regimen: Is Abciximab a Superior Way to Eliminate Elevated Thrombotic Risk in Diabetics (ISAR-SWEET) trial (n=701) did not show beneficial effects of abciximab over placebo (8.3% vs. 8.6%) on the risk of 1-year death and MI in diabetic patients undergoing elective PCI after high-dose clopidogrel (600 mg) pretreatment (HR, 0.97; 95% CI, 0.58 to 1.62; P=0.91) [43]. The Intracoronary Stenting and Antithrombotic: Regimen Rapid Early Action for Coronary Treatment 2 (ISAR-REACT 2) trial (n=2,022) demonstrated a significant reduction of 30-day major adverse cardiac event (MACE) with the use of abciximab versus placebo in patients with NSTE-ACS undergoing PCI on top of 600-mg clopidogrel loading (8.9% vs. 11.9%; OR, 0.75; 95% CI, 0.58 to 0.97; P=0.03) [44], which benefit was restricted to patients with elevated troponin levels (OR, 0.71; 95% CI, 0.54 to 0.95; P=0.02) and was observed across all subgroups, including diabetic patients. The Early Glycoprotein IIb/IIIa Inhibition in Non-ST-Segment Elevation Acute Coronary Syndrome (EARLY-ACS) trial compared strategy of early (~24 hours before PCI) routine administration with delayed provisional administration of eptifibatide (n=9,492) [45], in which the rate of 30-day death or MI did not differ (11.2% vs. 12.3%; OR, 0.89; 95% CI, 0.79 to 1.01; P=0.08) with the expense of higher risks of bleeding and red-cell transfusion in the early eptifibatide group; absolute reduction of MACE at 96 hours with early eptifibatide treatment was more pronounced in patients with versus without DM (2.1% vs. 0.8%). Additionally, a meta-analysis evaluating the effects of GP IIb/IIIa inhibitors in the setting of primary PCI for STEMI suggested a decrease in mortality, but not in re-infarction in diabetic patients [46].
- In the era of potent P2Y12 inhibitor, it may be questionable whether diabetic patients may achieve further clinical benefit from the routine use of GP IIb/IIIa inhibitor in ACS patients. In TRITON, the benefit of prasugrel over clopidogrel on primary ischemic endpoint was irrespective of GP IIb/IIIa inhibitor during the index hospitalization (11% and 16% risk reduction in patients with and without GP IIb/IIIa inhibitor) [37]. In the subset of the PLATO trial planned for an invasive strategy, the clinical benefit of ischemic endpoints with ticagrelor versus clopidogrel was numerically lower in patients receiving GP IIb/IIIa inhibitor (10% and 19% risk reduction in patients with and without GP IIb/IIIa inhibitor) (Pinteraction=0.37) [47]. A major concern with routine use of GP IIb/IIIa inhibitor on top of potent P2Y12 inhibitor is the increase in the risk of serious bleeding. The provisional injection of GP IIb/IIIa inhibitor (intravenous or intracoronary) with short-term infusion (~6 hours) in the selected cases as a bridging strategy (e.g., angiographic evidence of massive thrombus, slow or no-reflow, or a thrombotic complication) may be optimal strategy to maximize clinical efficacy and safety during potent P2Y12 inhibitor therapy.
- Phosphodiesterase inhibitor
- Mammalian phosphodiesterases (PDEs) are the important targets for pharmacologic intervention in the treatment of a number of diseases such as erectile dysfunction, pulmonary hypertension, intermittent claudication, and chronic pulmonary obstructive disease [48]. Therefore, many new PDE inhibitors are being developed for treatment of these disorders. The superfamily of PDEs is comprised of 11 families of enzymes, and individual isozymes modulate distinct regulatory pathways in different cells. For example, PDE5 isozymes are found in platelets, vascular smooth muscle and endothelial cells, with observed high expression in corpus cavernosum and lung. PDE2, PDE3, and PDE5 isozymes are accountable for the majority of platelet PDE activity (>90%) [49]. In platelets, cyclic adenosine 3',5'-monophosphate (cAMP) is hydrolysed by PDE3 and PDE2, whereas cyclic guanosine 3',5'-monophosphate is hydrolysed by PDE5 and PDE2. Dual mechanism with increased production of cAMP (by clopidogrel) and decreased degradation of cAMP (by PDE inhibitor) synergistically enhances the level of intraplatelet vasodilator-stimulated phosphoprotein-phosphorylation and thus stabilize platelet activation. "Triple therapy" with adjunctive PDE3 inhibitor cilostazol to DAPT (aspirin+clopidogrel) significantly enhances platelet inhibition compared with double-dose clopidogrel in high-risk patients (e.g., HPR, AMI, DM, and so on) [50]. On the other hand, other PDE inhibitors pentoxifylline (nonselective) and dipyridamole (PDE5) did not enhance ADP-mediated platelet inhibition similar to cilostazol [51]. The latter finding may be mainly related to different effect of PDE inhibitors on intraplatelet cAMP levels. Contrary to cilostazol, pentoxifylline and dipyridamole have weak effect on intraplatelet cAMP levels, which may be associated with their low PDE3 selectivity.
- Cilostazol is a dual inhibitor of PDE3 and adenosine reuptake that may have an important role in reducing ischemic events associated with CAD [50-53]. Cilostazol is a widely used selective and reversible PDE3 inhibitor, which is highly expressed in myocardial and vascular smooth muscle cells (VSMCs) and platelets. It also inhibits adenosine reuptake into erythrocytes, endothelial cells, muscle cells, and platelets, thereby increasing interstitial and circulatory adenosine levels at clinically relevant concentrations (~3 µmol/L). Adenosine activates G-protein-coupled adenosine receptors, possesses a wide range of biological activities and influences cell survival through pre- and post-conditioning processes in experimental studies. In platelets and VSMCs, the interaction of adenosine with Gs-coupled adenosine A2 receptors results in increased intracellular cAMP. Thus, cilostazol can increase the production and also inhibit the breakdown of cAMP in platelets and VSMCs. The unique feature of cilostazol may contribute to the observed efficacy profile of cilostazol in platelet reactivity and atheroma progression among DM patients. For example, the Diabetic Atherosclerosis Prevention by Cilostazol (DAPC) trial compared prevention by cilostazol (100 to 200 mg daily) versus aspirin (81 to 100 mg daily) of progression in carotid intima-media thickness in type 2 diabetic patients during a 2-year observation period [54]. The regression in maximum left and right common carotid artery intima-media thickness was significantly greater with cilostazol compared with aspirin (-0.088±0.260 mm vs. 0.059±0.275 mm, P<0.001; -0.042± 0.274 mm vs. 0.045±0.216 mm, P=0.003). In the Adjunctive Cilostazol versus double-dose ClopidogrEL in Diabetes Mellitus (ACCEL-DM) trial, adjunctive cilostazol to DAPT showed the greater inhibition of platelet aggregation and the lower prevalence of HPR than double-dose clopidogrel in type 2 diabetic patients undergoing PCI [50]. More interestingly, compared with clopidogrel (75 mg daily) on top of aspirin, adjunctive cilostazol (100 mg twice daily) to aspirin showed the similar inhibition of ADP-induced platelet aggregation [52,53]. In addition, the cilostazol treatment achieved the lower level of platelet function after the stimuli with collagen and arachidonic acid compared with the clopidogrel treatment, which implicates the unique character of antiplatelet effect by cilostazol.
- The benefit of this triple therapy strategy has been mostly observed in PCI-treated patients, mainly as a reduction in the rates of target lesion revascularization and even in stent thrombosis [55,56,57]. In a recent meta-analysis, adjunctive cilostazol reduced the risk of angiographic restenosis irrespective of stent type (51% and 37% relative reduction after bare-metal stent and drug-eluting stent, respectively) and decreased numerically the risk of stent thrombosis by 43% (95% CI, 0.41 to 1.67), without the increase of major bleeding (OR, 1.00) [55]. The clinical efficacy of cilostazol in ischemic events may be more prominent in the setting of ACS. In a Chinese clinical trial including ACS patients (n=1,212), triple antiplatelet therapy with the addition of 6-month cilostazol after successful PCI was associated with a significantly lower incidence of the primary endpoint (composite of cardiac death, nonfatal MI, stroke, or target vessel revascularization at 1 year) (10.3% vs. 15.1%; HR, 0.65; 95% CI, 0.41 to 0.91; P=0.011), and no differences in the risks of TIMI major or minor bleeding were found (0.2% vs. 0.2%) [56]. Of note, the DM subgroup showed a more pronounced benefit with triple therapy (53% reduction) (Fig. 5). However, the use of cilostazol is limited by the high frequency of side effects (e.g., headache, palpitations, and GI disturbances) and increased risk of withdrawal.
- PAR-1 inhibitor
- Thrombin is the serine protease enzyme linked between plasmatic and cellular components of the thrombotic process and it plays a crucial role in the platelet activation and coagulation cascade [58]. Platelet PAR-1 and PAR-4 account for the thrombin-mediated signaling in platelets. PAR-1 mediates platelet responses at subnanomolar concentrations of thrombin, whereas PAR-4 mediates platelet activation at higher thrombin concentrations. Activation of either one is sufficient to trigger platelet secretion and aggregation, whereas PAR-1 is likely to be the most important receptor. In addition to platelet-mediated effects and fibrin polymerization during clot generation, thrombin exerts diverse effects on various cells. The PAR-1 receptor is present in platelets, endothelial cells, VSMCs, mononuclear cells, fibroblasts, and cells of atherosclerotic plaque, suggesting a major role in tissue response to injury, angiogenesis, inflammation, and thrombosis. In addition to its role during initial thrombus generation by stimulating platelet aggregation, thrombin that is produced in large quantities following thrombus generation may stimulate the secretion of platelet-derived growth factor and induce angiogenesis. The latter response to thrombin may contribute to vascular remodeling and also restenosis.
- Five PAR-1 antagonists have been developed, of which only one drug (vorapaxar) has been investigated in phase III clinical trial and approved for treatment in ACS patients. Vorapaxar is an oral competitive PAR-1 antagonist that blocks thrombin-mediated platelet activation without interfering with thrombin-mediated cleavage of fibrinogen [58]. It is rapidly absorbed (peak level in 60 to 90 minutes), has high bioavailability and a half-life of approximately 311 hours. Following promising findings from early phase clinical investigations, vorapaxar was tested in two large-scale, phase III clinical trials.
- In the Thrombin Receptor Antagonist for Clinical Event Reduction in Acute Coronary Syndrome (TRACER) trial (n= 12,944) [59], the clinical efficacy and safety of vorapaxar (40 mg loading dose and a 2.5 mg daily maintenance dose) versus placebo in addition to standard antiplatelet therapy (96% on aspirin and 91.8% on clopidogrel) was evaluated in patients with NSTE-ACS. Vorapaxar versus placebo treatment was associated with a significant decrease in the composite of cardiovascular death, MI, or stroke at 2 years (14.7% vs.16.4%; HR, 0.89; 95% CI, 0.81 to 0.98; P=0.02), at the expense of the increase in the rate of GUSTO moderate or severe bleeding (7.2% vs. 5.2%; HR, 1.35; 95% CI, 1.16 to 1.58; P<0.001), and a 3-fold increase in intracranial bleeding (1.1% vs. 0.2%, P<0.001). The excess prevalence of intracranial hemorrhage in patients with a history of stroke led to an unplanned safety review, which recommended early termination of this trial.
- In the Trial to Assess the Effects of Vorapaxar in Preventing Heart Attack and Stroke in Patients With Atherosclerosis-TIMI 50 (TRA 2P-TIMI 50) trial (n=26,449) [60], secondary prevention by adjunctive vorapaxar (2.5 mg daily) versus placebo in addition to standard-of-care therapy (58% on DAPT) was assessed among patients with known atherothrombotic disease (a history of MI, ischemic stroke, or peripheral arterial disease). Vorapaxar significantly reduced the primary endpoint (composite of cardiovascular death, MI, or stroke) compared with placebo at 30-month follow-up (9.3% vs. 10.5%; HR, 0.87; 95% CI, 0.80 to 0.94; P<0.001), which was largely driven by a 17% reduction in the MI risk. GUSTO moderate or severe bleeding occurred in 4.2% of patients who received vorapaxar and 2.5% of those who received placebo (HR, 1.66; 95% CI, 1.43 to 1.93; P<0.001) and intracranial bleeding was a twofold increase by vorapaxar (1.0% vs. 0.5%, P<0.001). Contrary to patients with a history of stroke, patients with previous MI (n=17,779) treated with vorapaxar exhibited a reduction in the primary endpoint at 3-year compared with placebo (8.1% vs. 9.7%; HR, 0.80; 95% CI, 0.72 to 0.89; P<0.0001) [61]. Despite an overall increase in bleeding complications, intracranial bleeding were not significantly higher in the vorapaxar versus placebo group (0.6% vs. 0.4%, P=0.076). The clinical benefit by vorapaxar was even more pronounced after exclusion of elderly patients (>75-year old), individuals with a history of stroke, and those with a low body weight (<60 kg). In diabetic patients with a prior MI (n=3,623) [62], vorapaxar significantly reduced the primary endpoint (11.4% vs.14.3%; HR, 0.73; 95% CI, 0.60 to 0.89; P=0.002) with a number needed to treat to avoid 1 major cardiovascular event of 29. The incidence of GUSTO moderate or severe bleeding was increased with vorapaxar in DM patients (4.4% vs. 2.6%; HR, 1.60; 95% CI, 1.07 to 2.40). However, net clinical outcome integrating these two endpoints (efficacy and safety) was improved with vorapaxar (HR, 0.79; 95% CI, 0.67 to 0.93).
- Based on the subanalysis, the FDA approved clinical use of vorapaxar (2.5 mg daily) in addition to standard-of-care therapy (aspirin, clopidogrel, or both) among patients with a history of MI or with peripheral arterial disease. Vorapaxar is contraindicated in patients with a history of stroke, transient ischemic attack, or intracranial hemorrhage, and in those with active pathological bleeding.
CLINICAL EVIDENCES OF ANTIPLATELET REGIMEN IN DIABETES MELLITUS
(1) Clopidogrel versus aspirin
(2) Clopidogrel+aspirin versus placebo+aspirin
(3) High-dose clopidogrel+aspirin versus standard-dose clopidogrel+aspirin
(4) Prasugrel+aspirin versus clopidogrel+aspirin
(5) Ticagrelor+aspirin versus clopidogrel+aspirin
(6) Cangrelor
- Numerous data have demonstrated a close relationship between HPR or "antiplatelet resistance" and atherothrombotic events in high-risk patients (e.g., PCI-treated patients with ACS or DM) [24]. In "laboratory resistant" patients, antiplatelet drug fails to block its specific platelet target (e.g., aspirin against COX-1 enzyme and clopidogrel against P2Y12 receptor) and it is only meaningful when "laboratory resistance" is translated "treatment failure" (the recurrence of ischemic events despite treatment).
- Prevalence of aspirin resistance is widely variable across the studies that may be due to differences in platelet function testing used, definition of resistance, aspirin dose, and patient cohort. When COX-1-dependent tests (by determination of serum/urine thromboxane and assays with arachidonic acid as agonist) are used, aspirin resistance is a rare phenomenon (<5% of patients) [63,64] and the main cause of this aspirin resistance is poor compliance. However, when COX-1-independent tests assays are used, prevalence of aspirin resistance appears higher. The Aspirin-Induced Platelet Effect (ASPECT) study demonstrated that aspirin inhibited platelet aggregation stimulated by agonists other than arachidonic acid in a dose-dependent manner among stable CAD patients [63]; significant effects were observed for collagen- and shear-induced aggregation and 11-dehydrothromboxane B2 production. The latter finding may be due to effects of aspirin beyond inhibition of its primary target COX-1 by acetylation and was termed a non-COX-1 effect.
- The attenuated antiplatelet effect of aspirin therapy in DM patients can be explained by various mechanisms such as reduced drug bioavailability, accelerated platelet turnover, and glycosylation of platelet membrane proteins [6]. When platelet turnover is heightened, an increased proportion of immature platelets capable of protein synthesis are released from the bone marrow and can be identified as a marker of accelerated thrombopoiesis. In a post hoc analysis of ASPECT, greater platelet reactivity and a higher prevalence of aspirin resistance were present in the patients with DM [65]. Aspirin doses of >81 mg daily (162 to 325 mg daily) were associated with similar rates of resistance and platelet function in patients with and without DM. A higher aspirin dosing strategy than 81 mg daily in DM patients may be associated with enhanced platelet inhibition (mainly by COX-1-dependent methods) and possibly better protection against atherothrombotic event. Elevated TXA2 synthesis may be related with increased platelet turnover in DM patients; the introduction of newly generated platelets not exposed to aspirin into the systemic circulation continues to generate TXA2, which may activate thromboxane and prostaglandin endoperoxide (TP) receptor. TP receptor activation has led to interest in developing TP receptor blockers [6].
- In a post hoc analysis of ASPECT, a higher aspirin dose (162 to 325 mg daily) than 81 mg daily did not decrease the level of ADP-mediated platelet function and closure time in PFA-100 collagen/epinephrine assay among stable CAD patients with DM [65]. In aspirin-treated patients presenting for angiographic evaluation of CAD (n=562), both serum thromboxane B2 >3.1 ng/mL and PFA-100 collagen-ADP closure time <65 seconds (OR, 3.5; 95% CI, 1.2 to 10.4; P=0.027) were associated with MACEs at 2-year follow-up [64]. This finding suggests that multiple mechanisms, including but not confined to inadequate inhibition of COX-1, are responsible for poor clinical outcomes in aspirin-treated patients. The addition of other pathway blockade (e.g., P2Y12 inhibitor) can be plausible strategy to overcome the combined risk of aspirin resistance in DM patients. Since enhanced inhibition of platelet activation by combination regimen can increased the risk of serious bleeding, the potency of antiplatelet therapy must be determined on the risk profile of the patient cohort. In the primary prevention subgroup with multiple risk factors from CHARISMA (n=3,284, 80.8% were diabetics) [29], clopidogrel versus placebo on top of aspirin did not decrease the rate of the primary endpoint (6.6% vs. 5.5%, P=0.20) and increased the risk of severe bleeding (2.0% vs.1.2%, P=0.07).
- DAPT with clopidogrel and aspirin is the standard antiplatelet regimen in high-risk DM patients (e.g., ACS or PCI). However, a substantial portion of DM patients suffers from recurrent cardiovascular events. The prevalence of "clopidogrel resistance" varies considerably and is related to differences in definitions, type of test used, clopidogrel dose, and cohort character [24]. Genetic, cellular, and clinical mechanisms have been associated with inadequate responsiveness to clopidogrel. The presence of DM is an important clinical factor that contributes to "clopidogrel resistance." Numerous mechanisms have been suggested to explain the inadequate clopidogrel response observed in DM patients: low bioavailability of clopidogrel, lack of response to insulin in platelets, alterations in calcium metabolism, upregulation of P2Y12 receptor signaling, increased exposure to ADP, and increased platelet turnover [6]. Several antiplatelet treatment strategies have been developed to optimize platelet inhibition: (1) dose modification of clopidogrel; (2) use of potent P2Y12 inhibitor agents; and (3) addition of a third antiplatelet drug (triple therapy) (e.g., cilostazol, PAR-1 inhibitor) [9]. There is an accompanying increased risk of bleeding with more potent platelet inhibition. It could be an important issue in the future trials whether a therapeutic window exists for antiplatelet strategy to simultaneously limit thrombotic and bleeding events.
RESISTANCE TO ANTIPLATELET AGENT IN DIABETIC PATIENTS
- Diabetes itself is a hypercoagulable state and hyperreactive platelets in DM patients remarkably contribute to the increased risk of ischemic events occurrence. Furthermore, DM patients have shown low response to commonly used antiplatelet regimen (aspirin and clopidogrel). Understanding mechanism of "treatment failure" in DM patients during antiplatelet therapy may enable more reasonable approaches to maximize clinical efficacy and safety. Because the role of aspirin in primary prevention among DM patients still remains questionable, upcoming results from ongoing aspirin trials in primary prevention and clinical evidences from other treatment strategies (e.g. statin, P2Y12 antagonist, and polypill) are warranted. For secondary prevention in high-risk DM patients (e.g. ACS), the development of more potent or new combination antithrombotic strategies may control the enhanced hypercoagulable state in diverse pathways and therefore improve clinical outcomes. Large-scale randomized trials specifically designed to evaluate these new antithrombotic strategies in DM patients are warranted to determine their efficacy and safety.
CONCLUSIONS
-
Acknowledgements
- This study was supported by grants from Institute of the Health Sciences, Gyeongsang National University and from the National Institute of Health (NIHP50 HL110789).
ACKNOWLEDGMENTS
-
CONFLICTS OF INTEREST: Dr. Gurbel reports serving as a consultant fees/receiving honoraria from Daiichi Sankyo, Bayer, AstraZeneca, Merck, Boehringer, Janssen, and CSL; receiving grants from the National Institutes of Health, Daiichi Sankyo, CSL, AstraZeneca, Harvard Clinical Research Institute, Bayer, Haemonetics, Duke Clinical Research Institute, Sinnowa, an Coramed. Dr. Jeong has received honoraria for lectures from AstraZeneca, Sanofi-Aventis, Daiichi Sankyo/Lilly, Haemonetics, Otsuka and Yuhan Pharmaceuticals; and research grants or support from AstraZeneca, Korean Society of Interventional Cardiology, Han-mi Pharmaceuticals, and Haemonetics.
NOTES
- 1. World Health Organization. Global status report on noncommunicable diseases 2010 [Internet] updated 2015 Mar 16. Available from: http://www.who.int/nmh/publications/ncd_report_full_en.pdf.
- 2. Emerging Risk Factors Collaboration. Sarwar N, Gao P, Seshasai SR, Gobin R, Kaptoge S, Di Angelantonio E, Ingelsson E, Lawlor DA, Selvin E, Stampfer M, Stehouwer CD, Lewington S, Pennells L, Thompson A, Sattar N, White IR, Ray KK, Danesh J. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet 2010;375:2215-2222. ArticlePubMedPMC
- 3. Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 1998;339:229-234. ArticlePubMed
- 4. Morel O, Kessler L, Ohlmann P, Bareiss P. Diabetes and the platelet: toward new therapeutic paradigms for diabetic atherothrombosis. Atherosclerosis 2010;212:367-376. ArticlePubMed
- 5. Patti G, Proscia C, Di Sciascio G. Antiplatelet therapy in patients with diabetes mellitus and acute coronary syndrome. Circ J 2014;78:33-41. ArticlePubMed
- 6. Ferreiro JL, Angiolillo DJ. Diabetes and antiplatelet therapy in acute coronary syndrome. Circulation 2011;123:798-813. ArticlePubMed
- 7. Angiolillo DJ, Bernardo E, Sabate M, Jimenez-Quevedo P, Costa MA, Palazuelos J, Hernandez-Antolin R, Moreno R, Escaned J, Alfonso F, Banuelos C, Guzman LA, Bass TA, Macaya C, Fernandez-Ortiz A. Impact of platelet reactivity on cardiovascular outcomes in patients with type 2 diabetes mellitus and coronary artery disease. J Am Coll Cardiol 2007;50:1541-1547. ArticlePubMed
- 8. Mangiacapra F, Peace A, Barbato E, Patti G, Gatto L, Ricottini E, De Bruyne B, Di Sciascio G, Wijns W. Thresholds for platelet reactivity to predict clinical events after coronary intervention are different in patients with and without diabetes mellitus. Platelets 2014;25:348-356. ArticlePubMed
- 9. Gurbel PA, Tantry US. Combination antithrombotic therapies. Circulation 2010;121:569-583. ArticlePubMed
- 10. Franchi F, Angiolillo DJ. Novel antiplatelet agents in acute coronary syndrome. Nat Rev Cardiol 2015;12:30-47. ArticlePubMedPDF
- 11. Patrignani P, Filabozzi P, Patrono C. Selective cumulative inhibition of platelet thromboxane production by low-dose aspirin in healthy subjects. J Clin Invest 1982;69:1366-1372. ArticlePubMedPMC
- 12. Sanderson S, Emery J, Baglin T, Kinmonth AL. Narrative review: aspirin resistance and its clinical implications. Ann Intern Med 2005;142:370-380. ArticlePubMed
- 13. Halvorsen S, Andreotti F, ten Berg JM, Cattaneo M, Coccheri S, Marchioli R, Morais J, Verheugt FW, De Caterina R. Aspirin therapy in primary cardiovascular disease prevention: a position paper of the European Society of Cardiology working group on thrombosis. J Am Coll Cardiol 2014;64:319-327. PubMed
- 14. Seshasai SR, Wijesuriya S, Sivakumaran R, Nethercott S, Erqou S, Sattar N, Ray KK. Effect of aspirin on vascular and nonvascular outcomes: meta-analysis of randomized controlled trials. Arch Intern Med 2012;172:209-216. ArticlePubMed
- 15. Antithrombotic Trialists' (ATT) Collaboration. Baigent C, Blackwell L, Collins R, Emberson J, Godwin J, Peto R, Buring J, Hennekens C, Kearney P, Meade T, Patrono C, Roncaglioni MC, Zanchetti A. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet 2009;373:1849-1860. ArticlePubMedPMC
- 16. Vandvik PO, Lincoff AM, Gore JM, Gutterman DD, Sonnenberg FA, Alonso-Coello P, Akl EA, Lansberg MG, Guyatt GH, Spencer FA. American College of Chest Physicians. Primary and secondary prevention of cardiovascular disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012;141:e637S-e668S. ArticlePubMedPMC
- 17. He J, Whelton PK, Vu B, Klag MJ. Aspirin and risk of hemorrhagic stroke: a meta-analysis of randomized controlled trials. JAMA 1998;280:1930-1935. ArticlePubMed
- 18. Pignone M, Alberts MJ, Colwell JA, Cushman M, Inzucchi SE, Mukherjee D, Rosenson RS, Williams CD, Wilson PW, Kirkman MS. American Diabetes Association. American Heart Association. American College of Cardiology Foundation. Aspirin for primary prevention of cardiovascular events in people with diabetes: a position statement of the American Diabetes Association, a scientific statement of the American Heart Association, and an expert consensus document of the American College of Cardiology Foundation. Diabetes Care 2010;33:1395-1402. PubMedPMC
- 19. American Diabetes A. Standards of medical care in diabetes: 2014. Diabetes Care 2014;(Suppl 1):37:S14-S80. ArticlePubMedPDF
- 20. Antiplatelet Trialists' Collaboration. Collaborative overview of randomised trials of antiplatelet therapy. I: prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients. BMJ 1994;308:81-106. ArticlePubMedPMC
- 21. CURRENT-OASIS 7 Investigators. Mehta SR, Bassand JP, Chrolavicius S, Diaz R, Eikelboom JW, Fox KA, Granger CB, Jolly S, Joyner CD, Rupprecht HJ, Widimsky P, Afzal R, Pogue J, Yusuf S. Dose comparisons of clopidogrel and aspirin in acute coronary syndromes. N Engl J Med 2010;363:930-942. ArticlePubMed
- 22. Mehta SR, Tanguay JF, Eikelboom JW, Jolly SS, Joyner CD, Granger CB, Faxon DP, Rupprecht HJ, Budaj A, Avezum A, Widimsky P, Steg PG, Bassand JP, Montalescot G, Macaya C, Di Pasquale G, Niemela K, Ajani AE, White HD, Chrolavicius S, Gao P, Fox KA, Yusuf S. CURRENT-OASIS 7 trial investigators. Double-dose versus standard-dose clopidogrel and high-dose versus low-dose aspirin in individuals undergoing percutaneous coronary intervention for acute coronary syndromes (CURRENT-OASIS 7): a randomised factorial trial. Lancet 2010;376:1233-1243. ArticlePubMed
- 23. Levine GN, Jeong YH, Goto S, Anderson JL, Huo Y, Mega JL, Taubert K, Smith SC Jr. Expert consensus document: World Heart Federation expert consensus statement on antiplatelet therapy in East Asian patients with ACS or undergoing PCI. Nat Rev Cardiol 2014;11:597-606. ArticlePubMedPDF
- 24. Tantry US, Bonello L, Aradi D, Price MJ, Jeong YH, Angiolillo DJ, Stone GW, Curzen N, Geisler T, Ten Berg J, Kirtane A, Siller-Matula J, Mahla E, Becker RC, Bhatt DL, Waksman R, Rao SV, Alexopoulos D, Marcucci R, Reny JL, Trenk D, Sibbing D, Gurbel PA. Working Group on On-Treatment Platelet Reactivity. Consensus and update on the definition of on-treatment platelet reactivity to adenosine diphosphate associated with ischemia and bleeding. J Am Coll Cardiol 2013;62:2261-2273. ArticlePubMed
- 25. Jeong YH, Kim IS, Park Y, Kang MK, Koh JS, Hwang SJ, Kwak CH, Hwang JY. Carriage of cytochrome 2C19 polymorphism is associated with risk of high post-treatment platelet reactivity on high maintenance-dose clopidogrel of 150 mg/day: results of the ACCEL-DOUBLE (Accelerated Platelet Inhibition by a Double Dose of Clopidogrel According to Gene Polymorphism) study. JACC Cardiovasc Interv 2010;3:731-741. PubMed
- 26. Angiolillo DJ, Badimon JJ, Saucedo JF, Frelinger AL, Michelson AD, Jakubowski JA, Zhu B, Ojeh CK, Baker BA, Effron MB. A pharmacodynamic comparison of prasugrel vs. high-dose clopidogrel in patients with type 2 diabetes mellitus and coronary artery disease: results of the Optimizing anti-Platelet Therapy In diabetes MellitUS (OPTIMUS)-3 Trial. Eur Heart J 2011;32:838-846. ArticlePubMedPMC
- 27. Alexopoulos D, Xanthopoulou I, Mavronasiou E, Stavrou K, Siapika A, Tsoni E, Davlouros P. Randomized assessment of ticagrelor versus prasugrel antiplatelet effects in patients with diabetes. Diabetes Care 2013;36:2211-2216. ArticlePubMedPMCPDF
- 28. Bhatt DL, Fox KA, Hacke W, Berger PB, Black HR, Boden WE, Cacoub P, Cohen EA, Creager MA, Easton JD, Flather MD, Haffner SM, Hamm CW, Hankey GJ, Johnston SC, Mak KH, Mas JL, Montalescot G, Pearson TA, Steg PG, Steinhubl SR, Weber MA, Brennan DM, Fabry-Ribaudo L, Booth J, Topol EJ. CHARISMA Investigators. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med 2006;354:1706-1717. ArticlePubMed
- 29. Wang TH, Bhatt DL, Fox KA, Steinhubl SR, Brennan DM, Hacke W, Mak KH, Pearson TA, Boden WE, Steg PG, Flather MD, Montalescot G, Topol EJ. CHARISMA Investigators. An analysis of mortality rates with dual-antiplatelet therapy in the primary prevention population of the CHARISMA trial. Eur Heart J 2007;28:2200-2207. ArticlePubMed
- 30. Hayden MR, Tyagi SC. Vasa vasorum in plaque angiogenesis, metabolic syndrome, type 2 diabetes mellitus, and atheroscleropathy: a malignant transformation. Cardiovasc Diabetol 2004;3:1PubMedPMC
- 31. CAPRIE Steering Committee. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). Lancet 1996;348:1329-1339. ArticlePubMed
- 32. Bhatt DL, Marso SP, Hirsch AT, Ringleb PA, Hacke W, Topol EJ. Amplified benefit of clopidogrel versus aspirin in patients with diabetes mellitus. Am J Cardiol 2002;90:625-628. ArticlePubMed
- 33. Bhatt DL, Flather MD, Hacke W, Berger PB, Black HR, Boden WE, Cacoub P, Cohen EA, Creager MA, Easton JD, Hamm CW, Hankey GJ, Johnston SC, Mak KH, Mas JL, Montalescot G, Pearson TA, Steg PG, Steinhubl SR, Weber MA, Fabry-Ribaudo L, Hu T, Topol EJ, Fox KA. CHARISMA Investigators. Patients with prior myocardial infarction, stroke, or symptomatic peripheral arterial disease in the CHARISMA trial. J Am Coll Cardiol 2007;49:1982-1988. ArticlePubMed
- 34. Mehta SR, Yusuf S, Peters RJ, Bertrand ME, Lewis BS, Natarajan MK, Malmberg K, Rupprecht H, Zhao F, Chrolavicius S, Copland I, Fox KA. Clopidogrel in Unstable angina to prevent Recurrent Events trial (CURE) Investigators. Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: the PCI-CURE study. Lancet 2001;358:527-533. ArticlePubMed
- 35. Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK. Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial Investigators. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med 2001;345:494-502. ArticlePubMed
- 36. Steinhubl SR, Berger PB, Mann JT 3rd, Fry ET, DeLago A, Wilmer C, Topol EJ. CREDO Investigators Clopidogrel for the Reduction of Events During Observation. Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: a randomized controlled trial. JAMA 2002;288:2411-2420. ArticlePubMed
- 37. Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, Neumann FJ, Ardissino D, De Servi S, Murphy SA, Riesmeyer J, Weerakkody G, Gibson CM, Antman EM. TRITON-TIMI 38 Investigators. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2007;357:2001-2015. ArticlePubMed
- 38. Wiviott SD, Braunwald E, Angiolillo DJ, Meisel S, Dalby AJ, Verheugt FW, Goodman SG, Corbalan R, Purdy DA, Murphy SA, McCabe CH, Antman EM. TRITON-TIMI 38 Investigators. Greater clinical benefit of more intensive oral antiplatelet therapy with prasugrel in patients with diabetes mellitus in the trial to assess improvement in therapeutic outcomes by optimizing platelet inhibition with prasugrel-Thrombolysis in Myocardial Infarction 38. Circulation 2008;118:1626-1636. ArticlePubMed
- 39. Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, Horrow J, Husted S, James S, Katus H, Mahaffey KW, Scirica BM, Skene A, Steg PG, Storey RF, Harrington RA. PLATO Investigators. Freij A Thorsen M . Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2009;361:1045-1057. ArticlePubMed
- 40. Ferreiro JL, Ueno M, Tello-Montoliu A, Tomasello SD, Capodanno D, Capranzano P, Dharmashankar K, Darlington A, Desai B, Rollini F, Guzman LA, Bass TA, Angiolillo DJ. Effects of cangrelor in coronary artery disease patients with and without diabetes mellitus: an in vitro pharmacodynamic investigation. J Thromb Thrombolysis 2013;35:155-164. ArticlePubMedPDF
- 41. Steg PG, Bhatt DL, Hamm CW, Stone GW, Gibson CM, Mahaffey KW, Leonardi S, Liu T, Skerjanec S, Day JR, Iwaoka RS, Stuckey TD, Gogia HS, Gruberg L, French WJ, White HD, Harrington RA. CHAMPION Investigators. Effect of cangrelor on periprocedural outcomes in percutaneous coronary interventions: a pooled analysis of patient-level data. Lancet 2013;382:1981-1992. ArticlePubMed
- 42. Kristensen SD, Wurtz M, Grove EL, De Caterina R, Huber K, Moliterno DJ, Neumann FJ. Contemporary use of glycoprotein IIb/IIIa inhibitors. Thromb Haemost 2012;107:215-224. ArticlePubMed
- 43. Mehilli J, Kastrati A, Schuhlen H, Dibra A, Dotzer F, von Beckerath N, Bollwein H, Pache J, Dirschinger J, Berger PP, Schomig A. Intracoronary Stenting and Antithrombotic Regimen: Is Abciximab a Superior Way to Eliminate Elevated Thrombotic Risk in Diabetics (ISAR-SWEET) Study Investigators. Randomized clinical trial of abciximab in diabetic patients undergoing elective percutaneous coronary interventions after treatment with a high loading dose of clopidogrel. Circulation 2004;110:3627-3635. ArticlePubMed
- 44. Kastrati A, Mehilli J, Neumann FJ, Dotzer F, ten Berg J, Bollwein H, Graf I, Ibrahim M, Pache J, Seyfarth M, Schuhlen H, Dirschinger J, Berger PB, Schomig A. Intracoronary Stenting and Antithrombotic: Regimen Rapid Early Action for Coronary Treatment 2 (ISAR-REACT 2) Trial Investigators. Abciximab in patients with acute coronary syndromes undergoing percutaneous coronary intervention after clopidogrel pretreatment: the ISAR-REACT 2 randomized trial. JAMA 2006;295:1531-1538. ArticlePubMed
- 45. Giugliano RP, White JA, Bode C, Armstrong PW, Montalescot G, Lewis BS, van 't Hof A, Berdan LG, Lee KL, Strony JT, Hildemann S, Veltri E, Van de Werf F, Braunwald E, Harrington RA, Califf RM, Newby LK. EARLY ACS Investigators. Early versus delayed, provisional eptifibatide in acute coronary syndromes. N Engl J Med 2009;360:2176-2190. ArticlePubMed
- 46. De Luca G, Navarese E, Marino P. Risk profile and benefits from Gp IIb-IIIa inhibitors among patients with ST-segment elevation myocardial infarction treated with primary angioplasty: a meta-regression analysis of randomized trials. Eur Heart J 2009;30:2705-2713. ArticlePubMedPMC
- 47. Cannon CP, Harrington RA, James S, Ardissino D, Becker RC, Emanuelsson H, Husted S, Katus H, Keltai M, Khurmi NS, Kontny F, Lewis BS, Steg PG, Storey RF, Wojdyla D, Wallentin L. PLATelet inhibition and patient Outcomes Investigators. Comparison of ticagrelor with clopidogrel in patients with a planned invasive strategy for acute coronary syndromes (PLATO): a randomised double-blind study. Lancet 2010;375:283-293. ArticlePubMed
- 48. Francis SH, Blount MA, Corbin JD. Mammalian cyclic nucleotide phosphodiesterases: molecular mechanisms and physiological functions. Physiol Rev 2011;91:651-690. ArticlePubMed
- 49. Gresele P, Momi S, Falcinelli E. Anti-platelet therapy: phosphodiesterase inhibitors. Br J Clin Pharmacol 2011;72:634-646. ArticlePubMedPMC
- 50. Jeong YH, Tantry US, Park Y, Kwon TJ, Park JR, Hwang SJ, Bliden KP, Koh EH, Kwak CH, Hwang JY, Kim S, Gurbel PA. Pharmacodynamic effect of cilostazol plus standard clopidogrel versus double-dose clopidogrel in patients with type 2 diabetes undergoing percutaneous coronary intervention. Diabetes Care 2012;35:2194-2197. ArticlePubMedPMCPDF
- 51. Park Y, Jeong YH, Tantry US, Ahn JH, Kim KH, Koh JS, Park JR, Hwang SJ, Kwak CH, Hwang JY, Gurbel PA. Effect of adjunctive dipyridamole to DAPT on platelet function profiles in stented patients with high platelet reactivity. The result of the ACCEL-DIP Study. Thromb Haemost 2014;112:1198-1208. ArticlePubMed
- 52. Jeong YH, Park Y, Muse WC, Kwon TJ, Koh JS, Hwang SJ, Kwak CH, Hwang JY. Pharmacodynamic effect of clopidogrel therapy and switching to cilostazol in patients with the CYP2C19 loss-of-function allele (ACCEL-SWITCH) study. J Thromb Haemost 2012;10:1685-1688. ArticlePubMed
- 53. Park Y, Jung JM, Tantry US, Kim K, Koh JS, Park JR, Hwang SJ, Kwak CH, Hwang JY, Kim S, Gurbel PA, Jeong YH. Pharmacodynamic effects of cilostazol versus clopidogrel in stented patients under proton pump inhibitor co-administration: the ACCEL-PARAZOL study. J Atheroscler Thromb 2014;21:1121-1139. ArticlePubMed
- 54. Katakami N, Kim YS, Kawamori R, Yamasaki Y. The phosphodiesterase inhibitor cilostazol induces regression of carotid atherosclerosis in subjects with type 2 diabetes mellitus: principal results of the Diabetic Atherosclerosis Prevention by Cilostazol (DAPC) study: a randomized trial. Circulation 2010;121:2584-2591. ArticlePubMed
- 55. Jang JS, Jin HY, Seo JS, Yang TH, Kim DK, Kim DS, Kim DK, Seol SH, Kim DI, Cho KI, Kim BH, Park YH, Je HG, Jeong YH, Kim WJ, Lee JY, Lee SW. A meta-analysis of randomized controlled trials appraising the efficacy and safety of cilostazol after coronary artery stent implantation. Cardiology 2012;122:133-143. ArticlePubMedPDF
- 56. Han Y, Li Y, Wang S, Jing Q, Wang Z, Wang D, Shu Q, Tang X. Cilostazol in addition to aspirin and clopidogrel improves long-term outcomes after percutaneous coronary intervention in patients with acute coronary syndromes: a randomized, controlled study. Am Heart J 2009;157:733-739. ArticlePubMed
- 57. Lee SW, Park SW, Kim YH, Yun SC, Park DW, Lee CW, Kang SJ, Park SJ, Lee JH, Choi SW, Seong IW, Lee NH, Cho YH, Shin WY, Lee SJ, Lee SW, Hyon MS, Bang DW, Choi YJ, Kim HS, Lee BK, Lee K, Park HK, Park CB, Lee SG, Kim MK, Park KH, Park WJ. DECLARE-LONG II Study Investigators. A randomized, double-blind, multicenter comparison study of triple antiplatelet therapy with dual antiplatelet therapy to reduce restenosis after drug-eluting stent implantation in long coronary lesions: results from the DECLARE-LONG II (Drug-Eluting Stenting Followed by Cilostazol Treatment Reduces Late Restenosis in Patients with Long Coronary Lesions) trial. J Am Coll Cardiol 2011;57:1264-1270. PubMed
- 58. Gurbel PA, Jeong YH, Tantry US. Vorapaxar: a novel protease-activated receptor-1 inhibitor. Expert Opin Investig Drugs 2011;20:1445-1453.ArticlePubMed
- 59. Tricoci P, Huang Z, Held C, Moliterno DJ, Armstrong PW, Van de Werf F, White HD, Aylward PE, Wallentin L, Chen E, Lokhnygina Y, Pei J, Leonardi S, Rorick TL, Kilian AM, Jennings LH, Ambrosio G, Bode C, Cequier A, Cornel JH, Diaz R, Erkan A, Huber K, Hudson MP, Jiang L, Jukema JW, Lewis BS, Lincoff AM, Montalescot G, Nicolau JC, Ogawa H, Pfisterer M, Prieto JC, Ruzyllo W, Sinnaeve PR, Storey RF, Valgimigli M, Whellan DJ, Widimsky P, Strony J, Harrington RA, Mahaffey KW. TRACER Investigators. Thrombin-receptor antagonist vorapaxar in acute coronary syndromes. N Engl J Med 2012;366:20-33. ArticlePubMed
- 60. Morrow DA, Braunwald E, Bonaca MP, Ameriso SF, Dalby AJ, Fish MP, Fox KA, Lipka LJ, Liu X, Nicolau JC, Ophuis AJ, Paolasso E, Scirica BM, Spinar J, Theroux P, Wiviott SD, Strony J, Murphy SA. TRA 2P-TIMI 50 Steering Committee and Investigators. Vorapaxar in the secondary prevention of atherothrombotic events. N Engl J Med 2012;366:1404-1413. ArticlePubMed
- 61. Scirica BM, Bonaca MP, Braunwald E, De Ferrari GM, Isaza D, Lewis BS, Mehrhof F, Merlini PA, Murphy SA, Sabatine MS, Tendera M, Van de Werf F, Wilcox R, Morrow DA. TRA 2°P-TIMI 50 Steering Committee Investigators. Vorapaxar for secondary prevention of thrombotic events for patients with previous myocardial infarction: a prespecified subgroup analysis of the TRA 2 degrees P-TIMI 50 trial. Lancet 2012;380:1317-1324. PubMed
- 62. Cavender MA, Scirica BM, Bonaca MP, Angiolillo DJ, Dalby AJ, Dellborg M, Morais J, Murphy SA, Oude Ophius T, Tendera M, Braunwald E, Morrow DA. Vorapaxar in patients with diabetes and prior MI: findings from the TRA 2 degrees P-TIMI 50 trial. Circulation 2015;131:1047-1053. ArticlePubMedPMC
- 63. Gurbel PA, Bliden KP, DiChiara J, Newcomer J, Weng W, Neerchal NK, Gesheff T, Chaganti SK, Etherington A, Tantry US. Evaluation of dose-related effects of aspirin on platelet function: results from the Aspirin-Induced Platelet Effect (ASPECT) study. Circulation 2007;115:3156-3164. ArticlePubMed
- 64. Frelinger AL 3rd, Li Y, Linden MD, Barnard MR, Fox ML, Christie DJ, Furman MI, Michelson AD. Association of cyclooxygenase-1-dependent and -independent platelet function assays with adverse clinical outcomes in aspirin-treated patients presenting for cardiac catheterization. Circulation 2009;120:2586-2596. ArticlePubMed
- 65. DiChiara J, Bliden KP, Tantry US, Hamed MS, Antonino MJ, Suarez TA, Bailon O, Singla A, Gurbel PA. The effect of aspirin dosing on platelet function in diabetic and nondiabetic patients: an analysis from the aspirin-induced platelet effect (ASPECT) study. Diabetes 2007;56:3014-3019. PubMed
REFERENCES
Proposed mechanism of atherothrombosis in diabetes mellitus [4,5]. PKC, protein kinase C; RAGE, receptor for advanced glycation endproducts; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; PAI-1, plasminogen activator inhibitor-1; VSMC, vascular smooth muscle cell.
Antiplatelet agents currently available or under development [9,10]. PAR, protease-activated receptor; TXA, thromboxane; COX-1, cyclooxygenase-1; PDE, phosphodiesterase; AC, adenylyl cyclase; ADP, adenosine diphosphate; cAMP, cyclic adenosine monophosphate; 5-HT2A, 5-hydroxytryptamine (serotonin) receptor 2A; VASP-P, vasodilator-stimulated phosphoprotein-phosphorylation; PKA, protein kinase A; GP, glycoprotein; TNF, tumor necrosis factor.
Relationship between clinical benefit and bleeding risk according to absolute cardiovascular risk in primary prevention trials of aspirin. Adapted from Halvorsen et al. [13]. To examine the association between treatment effects of aspirin on cardiovascular events, major gastrointestinal bleeding, and total major bleeding according to the level of cardiovascular risk (per 100 person-years in the control arm), univariate inverse variance-weighted linear regressions of the risk difference was fitted for the outcome events per 100 person-years between the two experimental arms. The size of circles is proportional to the inverse of variance of the risk difference. Red arrow denotes the area where benefit likely equals risk, yellow area denotes area of prescription uncertainty, and green arrow denotes the area where benefit most likely exceeds risk. Continuous line=linear regression; dotted line=lower and higher 95% confidence interval. CV, cardiovascular; GI, gastrointestinal; SAPAT, Swedish Angina Pectoris Aspirin Trial; JPAD, Japanese Primary Prevention of Atherosclerosis with Aspirin for Diabetes; PPP, Primary Prevention Project; HOT, Hypertension Optimal Treatment; WHS, Women's Health Study; BDT, British Doctors Trial; PHS, Physicians Health Study; AAA, Aspirin for Asymptomatic Atherosclerosis; TPT, Thrombosis Prevention Trial; POPAPDAD, prevention of progression of arterial disease and diabetes.
Metabolic pathway of P2Y12 receptor inhibitors. Adapted from Levine et al., with permission from Nature Publishing Group [23]. MDR1, multidrug resistance protein 1; hCE, human carboxylesterase; CYP, cytochrome P450; ADP, adenosine diphosphate; GP, glycoprotein.
Randomized clinical trials evaluating primary efficacy of intensified antiplatelet regimen versus clopidogrel in diabetic patients with acute coronary syndrome [22,37,39,56]. CI, confidence interval; CURRENT-OASIS 7, Clopidogrel Optimal Loading Dose Usage to Reduce Recurrent EveNTs-Optimal Antiplatelet Strategy for InterventionS; PCI, percutaneous coronary intervention; ACS, acute coronary syndrome; TRITON-TIMI 38, Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel-Thrombolysis in Myocardial Infarction 38; PLATO, platelet inhibition and patient outcomes.
Clinical trials of aspirin in primary prevention for diabetes
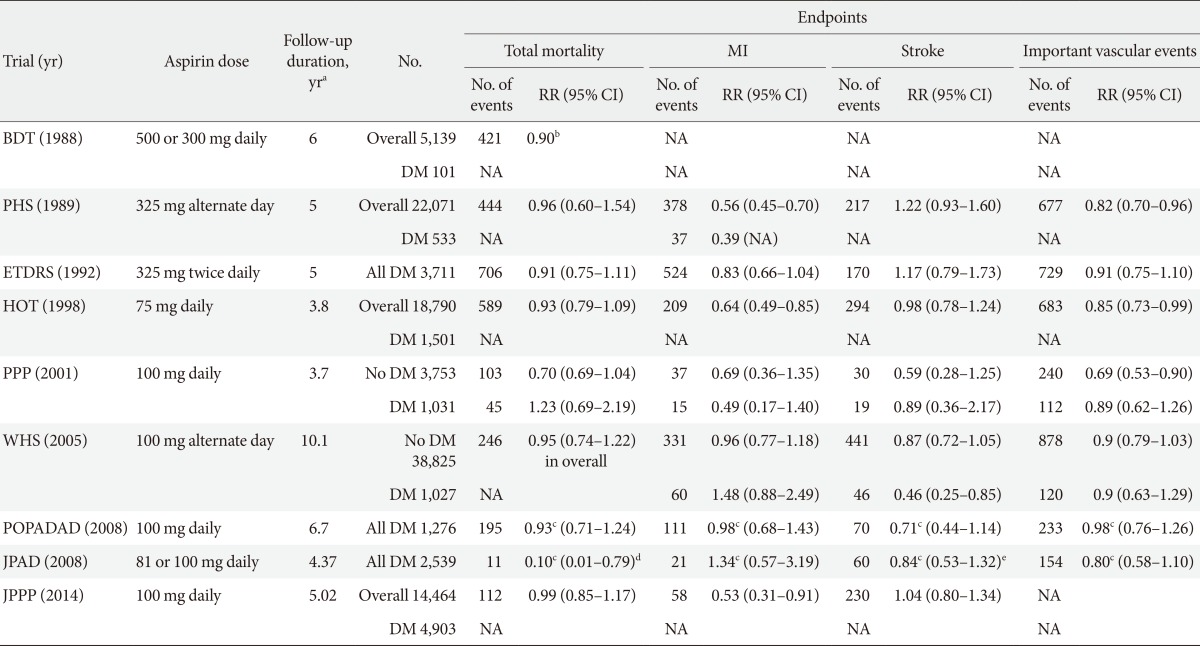
MI, myocardial infarction; RR, relative risk; CI, confidence interval; BDT, British Doctors Trial; DM, diabetes mellitus; NA, not available; PHS, Physicians Health Study; ETDRS, Early Treatment Diabetic Retinopathy Study; HOT, Hypertension Optimal Treatment; PPP, Primary Prevention Project; WHS, Women's Health Study; POPADAD, Prevention of Progression of Arterial Disease and Diabetes; JPAD, Japanese Primary Prevention of Atherosclerosis with Aspirin for Diabetes; JPPP, Japanese Primary Prevention Project.
aDuration of follow-up represents median follow-up for POPADAD, JPAD, and JPPP, mean follow-up for the other trials, bRatio of events to person-years, cHazard ratio, dCoronary and cerebrovascular mortality, eCerebrovascular disease (fatal+nonfatal).
Main outcomes of randomized clinical trials investigating clinical efficacy of oral antiplatelet treatment in diabetic patients with acute coronary syndrome or undergoing percutaneous coronary intervention
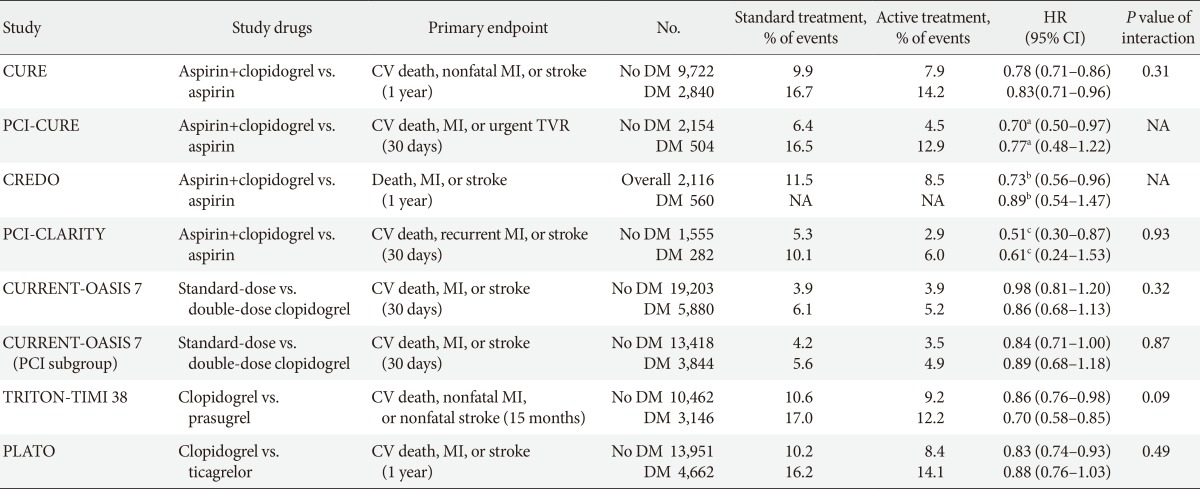
HR, hazard ratio; CI, confidence interval; CURE, Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial; CV, cardiovascular; MI, myocardial infarction; DM, diabetes mellitus; PCI, percutaneous coronary intervention; TVR, target vessel revascularization; NA, not available; CREDO, Clopidogrel for the Reduction of Events During Observation; CLARITY, Clopidogrel as Adjunctive Reperfusion Therapy; CURRENT-OASIS 7, Clopidogrel Optimal Loading Dose Usage to Reduce Recurrent EveNTs-Optimal Antiplatelet Strategy for InterventionS; TRITON-TIMI 38, Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition With Prasugrel-Thrombolysis in Myocardial Infarction 38; PLATO, PLATelet inhibition and patient Outcomes.
aRelative risk, bRelative risk reduction, cOdds ratio.
Figure & Data
References
Citations

- Mice expressing nonpolymerizable fibrinogen have reduced arterial and venous thrombosis with preserved hemostasis
Woosuk S. Hur, Tomohiro Kawano, Jean Marie N. Mwiza, David S. Paul, Robert H. Lee, Emily G. Clark, Emma G. Bouck, Ananya Dutta, Can Cai, Stephen R. Baker, Martin Guthold, Nigel Mackman, Pierre Mangin, Alisa S. Wolberg, Wolfgang Bergmeier, Matthew J. Flick
Blood.2024; 143(2): 105. CrossRef - On‐Treatment Platelet Reactivity and Ischemic Outcomes in Patients With Diabetes Mellitus: Two‐Year Results From ADAPT‐DES
Bahira Shahim, Björn Redfors, Thomas D. Stuckey, Mengdan Liu, Zhipeng Zhou, Bernhard Witzenbichler, Giora Weisz, Michael J. Rinaldi, Franz‐Josef Neumann, D. Christopher Metzger, Timothy D. Henry, David A. Cox, Peter L. Duffy, Bruce R. Brodie, Iva Srdanovi
Journal of the American Heart Association.2023;[Epub] CrossRef - Platelet Glycoprotein-Ib (GPIb) May Serve as a Bridge between Type 2 Diabetes Mellitus (T2DM) and Atherosclerosis, Making It a Potential Target for Antiplatelet Agents in T2DM Patients
Muttia Amalia, Meidi Utami Puteri, Fadlina Chany Saputri, Rani Sauriasari, Bambang Widyantoro
Life.2023; 13(7): 1473. CrossRef - Polymorphisms of genes related to phase II metabolism and resistance to clopidogrel
Abdullah Alkattan, Ahmed Alkhalifah, Eman Alsalameen, Fatimah Alghanim, Nashwa Radwan
Pharmacogenomics.2022; 23(1): 61. CrossRef - Prolonged dual antiplatelet therapy after drug-eluting stent implantation in patients with diabetes mellitus: A nationwide retrospective cohort study
Seung-Jun Lee, Dong-Woo Choi, Choongki Kim, Yongsung Suh, Sung-Jin Hong, Chul-Min Ahn, Jung-Sun Kim, Byeong-Keuk Kim, Young-Guk Ko, Donghoon Choi, Eun-Cheol Park, Yangsoo Jang, Chung-Mo Nam, Myeong-Ki Hong
Frontiers in Cardiovascular Medicine.2022;[Epub] CrossRef - New opportunities of antithrombotic therapy in patients with type 2 diabetes mellitus and stable coronary heart disease for reducing the cardiovascular risk and cardiovascular complications: THEMIS, THEMIS-PCI trials
Victor I. Kalashnikov, Marina S. Michurova
Terapevticheskii arkhiv.2022; 94(10): 1204. CrossRef - Ischemic and Bleeding Events of Ticagrelor Monotherapy in Korean Patients With and Without Diabetes Mellitus: Insights From the TICO Trial
Kyeong Ho Yun, Jae Young Cho, Seung Yul Lee, Sang Jae Rhee, Byeong Keuk Kim, Myeong Ki Hong, Yangsoo Jang, Seok Kyu Oh
Frontiers in Pharmacology.2021;[Epub] CrossRef - Long-term outcomes of ischaemic stroke patients with diabetes in a multi-ethnic cohort in Singapore
Ei Zune The, Mei Yen Ng, Geelyn JL Ng, Bernadette GC Er, Amy ML Quek, Prakash Paliwal, Leonard L Yeo, Bernard PL Chan, Vijay K Sharma, Hock Luen Teoh, Eric YH Khoo, Raymond CS Seet
Annals of the Academy of Medicine, Singapore.2021; 50(1): 16. CrossRef - Protective Role of Platelets in Myocardial Infarction and Ischemia/Reperfusion Injury
Kornela Hałucha, Alina Rak-Pasikowska, Iwona Bil-Lula, David J. Chambers
Cardiology Research and Practice.2021; 2021: 1. CrossRef - Pre- and Post-Conditioning of the Heart: An Overview of Cardioprotective Signaling Pathways
Denise Coutinho de Miranda, Gabriela de Oliveira Faria, Milla Marques Hermidorff, Fernanda Cacilda dos Santos Silva, Leonardo Vinícius Monteiro de Assis, Mauro César Isoldi
Current Vascular Pharmacology.2021; 19(5): 499. CrossRef - Post-Transcriptional Expression Control in Platelet Biogenesis and Function
Carolin T. Neu, Tony Gutschner, Monika Haemmerle
International Journal of Molecular Sciences.2020; 21(20): 7614. CrossRef - The impact of metformin and aspirin on T-cell mediated inflammation: A systematic review of in vitro and in vivo findings
Tawanda Maurice Nyambuya, Phiwayinkosi Vusi Dludla, Vuyolwethu Mxinwa, Kabelo Mokgalaboni, Siphamandla Raphael Ngcobo, Luca Tiano, Bongani Brian Nkambule
Life Sciences.2020; 255: 117854. CrossRef - Short- versus long-term dual antiplatelet therapy after second-generation drug-eluting stent implantation in patients with diabetes mellitus: A meta-analysis of randomized controlled trials
Hongyu Zhang, Junsong Ke, Jun Huang, Kai Xu, Yun Chen, Timir Paul
PLOS ONE.2020; 15(12): e0242845. CrossRef - Long non-coding RNA metallothionein 1 pseudogene 3 promotes p2y12 expression by sponging miR-126 to activate platelet in diabetic animal model
Ming Zhou, Meng Gao, Yanwei Luo, Rong Gui, Hongwen Ji
Platelets.2019; 30(4): 452. CrossRef - Circulating blood cells and extracellular vesicles in acute cardioprotection
Sean M Davidson, Ioanna Andreadou, Lucio Barile, Yochai Birnbaum, Hector A Cabrera-Fuentes, Michael V Cohen, James M Downey, Henrique Girao, Pasquale Pagliaro, Claudia Penna, John Pernow, Klaus T Preissner, Péter Ferdinandy
Cardiovascular Research.2019; 115(7): 1156. CrossRef - Comparison of 1-year clinical outcomes between prasugrel and ticagrelor versus clopidogrel in type 2 diabetes patients with acute myocardial infarction underwent successful percutaneous coronary intervention
Kye Taek Ahn, Seok-Woo Seong, Ung Lim Choi, Seon-Ah Jin, Jun Hyung Kim, Jae-Hwan Lee, Si Wan Choi, Myung Ho Jeong, Shung Chull Chae, Young Jo Kim, Chong Jin Kim, Hyo-Soo Kim, Myeong-Chan Cho, Hyeon-Cheol Gwon, Jin-Ok Jeong, In-Whan Seong
Medicine.2019; 98(11): e14833. CrossRef - Fibrin Clot Strength in Patients with Diabetes Mellitus Measured by Thrombelastography
Benjamin T. Maatman, Glen Schmeisser, Rolf P. Kreutz
Journal of Diabetes Research.2018; 2018: 1. CrossRef - Role of proprotein convertase subtilisin/kexin type 9 inhibitors in patients with coronary artery disease undergoing percutaneous coronary intervention
Eliano P. Navarese, Michalina Kołodziejczak, Aniela Petrescu, Bernhard Wernly, Michael Lichtenauer, Alexander Lauten, Antonino Buffon, Wojciech Wanha, Vincenzo Pestrichella, Gennaro Sardella, Gaetano Contegiacomo, Udaya Tantry, Kevin Bliden, Jacek Kubica,
Expert Review of Cardiovascular Therapy.2018; 16(6): 419. CrossRef - Cardioprotective Properties of Human Platelets Are Lost in Uncontrolled Diabetes Mellitus: A Study in Isolated Rat Hearts
Isabella Russo, Saveria Femminò, Cristina Barale, Francesca Tullio, Stefano Geuna, Franco Cavalot, Pasquale Pagliaro, Claudia Penna
Frontiers in Physiology.2018;[Epub] CrossRef - Aortic thrombosis in dogs
Trevor P.E. Williams, Scott Shaw, Adam Porter, Larry Berkwitt
Journal of Veterinary Emergency and Critical Care.2017; 27(1): 9. CrossRef - Challenges in Patients with Diabetes: Improving Clinical Outcomes After Percutaneous Coronary Intervention Through EVOlving Stent Technology
Robert A Byrne, Shmuel Banai, Roisin Colleran, Antonio Colombo
Interventional Cardiology Review.2017; 13(01): 40. CrossRef - Adding CABG to the Dual Antiplatelet Salad
Glenn N. Levine, Faisal G. Bakaeen
Journal of the American College of Cardiology.2017; 69(2): 128. CrossRef - The association between arterial stiffness and tongue manifestations of blood stasis in patients with type 2 diabetes
Po-Chi Hsu, Yu-Chuen Huang, John Y. Chiang, Hen-Hong Chang, Pei-Yung Liao, Lun-Chien Lo
BMC Complementary and Alternative Medicine.2016;[Epub] CrossRef - Platelet phenotype changes associated with breast cancer and its treatment
Chris E. Holmes, Jamie E. Levis, David J. Schneider, Nadia M. Bambace, Deva Sharma, Inder Lal, Marie E. Wood, Hyman B. Muss
Platelets.2016; 27(7): 703. CrossRef
- Figure
- Related articles
-
- The Role of Echocardiography in Evaluating Cardiovascular Diseases in Patients with Diabetes Mellitus
- Intensified Multifactorial Intervention in Patients with Type 2 Diabetes Mellitus
- Not Control but Conquest: Strategies for the Remission of Type 2 Diabetes Mellitus
- Current Status of Low-Density Lipoprotein Cholesterol Target Achievement in Patients with Type 2 Diabetes Mellitus in Korea Compared with Recent Guidelines
- Do-It-Yourself Open Artificial Pancreas System in Children and Adolescents with Type 1 Diabetes Mellitus: Real-World Data

 KDA
KDA PubReader
PubReader Cite
Cite