
- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Articles
- Page Path
- HOME > Diabetes Metab J > Volume 39(3); 2015 > Article
-
Original ArticleComplications Serum Ceruloplasmin Level as a Predictor for the Progression of Diabetic Nephropathy in Korean Men with Type 2 Diabetes Mellitus
- Min Jung Lee, Chang Hee Jung, Yu Mi Kang, Jung Eun Jang, Jaechan Leem, Joong-Yeol Park, Woo Je Lee
-
Diabetes & Metabolism Journal 2015;39(3):230-239.
DOI: https://doi.org/10.4093/dmj.2015.39.3.230
Published online: April 22, 2015
Department of Internal Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- Corresponding author: Woo Je Lee. Department of Internal Medicine, Asan Medical Center, University of Ulsan College of Medicine, 88 Olympic-ro 43-gil, Songpa-gu, Seoul 138-736, Korea. lwjatlas@amc.seoul.kr
- *Min Jung Lee and Chang Hee Jung contributed equally to this study as first authors.
Copyright © 2015 Korean Diabetes Association
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
-
Background
- Oxidative stress is known to be associated with progression of diabetic kidney disease. Ceruloplasmin acts as a pro-oxidant under conditions of severe oxidative stress. Thus, we conducted a longitudinal observational study to evaluate whether the serum ceruloplasmin level is a predictive biomarker for progression of diabetic nephropathy.
-
Methods
- A total of 643 Korean men with type 2 diabetes mellitus were enrolled. Serum ceruloplasmin was measured using a nephelometric method. Progression of diabetic nephropathy was defined as transition in albuminuria class (i.e., normoalbuminuria to microalbuminuria, microalbuminuria to macroalbuminuria, or normoalbuminuria to macroalbuminuria) and/or a greater than 2-fold increase of serum creatinine at follow-up compared with the baseline value.
-
Results
- During the follow-up period (median, 2.7 years; range, 0.3 to 4.4 years), 49 of 643 patients (7.6%) showed the progression of diabetic nephropathy and three patients (0.5%) developed end-stage renal disease. Baseline ceruloplasmin levels were higher in the progressors than in the nonprogressors (262.6±40.9 mg/L vs. 233.3±37.8 mg/L, P<0.001). Kaplan-Meier analysis showed a significantly higher incidence of nephropathy progression according to ceruloplasmin tertile (log-rank test, P<0.001). The hazard ratio (HR) for progression of diabetic nephropathy was significantly higher in the highest ceruloplasmin tertile category compared with the lowest ceruloplasmin tertile category, even after adjusting for confounding variables (HR, 3.32; 95% confidence interval, 1.28 to 8.61; P=0.003).
-
Conclusion
- Baseline serum ceruloplasmin is an independent predictive factor for the progression of diabetic nephropathy in patients with type 2 diabetes mellitus.
- Diabetic kidney disease is the most common cause of chronic kidney disease, leading to end-stage renal disease (ESRD) and premature death [1]. In addition, it negatively affects a patient's quality of life and social environment, and poses a burden on national health care budgets [2]. Although various therapeutic approaches, such as hypoglycemic agents, antihypertensive drugs, and renin-angiotensin system inhibitors, have been tried to slow the progression of nephropathy, the number of patients with diabetic kidney disease continues to rise with the prevalence of type 2 diabetes mellitus [3]. Thus, early identification of patients at risk of developing diabetic nephropathy and initiation of appropriate therapy is important to improve patient outcomes.
- Ceruloplasmin, a copper-carrying metalloenzyme, acts as an antioxidant through its ferroxidase activity [4]. However, in conditions of elevated oxidative stress, it may act as a pro-oxidant by donating of free copper ions, which induces reactive oxygen species (ROS) formation and low density lipoprotein (LDL) oxidation [5]. In addition, as an acute phase protein, the ceruloplasmin level reflects acute and chronic inflammation in an organism [5]. Elevated serum ceruloplasmin levels have been observed in both type 1 and type 2 diabetes [67] and in patients with diabetic retinopathy [8]. In line with that, in our previous cross-sectional study, we observed a positive association between serum ceruloplasmin level and albuminuria in patients with type 2 diabetes mellitus [9]. However, longitudinal studies investigating the association between serum ceruloplasmin and development of diabetic nephropathy had not been conducted. Thus, to evaluate the role of serum ceruloplasmin as a biomarker for predicting progression of diabetic nephropathy, we conducted this longitudinal observational study.
INTRODUCTION
- Study population
- Study subjects were recruited from an outpatient clinic at a major referral center, Asan Medical Center (Seoul, Korea). A total of 703 patients whose serum ceruloplasmin levels were measured were followed up from January 2009 through December 2013. Ceruloplasmin were measured in patients with type 2 diabetes mellitus who visited our diabetes clinic for the first time after registration at the Asan Diabetes Registry as well as in patients with type 2 diabetes mellitus who underwent screening tests for diabetic microvascular complications regularly every 1 to 2 years. There was no doctor's intention in the recruitment process.
- All patients visited the diabetes clinic on a regular basis (every 3 or 6 months). Subjects with histories of chronic inflammatory processes (n=1, ulcerative colitis; n=2, rheumatoid arthritis) or liver cirrhosis (n=6; due to hepatitis B infection in four cases and alcohol use in two cases), and those with leukocytosis or leukopenia (blood leukocyte count >10.0×103/mm3 or <4.0×103/mm3, n=51) at baseline were excluded. After the exclusion of ineligible subjects, 643 subjects with a mean age of 57.0 years (range, 24 to 86 years) were included in the analysis. The Institutional Review Board of Asan Medical Center approved this retrospective observational study, and waived for requirement of written informed consent because personal information was not included in this analysis and risk of this study was considered to be negligible.
- Lifestyle factors and measurements
- All participants were interviewed by a specially trained nurse, and information on medication and history of previous medical or surgical diseases was obtained. Smoking habits were categorized as "never," "previous," or "current." A history of cardiovascular disease (CVD) was defined as a history of angina, myocardial infarction, and/or cerebrovascular incidents. Antidiabetic treatments were categorized as none, oral hypoglycemic agents (OHAs), insulin, or insulin combined with OHAs. The antihypertensive medications that subjects were taking were categorized as angiotensin-converting enzyme inhibitors (ACEi), angiotensin II receptor blockers (ARBs), or others (β-blockers, calcium-channel blockers, diuretics, or α-blockers). Height (m) and weight (kg) were measured while subjects were wearing light clothing without shoes. Body mass index (BMI, kg/m2) was calculated as weight in kilograms divided by the square of height in meters. Blood pressure was measured using an automatic manometer with an appropriate cuff size on the right arm after a resting period of ≥5 minutes at intervals of 6 months, and mean systolic blood pressure (SBP) and diastolic blood pressure (DBP) values for the observation period were used as follow-up data. The presence of retinopathy was assessed by retinal photography (two fields per eye) with a wide-angle camera and scored centrally by an ophthalmologist.
- After overnight fasting, early morning blood samples were drawn from the antecubital vein. Fasting total cholesterol, high density lipoprotein cholesterol, LDL cholesterol (LDL-C), triglyceride (TG), and uric acid levels were measured using an enzymatic colorimetric method (Toshiba Medical System Co., Ltd., Tokyo, Japan). High sensitivity C-reactive protein (hsCRP) and fasting plasma glucose (FPG) concentrations were measured using immunoturbidimetric (Toshiba) and enzymatic colorimetric (Toshiba) methods, respectively. Glycosylated hemoglobin (HbA1c) was measured using an immunoturbidimetric method and an Integra 800 System (Roche Diagnostics, Basel, Switzerland) every 6 months, and calculated mean HbA1c values for the observation period were used as follow-up data.
- The extent of albuminuria was determined from the urinary albumin-to-creatinine ratio (UACR), which was measured by a photometric method using the Integra 800 system (Roche Diagnostics) in a random spot urine collection. The category of albuminuria was defined as normoalbuminuria if the UACR was <30 mg/g, microalbuminuria if the UACR was 30 to 299 mg/g, and macroalbuminuria if the UACR was ≥300 mg/g [10]. Creatinine was measured using the Jaffe method, and estimated glomerular filtration rate (eGFR) was calculated using the Modification of Diet in Renal Disease study equation [11]. Serum ceruloplasmin level was measured using a nephelometric method (BN II System; Siemens, Marburg, Germany).
- Definition of diabetic nephropathy progression
- The outcome in terms of "progression of diabetic nephropathy" was defined as transition in albuminuria class (i.e., normo- to microalbuminuria, micro- to macroalbuminuria, or normo- to macroalbuminuria) and/or a greater than 2-fold increase in serum creatinine at follow-up compared with the baseline value. Transition in albuminuria class and doubling of creatinine value were confirmed by at least two consecutive tests to reduce misclassification.
- Statistical analyses
- Subjects were categorized into three tertile groups (i.e., Q1 ≤219 mg/L, Q2=220 to 249 mg/L, and Q3 ≥250 mg/L) according to the baseline ceruloplasmin concentration. Continuous variables with a normal distribution were expressed as the mean±standard deviation, and continuous variables with a skewed distribution were expressed as the median (interquartile range). Categorical variables were expressed as percentages (%). Characteristics of the study population according to ceruloplasmin tertile categories were compared using one-way analysis of variance or the Kruskal-Wallis test for continuous variables, and the chi-square test for categorical variables.
- Demographic and biochemical characteristics of the study population with respect to the progression of diabetic nephropathy were compared using an independent t-test or the Mann-Whitney U test for continuous variables, and the chi-square test for categorical variables. Time to progression of diabetic nephropathy was estimated by the Kaplan-Meier method and statistical differences among groups were compared by the log-rank test. The follow-up time was censored if diabetic nephropathy progressed or if the patient was lost to follow-up. To calculate the hazard ratios (HRs) and 95% confidence intervals (CIs) of each ceruloplasmin tertile category for the development of diabetic nephropathy, multivariate Cox proportional hazard models were applied after adjustment for conventional risk factors of diabetic nephropathy. Traditional diabetic nephropathy risk factors were included in model 1 and factors that showed a statistically significant (P<0.05) or borderline significant (P<0.10) associations in the univariate analysis were included in model 2. All the variables in models 1 and 2 were included in model 3.
- All statistical analyses were carried out using SPSS version 19.0 (SPSS Inc., Chicago, IL, USA). A P value of <0.05 was considered statistically significant.
METHODS
- Table 1 shows the clinical and biochemical characteristic of the subjects according to serum ceruloplasmin tertile category at baseline. There were significant positive associations between ceruloplasmin tertile category and SBP, current smoking status, HbA1c, total cholesterol, LDL-C, hsCRP, UACR, and percentage of subjects using insulin therapy at baseline. There was a borderline significant positive association between ceruloplasmin tertile and the prevalence of retinopathy at baseline. Among follow-up data, UACR measured at the time of last follow-up or at event occurrence increased according to ceruloplasmin tertile.
- During the follow-up period (median, 2.7 years; range, 0.3 to 4.4 years), 49 of 643 patients (7.6%) showed the progression of diabetic nephropathy and three patients (0.5%) developed ESRD. The clinical and biochemical characteristics at baseline and during follow-up for progressors and non-progressors are shown in Table 2. Duration of diabetes was significantly longer in progressors compared with nonprogressors. Progressors tended to use of insulin therapy and ACEi or ARB at baseline more frequently than nonprogressors. The percentage of subjects taking other antihypertensive medication tended to be higher in progressors, with borderline statistical significance. Progressors had significantly higher levels of uric acid, hsCRP, UACR, and creatinine at baseline, but a lower eGFR compared with non-progressors. They had higher values of total cholesterol and TG at baseline, although the associations were borderline significant. Baseline ceruloplasmin levels were significantly higher in the progressors than in the non-progressors (262.6±40.9 mg/L vs. 233.3±37.8 mg/L, P<0.001). During the follow-up period, the two groups did not differ in terms of mean SBP and DBP, but mean HbA1c was significantly higher in progressors than nonprogressors. At the time of event occurrence or the last observation, the progressors had higher UACR and creatinine, but lower eGFR compared with nonprogressors (all P values <0.001).
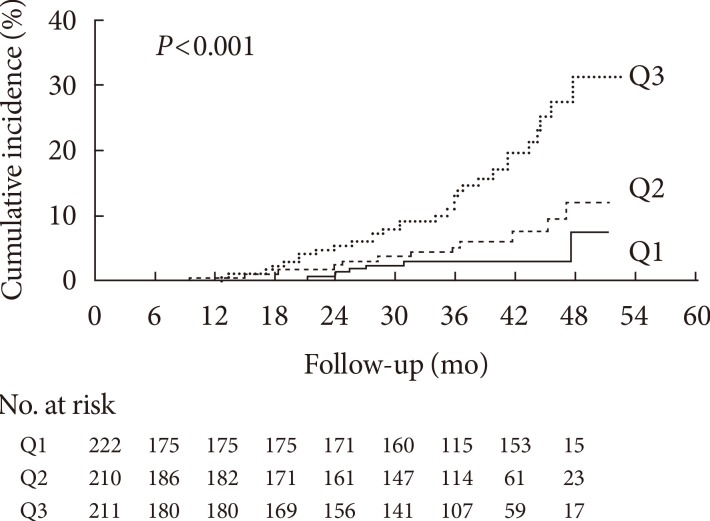
- Kaplan-Meier analysis demonstrated a significantly higher incidence of progression of diabetic nephropathy according to baseline ceruloplasmin tertile (log-rank test, P<0.001) (Fig. 1). Above all, subjects in Q3 showed a significantly increasing trend of diabetic nephropathy progression compared with Q1 and Q2 (Q1 vs. Q3, P<0.001; Q2 vs. Q3, P=0.002). No difference was found between Q1 and Q2 (P=0.167).
- When we performed multivariate Cox regression analysis adopting various models, the HRs for progression of diabetic nephropathy according to baseline ceruloplasmin tertile category consistently showed an increasing trend (Table 3). In the unadjusted model, the HRs and 95% CIs based on ceruloplasmin tertile groups were 1.95 (0.73 to 5.19) for Q2, and 5.41 (2.30 to 12.96) for Q3, respectively (P<0.001), with Q1 as reference. After adjusting for confounding variables including age, BMI, duration of diabetes, current smoker, past history of CVD, antidiabetic medication, ACEi or ARB use, other HTN medication, retinopathy, FPG, total cholesterol, uric acid, hsCRP, baseline UACR, baseline creatinine, follow-up mean HbA1c, follow-up mean SBP and follow-up mean DBP, the HRs (95% CI) for progression of diabetic nephropathy were 1.14 (0.38 to 3.40) for Q2 and 3.14 (1.21 to 8.10) for Q3, respectively (P=0.010). Besides baseline ceruloplasmin, baseline creatinine, baseline UACR, follow-up mean HbA1c, and uric acid were predictive factors for the development of diabetic nephropathy in model 3 (Table 4).
RESULTS
- Diabetic nephropathy is a leading cause of ESRD and greatly contributes to all-cause mortality in type 2 diabetes [1]. To slow the progression of diabetic nephropathy, early detection and treatment of those patients at risk of developing nephropathy is regarded as an important goal [12]. Recently, several novel biomarkers have been investigated to predict nephropathy in patients with type 2 diabetes. However there were few longitudinal studies with suitable methodological quality that properly adjusted for traditional conventional risk factors for diabetic nephropathy [12].
- In this longitudinal observational study, elevated ceruloplasmin levels at baseline were found to be positively associated with progression of diabetic nephropathy. After adjusting for conventional risk factors, ceruloplasmin levels still remained an independent risk factor for the progression of diabetic nephropathy. This result indicates that an elevated ceruloplasmin level might be a strong predictor of diabetic nephropathy progression.
- The exact mechanism underlying this positive association between ceruloplasmin levels and progression of diabetic nephropathy is largely unknown. One plausible explanation is that ceruloplasmin might act as a pro-oxidant under conditions of increased oxidative stress, such as in type 2 diabetes mellitus [8]. Although ceruloplasmin possesses antioxidant properties due to its ferroxidase activity [4], increased generation of ROS disrupts the binding of copper from ceruloplasmin which further induces ROS formation and LDL oxidation [5]. Considering that increased oxidative stress and oxidized LDL are known to be associated with progression of diabetic kidney disease [13], elevated ceruloplasmin level might reflect or augment progression of diabetic nephropathy.
- In addition, as an acute phase protein, ceruloplasmin might reflect subclinical inflammation, which plays a critical role in the pathogenesis of diabetic nephropathy [14]. Navarro et al. [15] reported that inflammatory parameters including hsCRP are independently associated with urinary albumin excretion. However, when we performed multivariate Cox regression analysis, serum hsCRP levels were not associated with the progression of diabetic nephropathy (HR, 0.98; 95% CI, 0.90 to 1.06; P=0.590) (Table 4), which contrasts with the results of a previous study [15]. Although the reasons for these discrepancies are unclear, we suspect that, compared with hsCRP, elevated ceruloplasmin might reflect chronic subclinical inflammation more sensitively or play a more important role in the progression of diabetic nephropathy.
- In multivariate Cox regression analysis, baseline creatinine, UACR, and follow-up mean HbA1c were found to be predictive factors for the progression of diabetic nephropathy, which is in line with previous studies [161718]. However, multivariate Cox regression analysis did not indicate that SBP was an independent predictor of progressive diabetic nephropathy, which was inconsistent with previous studies [19]. Since progressors tend to take ACEi or ARB and other hypertension medication at baseline more often than non-progressors, the difference in hypertensive medications between groups during the follow-up period might affect this outcome.
- Furthermore, in our study, an increased baseline uric acid level was predictive for the progression of diabetic nephropathy, which is in accordance with previous studies [1820]. Several studies showed that high uric acid level as well as ceruloplasmin was associated with increased oxidative stress [521], but further study is need to clarify the connections between those biomarkers and mechanisms of how they contributes to diabetic nephropathy.
- Although not statistically significant, we observed a tendency for eGFR to be increased in patients with the highest ceruloplasmin tertile category (Table 1). Since glomerular hyperfiltration usually occurs in early stages of diabetic nephropathy [22], it can be speculated that an elevated eGFR in the highest tertile category might reflect the early stages of renal dysfunction. Long-term extension studies will be required to evaluate the association between serum ceruloplasmin and changes in eGFR.
- This study has several limitations. First, we investigated the relationship between ceruloplasmin and progression of diabetic nephropathy only in men. Since medications such as oral contraceptives can affect ceruloplasmin levels [23], women were not included in this study and the result cannot be generalized to both sexes. Second, although we postulated that dissociated free copper, ROS, and oxidized LDL might play a role in ceruloplasmin-mediated diabetic nephropathy, we did not measure these values. Considering the previous report that ceruloplasmin was positively correlated with makers of ROS under the oxidative stress conditions [24], it might be better to measure oxidative stress makers as well as the level of ceruloplasmin in our study. Third, in this study, ceruloplasmin was measured by nephelometry. It has been suggested that the ratio of enzymatic to immunoreactive ceruloplasmin is a better indicator of copper status than either enzyme activity or immunoreactive ceruloplasmin level alone [25]. Thus, it might be better to assess ceruloplasmin using both methods. Forth, since increased urinary excretions of ceruloplasmin also predicted development of microalbuminuria in type 2 diabetic patients [26], concurrent measure of serum and urinary ceruloplasmin might be beneficial to evaluate the association between these markers, but we did not measure the level of urinary ceruloplasmin. In addition, we did not measure follow-up ceruloplasmin values and could not evaluate whether participants remained in initially allocated tertile category at the end of study. Lastly, to evaluate the long-term prognosis, it might be better to evaluate the occurrence of ESRD or death rather than transition of albuminuria class as the primary outcome. Although three patients developed ESRD (one event in group Q1, two events in group Q3) in this study, long-term follow-up of large numbers of subjects will be required to evaluate the ultimate outcome.
- Despite these limitations, our study has some robust features, in that we properly adjusted for various conventional risk factors that might affect the progression of diabetic nephropathy. It should be noted that the predictive value of ceruloplasmin in our Cox model was found to be equal to or even stronger than that of well-known risk factors for the progression of diabetic nephropathy, including hyperglycemia, baseline creatinine, UACR, and uric acid. Furthermore, we observed that the number of subjects who exceeded a 2-fold increase of creatinine at follow-up tended to rise with ceruloplasmin tertile group (one event in group Q1, three events in group Q2, and six events in group Q3). Although this subgroup analysis was not statistically significant, due to the small number of events, this result prompts further investigation of the possibility that an increased ceruloplasmin level predicts renal dysfunction as well as transition in albuminuria class.
- Albuminuria has been traditionally considered as the hallmark for diabetic nephropathy [27], but recent studies showed that one third to one half of type 2 diabetes with chronic kidney disease have no proteinuria [2829]. Although it is not widely available in clinical practice, measurements of serum ceruloplasmin might be used to compensate this limitation of 'albuminuria' as a predictive marker for diabetic nephropathy. To evaluate the predictive role of ceruloplasmin on progression of 'nonalbuminuric diabetic nephropathy,' further study will be required.
- In conclusion, these data suggest that an elevated serum ceruloplasmin is a strong prognostic marker for progression of diabetic nephropathy in patients with type 2 diabetes mellitus. This effect remains significant even after adjustment for conventional risk factors for diabetic nephropathy. Therefore, measuring ceruloplasmin levels in addition to UACR might be used for early identification of patients at risk of diabetic nephropathy in order to initiate appropriate therapy.
DISCUSSION
-
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.
NOTES
- 1. Afkarian M, Sachs MC, Kestenbaum B, Hirsch IB, Tuttle KR, Himmelfarb J, de Boer IH. Kidney disease and increased mortality risk in type 2 diabetes. J Am Soc Nephrol 2013;24:302-308. ArticlePubMedPMC
- 2. Gordois A, Scuffham P, Shearer A, Oglesby A. The health care costs of diabetic nephropathy in the United States and the United Kingdom. J Diabetes Complications 2004;18:18-26. ArticlePubMed
- 3. de Boer IH, Rue TC, Hall YN, Heagerty PJ, Weiss NS, Himmelfarb J. Temporal trends in the prevalence of diabetic kidney disease in the United States. JAMA 2011;305:2532-2539. ArticlePubMedPMC
- 4. Harris ZL. Aceruloplasminemia. J Neurol Sci 2003;207:108-109. ArticlePubMed
- 5. Shukla N, Maher J, Masters J, Angelini GD, Jeremy JY. Does oxidative stress change ceruloplasmin from a protective to a vasculopathic factor? Atherosclerosis 2006;187:238-250. ArticlePubMed
- 6. Cunningham J, Leffell M, Mearkle P, Harmatz P. Elevated plasma ceruloplasmin in insulin-dependent diabetes mellitus: evidence for increased oxidative stress as a variable complication. Metabolism 1995;44:996-999. ArticlePubMed
- 7. Daimon M, Susa S, Yamatani K, Manaka H, Hama K, Kimura M, Ohnuma H, Kato T. Hyperglycemia is a factor for an increase in serum ceruloplasmin in type 2 diabetes. Diabetes Care 1998;21:1525-1528. ArticlePubMedPDF
- 8. Memisogullari R, Bakan E. Levels of ceruloplasmin, transferrin, and lipid peroxidation in the serum of patients with type 2 diabetes mellitus. J Diabetes Complications 2004;18:193-197. ArticlePubMed
- 9. Jung CH, Lee WJ, Yu JH, Hwang JY, Shin MS, Koh EH, Kim MS, Park JY. Elevated serum ceruloplasmin levels are associated with albuminuria in Korean men with type 2 diabetes mellitus. Diabetes Res Clin Pract 2011;94:e3-e7. ArticlePubMed
- 10. Molitch ME, DeFronzo RA, Franz MJ, Keane WF, Mogensen CE, Parving HH, Steffes MW. American Diabetes Association. Nephropathy in diabetes. Diabetes Care 2004;27(Suppl 1):S79-S83. ArticlePubMedPDF
- 11. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. Modification of Diet in Renal Disease Study Group. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med 1999;130:461-470. ArticlePubMed
- 12. Hellemons ME, Kerschbaum J, Bakker SJ, Neuwirt H, Mayer B, Mayer G, de Zeeuw D, Lambers Heerspink HJ, Rudnicki M. Validity of biomarkers predicting onset or progression of nephropathy in patients with type 2 diabetes: a systematic review. Diabet Med 2012;29:567-577. ArticlePubMed
- 13. Ujihara N, Sakka Y, Takeda M, Hirayama M, Ishii A, Tomonaga O, Babazono T, Takahashi C, Yamashita K, Iwamoto Y. Association between plasma oxidized low-density lipoprotein and diabetic nephropathy. Diabetes Res Clin Pract 2002;58:109-114. ArticlePubMed
- 14. Wada J, Makino H. Inflammation and the pathogenesis of diabetic nephropathy. Clin Sci (Lond) 2013;124:139-152. ArticlePubMedPDF
- 15. Navarro JF, Mora C, Maca M, Garca J. Inflammatory parameters are independently associated with urinary albumin in type 2 diabetes mellitus. Am J Kidney Dis 2003;42:53-61. ArticlePubMed
- 16. UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352:837-853. ArticlePubMed
- 17. de Zeeuw D, Remuzzi G, Parving HH, Keane WF, Zhang Z, Shahinfar S, Snapinn S, Cooper ME, Mitch WE, Brenner BM. Proteinuria, a target for renoprotection in patients with type 2 diabetic nephropathy: lessons from RENAAL. Kidney Int 2004;65:2309-2320. ArticlePubMed
- 18. Hsu CY, Iribarren C, McCulloch CE, Darbinian J, Go AS. Risk factors for end-stage renal disease: 25-year follow-up. Arch Intern Med 2009;169:342-350. ArticlePubMedPMC
- 19. Bakris GL, Weir MR, Shanifar S, Zhang Z, Douglas J, van Dijk DJ, Brenner BM. RENAAL Study Group. Effects of blood pressure level on progression of diabetic nephropathy: results from the RENAAL study. Arch Intern Med 2003;163:1555-1565. ArticlePubMed
- 20. Jalal DI, Maahs DM, Hovind P, Nakagawa T. Uric acid as a mediator of diabetic nephropathy. Semin Nephrol 2011;31:459-465. ArticlePubMedPMC
- 21. Zhang Y, Yamamoto T, Hisatome I, Li Y, Cheng W, Sun N, Cai B, Huang T, Zhu Y, Li Z, Jing X, Zhou R, Cheng J. Uric acid induces oxidative stress and growth inhibition by activating adenosine monophosphate-activated protein kinase and extracellular signal-regulated kinase signal pathways in pancreatic beta cells. Mol Cell Endocrinol 2013;375:89-96. PubMed
- 22. Brenner BM, Lawler EV, Mackenzie HS. The hyperfiltration theory: a paradigm shift in nephrology. Kidney Int 1996;49:1774-1777. ArticlePubMed
- 23. Sontakke AN, More U. Changes in serum ceruloplasmin levels with commonly used methods of contraception. Indian J Clin Biochem 2004;19:102-104. ArticlePubMedPMCPDF
- 24. Kedziora-Kornatowska K, Kornatowski T, Bartosz G, Pawluk H, Czuczejko J, Kedziora J, Szadujkis-Szadurski L. Production of nitric oxide, lipid peroxidation and oxidase activity of ceruloplasmin in blood of elderly patients with primary hypertension. Effects of perindopril treatment. Aging Clin Exp Res 2006;18:1-6. ArticlePubMedPDF
- 25. Milne DB, Johnson PE. Assessment of copper status: effect of age and gender on reference ranges in healthy adults. Clin Chem 1993;39:883-887. ArticlePubMedPDF
- 26. Narita T, Sasaki H, Hosoba M, Miura T, Yoshioka N, Morii T, Shimotomai T, Koshimura J, Fujita H, Kakei M, Ito S. Parallel increase in urinary excretion rates of immunoglobulin G, ceruloplasmin, transferrin, and orosomucoid in normoalbuminuric type 2 diabetic patients. Diabetes Care 2004;27:1176-1181. ArticlePubMedPDF
- 27. Williams ME. Diabetic nephropathy: the proteinuria hypothesis. Am J Nephrol 2005;25:77-94. ArticlePubMedPDF
- 28. Kramer HJ, Nguyen QD, Curhan G, Hsu CY. Renal insufficiency in the absence of albuminuria and retinopathy among adults with type 2 diabetes mellitus. JAMA 2003;289:3273-3277. ArticlePubMed
- 29. Retnakaran R, Cull CA, Thorne KI, Adler AI, Holman RR. UKPDS Study Group. Risk factors for renal dysfunction in type 2 diabetes: UK Prospective Diabetes Study 74. Diabetes 2006;55:1832-1839. PubMed
REFERENCES
Kaplan-Meier curves for progression of diabetic nephropathy according to serum ceruloplasmin tertile categories.
Clinical and biochemical characteristics of the study subjects according to serum ceruloplasmin tertile categories at baseline
Values are presented as mean±standard deviation, median (range) unless otherwise indicated.
BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; CVD, cardiovascular disease; OHA, oral hypoglycemic agent; ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; HTN, hypertension; FPG, fasting plasma glucose; HbA1c, glycosylated hemoglobin; LDL-C, low density lipoprotein cholesterol; HDL-C, high density lipoprotein cholesterol; hsCRP, high sensitivity C-reactive protein; UACR, urinary albumin-to-creatinine ratio; eGFR, estimated glomerular filtration rate.
aLast UACR represent UACR in spot urine sample at last follow-up or at the time of event occurrence.
Baseline and follow-up clinical and biochemical characteristics of progressors and nonprogressors of diabetic nephropathy
Values are presented as mean±standard deviation, median (range) unless otherwise indicated.
BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; CVD, cardiovascular disease; OHA, oral hypoglycemic agent; ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; HTN, hypertension; FPG, fasting plasma glucose; HbA1c, glycosylated hemoglobin; LDL-C, low density lipoprotein cholesterol; HDL-C, high density lipoprotein cholesterol; hsCRP, high sensitivity C-reactive protein; UACR, urinary albumin-to-creatinine ratio; eGFR, estimated glomerular filtration rate.
aLast UACR represent UACR in spot urine sample at last follow-up or at the time of event occurrence.
HRs and 95% CI for progression of diabetic nephropathy based on serum ceruloplasmin tertile categories
Model 1: adjusted for conventional diabetic nephropathy risk factors (age, body mass index, duration of diabetes, current smoker, angiotensin-converting enzyme inhibitor (ACEi) or angiotensin II receptor blocker (ARB) use, other hypertension medication, high sensitivity C-reactive protein, baseline urinary albumin-to-creatinine ratio (UACR), baseline creatinine, follow-up mean glycosylated hemoglobin (HbA1c), follow-up mean systolic blood pressure (SBP), follow-up mean diastolic blood pressure). Model 2: adjusted for factors that showed a statistically significant (P<0.05, i.e., antidiabetic medication, ACEi or ARB use, fasting plasma glucose, total cholesterol, uric acid, baseline UACR, baseline creatinine, follow-up mean HbA1c) or borderline significant (P<0.10, i.e., duration of diabetes, baseline SBP, past history of cardiovascular disease, retinopathy) association in the univariate analysis. Model 3: adjusted for all the variables in models 1 and 2.
HR, hazard ratio; CI, confidence interval.
Other risk factors for progression of diabetic nephropathy demonstrated by multivariate Cox regression analysis
HR, hazard ratio; CI, confidence interval; UACR, urinary albumin-to-creatinine ratio; SBP, systolic blood pressure; DBP, diastolic blood pressure; ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; HTN, hypertension; HbA1c, glycosylated hemoglobin; LDL-C, low density lipoprotein cholesterol; HDL-C, high density lipoprotein cholesterol; hsCRP, high sensitivity C-reactive protein.
Figure & Data
References
Citations

- In-depth urinary and exosome proteome profiling analysis identifies novel biomarkers for diabetic kidney disease
Shichun Du, Linhui Zhai, Shu Ye, Le Wang, Muyin Liu, Minjia Tan
Science China Life Sciences.2023; 66(11): 2587. CrossRef - Serum Level of Ceruloplasmin, Angiotensin-Converting Enzyme and Transferrin as Markers of Severity in SARS-CoV-2 Infection in Patients with Type 2 Diabetes
Patricia-Andrada Reștea, Ștefan Țigan, Laura Grațiela Vicaș, Luminița Fritea, Eleonora Marian, Tunde Jurca, Annamaria Pallag, Iulius Liviu Mureșan, Corina Moisa, Otilia Micle, Mariana Eugenia Mureșan
Microbiology Research.2023; 14(4): 1670. CrossRef - The nephropathy of sickle cell trait and sickle cell disease
Kenneth I. Ataga, Santosh L. Saraf, Vimal K. Derebail
Nature Reviews Nephrology.2022; 18(6): 361. CrossRef - Integrated Analysis of Single-Cell RNA-seq and Bulk RNA-seq in the Identification of a Novel ceRNA Network and Key Biomarkers in Diabetic Kidney Disease
Yuejun Wang, Mingming Zhao, Yu Zhang
International Journal of General Medicine.2022; Volume 15: 1985. CrossRef - Molecular Functions of Ceruloplasmin in Metabolic Disease Pathology
Zhidong Liu, Miao Wang, Chunbo Zhang, Shigao Zhou, Guang Ji
Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy.2022; Volume 15: 695. CrossRef - A correlative study of copper, ceruloplasmin, iron, total iron binding capacity and total antioxidant capacity in diabetic nephropathy
Ramlingareddy, Shivashankara A Ramachandrayya, Jeena Jacob, Malathi Mala
Biomedicine.2022; 42(3): 469. CrossRef - Novel biomarkers for prognosticating diabetic kidney disease progression
Shilna Muttickal Swaminathan, Indu Ramachandra Rao, Srinivas Vinayak Shenoy, Attur Ravindra Prabhu, Pooja Basthi Mohan, Dharshan Rangaswamy, Mohan V Bhojaraja, Shivashankara Kaniyoor Nagri, Shankar Prasad Nagaraju
International Urology and Nephrology.2022; 55(4): 913. CrossRef - Evaluation of Serum Ceruloplasmin Levels as a Biomarker for Oxidative Stress in Patients With Diabetic Retinopathy
Gurunadh Satyanarayana, Narendra Keisham, Hitender S Batra, Subrahmanya Murti V, Mansur Khan, Sandeep Gupta, Vikram Mahindra
Cureus.2021;[Epub] CrossRef - Prospection of plasma proteins as biomarkers for diabetes mellitus monitoring
Liliane de Paula Silva, Fabiane Gomes de Moraes Rego, Geraldo Picheth, Marcelo Müller-Santos, Dayane Alberton
Journal of Diabetes & Metabolic Disorders.2021; 20(1): 611. CrossRef - Risk assessment for foot ulcers among Tunisian subjects with diabetes: a cross sectional outpatient study
B. Zantour, S. Bouchareb, Z. El Ati, F. Boubaker, W. Alaya, W. Kossomtini, M. H. Sfar
BMC Endocrine Disorders.2020;[Epub] CrossRef - Metalloproteins and apolipoprotein C: candidate plasma biomarkers of T2DM screened by comparative proteomics and lipidomics in ZDF rats
Shuai Wang, Zhiyuan Lu, Yuxin Wang, Tianran Zhang, Xiaodong He
Nutrition & Metabolism.2020;[Epub] CrossRef - Emerging vistas on electrochemical detection of diabetic retinopathy biomarkers
K.S. Shalini Devi, Madhurantakam Sasya, Uma Maheswari Krishnan
TrAC Trends in Analytical Chemistry.2020; 125: 115838. CrossRef - Oxidative stress in peritoneal dialysis patients: Association with the dialysis adequacy and technique survival
Natalia Stepanova, Lesya Korol, Olena Burdeyna
Indian Journal of Nephrology.2019; 29(5): 309. CrossRef - Recent advancements in biopolymer and metal nanoparticle-based materials in diabetic wound healing management
Veena Vijayakumar, Sushanta K. Samal, Smita Mohanty, Sanjay K. Nayak
International Journal of Biological Macromolecules.2019; 122: 137. CrossRef - Rat Böbrek Dokusunda Kurşunun Neden Olduğu Oksidatif Strese Karşı Kitosanın Koruyucu etkisi
Ugur ÖZDEK, Hasan TOZ, Ahmet Ufuk KÖMÜROĞLU, Leyla MİS, Zübeyir HUYUT, Yeter DEĞER
Van Veterinary Journal.2019; 30(3): 187. CrossRef - Changes in Trace Elements During Early Stages of Chronic Kidney Disease in Type 2 Diabetic Patients
Ching-Chiang Lin, Ching-Tang Shih, Chien-Hung Lee, Yeou-Lih Huang
Biological Trace Element Research.2018; 186(2): 330. CrossRef - The Divalent Elements Changes in Early Stages of Chronic Kidney Disease
Wan-Ju Kung, Ching-Tang Shih, Chien-Hung Lee, Ching-Chiang Lin
Biological Trace Element Research.2018; 185(1): 30. CrossRef - Long-term expression of glomerular genes in diabetic nephropathy
Dominik Chittka, Bernhard Banas, Laura Lennartz, Franz Josef Putz, Kathrin Eidenschink, Sebastian Beck, Thomas Stempfl, Christoph Moehle, Simone Reichelt-Wurm, Miriam C Banas
Nephrology Dialysis Transplantation.2018;[Epub] CrossRef - Incidence of chronic kidney disease among people with diabetes: a systematic review of observational studies
D. N. Koye, J. E. Shaw, C. M. Reid, R. C. Atkins, A. T. Reutens, D. J. Magliano
Diabetic Medicine.2017; 34(7): 887. CrossRef - Global epidemiology of diabetic foot ulceration: a systematic review and meta-analysis
Pengzi Zhang, Jing Lu, Yali Jing, Sunyinyan Tang, Dalong Zhu, Yan Bi
Annals of Medicine.2017; 49(2): 106. CrossRef - Biomarkers of diabetic nephropathy: A 2017 update
Nektaria Papadopoulou-Marketou, Christina Kanaka-Gantenbein, Nikolaos Marketos, George P. Chrousos, Ioannis Papassotiriou
Critical Reviews in Clinical Laboratory Sciences.2017; 54(5): 326. CrossRef - Serum Vascular Adhesion Protein-1 Predicts End-Stage Renal Disease in Patients with Type 2 Diabetes
Hung-Yuan Li, Hung-An Lin, Feng-Jung Nien, Vin-Cent Wu, Yi-Der Jiang, Tien-Jyun Chang, Hsien-Li Kao, Mao-Shin Lin, Jung-Nan Wei, Cheng-Hsin Lin, Shyang-Rong Shih, Chi-Sheng Hung, Lee-Ming Chuang, Emmanuel A Burdmann
PLOS ONE.2016; 11(2): e0147981. CrossRef
- Figure
- Related articles
-
- Risk Prediction and Management of Chronic Kidney Disease in People Living with Type 2 Diabetes Mellitus
- Implication of Sex Differences in Visceral Fat for the Assessment of Incidence Risk of Type 2 Diabetes Mellitus
- Comparison of Serum Ketone Levels and Cardiometabolic Efficacy of Dapagliflozin versus Sitagliptin among Insulin-Treated Chinese Patients with Type 2 Diabetes Mellitus

 KDA
KDA PubReader
PubReader Cite
Cite




