Gut Microbiota and Metabolic Disorders
Article information
Abstract
Gut microbiota plays critical physiological roles in the energy extraction and in the control of local or systemic immunity. Gut microbiota and its disturbance also appear to be involved in the pathogenesis of diverse diseases including metabolic disorders, gastrointestinal diseases, cancer, etc. In the metabolic point of view, gut microbiota can modulate lipid accumulation, lipopolysaccharide content and the production of short-chain fatty acids that affect food intake, inflammatory tone, or insulin signaling. Several strategies have been developed to change gut microbiota such as prebiotics, probiotics, certain antidiabetic drugs or fecal microbiota transplantation, which have diverse effects on body metabolism and on the development of metabolic disorders.
The Sulwon Award for Scientific Achievement is the Korean Diabetes Association's highest scientific award and honors an individual who has excellently contributed to the progress in the field of diabetes and metabolism. Sulwon Award is named after an emeritus professor Eung Jin Kim, who founded Korean Diabetes Association. Prof. Myung-Shik Lee received the fifth Sulwon Award at 2014 International Conference on Diabetes and Metabolism, October 16-18, 2014 at Gyeonggido, Korea.
INTRODUCTION
The adult gut microbiota comprises 10 to 100 trillion microorganisms, which is equivalent to ten times the number of our total somatic and germ cells [1]. Further, the collective genomes of gut microbiota (microbiome) contain 100- to 150-fold more genes than our own genome [2]. The gut microbiota has coevolved with humans and has shown profound effects on various host responses. Recent findings have suggested that an altered gut microbial composition is associated with metabolic diseases, including obesity, diabetes, or non-alcoholic fatty liver disease. These findings have indicated that the gut microbiota should be considered as an important factor to modulate host metabolism and metabolic disorders.
OBESITY-ASSOCIATED CHANGES IN THE COMPOSITION OF INTESTINAL MICROBIOTA
The gut microbiota plays an important role in the regulation of the host's metabolism and the extraction of energy from ingested food. Gut microbiota have not only the beneficial functions for the host but also the pathophysiological interactions with the host, particularly in the case of obesity and related metabolic disorders. Recent studies have shown that changes in the gut microbiota may be related in the pathogenesis of obesity and diabetes. For example, germ-free mice are protected from high fat diet (HFD)-induced obesity and metabolic dysfunction, including glucose intolerance, which is due to derepression of fasting-induced adipose factor (Fiaf), an inhibitor of lipoprotein lipase (LPL) [134]. Additionally, the colonization of germ-free animals with gut microbiota isolated from conventionally raised obese donors led to a significant increase in body fat content and insulin resistance in recipient mice [56]. Most bacterial species in the mouse and human gut belong to the phyla Bacteroidetes and Firmicutes. Compared with their lean counterparts, leptin-deficient ob/ob mice showed a decrease in Bacteroidetes and a corresponding increase in Firmicutes [7]. Interestingly, studies with humans also have shown that gut microbiota composition differs between obese and lean subjects [57]. Recently, two large metagenome-wide association studies reported that subjects with type 2 diabetes mellitus (T2DM) had a lower proportion of butyrate-producing Clostridiales (Roseburia and Faecalibacterium prausnitzii) and greater proportions of Clostridiales that do not produce butyrate, suggesting a protective role of butyrate-producing bacteria against T2DM [89].
Currently, it is not known whether these changes in the intestinal microbiota composition are secondary to the altered gastrointestinal motility and small intestinal bacterial overgrowth that are often seen in T2DM. Nevertheless, selected intestinal bacterial strains may function in the clinic as early diagnostic markers to better identify obese subjects who might be prone to develop T2DM, and provide a novel therapeutic modality against obesity or T2DM.
MECHANISMS BY WHICH INTESTINAL MICROBIOTA ARE ASSOCIATED WITH OBESITY AND DIABETES
Short-chain fatty acids
An essential role of gut microbiota is the fermentation of dietary polysaccharides that the host cannot otherwise digest. Dietary fibers constitute the indigestible portion of plant foods containing insoluble and soluble fibers. These soluble fibers are digested by enzymes derived from the gut microbiota into short-chain fatty acids (SCFAs). SCFAs (butyrate, acetate, and propionate) are absorbed in the intestines and used as energy by the host. In addition to their role as energy substrates, SCFAs function as regulators of food or energy intake [10] and inflammation [11]. It is well known that SCFAs are associated with increased satiety and reduced food intake [10]. SCFAs bind to G protein-coupled receptors, (GPCRs) such as GPR41 and GPR43, which are expressed in the enteroendocrine cells [12]. This action leads to the secretion of certain peptide hormones, such as peptide YY (PYY), that are released basolaterally into the systemic circulation, enabling a form of communication between the gut milieu and the host. Reduced food intake is, in part, due to the increases in the gut hormones, such as glucagon-like peptide (GLP)-1 and PYY [13] which decrease appetite and energy intake, and due to the decreased release of the gut peptide ghrelin, which increases food intake through effects on the hypothalamic and brainstem reward-related circuits (Fig. 1) [14]. SCFAs-mediated activation of GPR43 resulted in suppression of insulin signaling in the adipose tissue subsequently preventing fat accumulation [15]. Recent study reported that dietary soluble fibers exert their antiobesity and antidiabetic effects via the induction of intestinal gluconeogenesis (IGN), which is contrary to the general idea that gluconeogenesis impairs glucose tolerance [16]. Butyrate activates IGN gene expression through a cAMP-dependent mechanism, while propionate, itself a substrate of IGN, activates IGN gene expression via a gut-brain neural circuit [16]. Glucose released by IGN is detected by a portal vein glucose sensor that signals to the brain through the peripheral nervous system, thus exerting beneficial effects on food intake and improving glucose tolerance (Fig. 1)[1617].
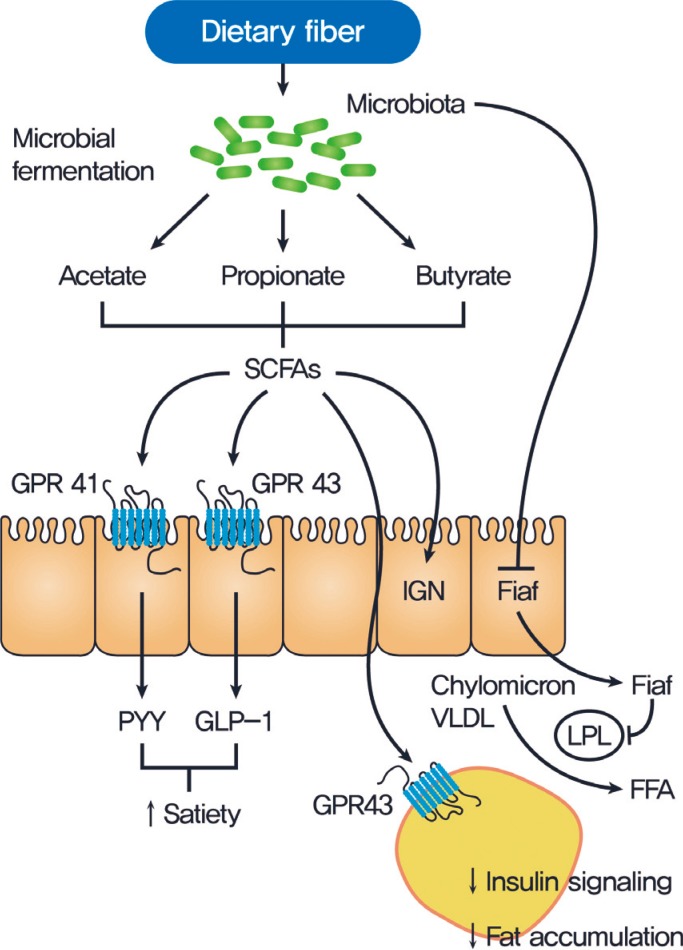
Gut microbiota regulation of host metabolism. Undigested carbohydrates are fermented by gut microbiota into short-chain fatty acids (SCFAs), primarily acetate, propionate, and butyrate. SCFAs affect the host metabolism in several ways. SCFAs can signal through G protein-coupled receptor 41 (GPR41) on enteroendocrine cells, inducing the secretion of peptide YY (PYY) which inhibits gut motility, increases intestinal transit rate, and reduces the harvest of energy from the diet. Engagement of GPR43 by SCFAs has been shown to trigger the glucogon-like peptide 1 (GLP-1) to increase insulin sensitivity. Gut microbiota efficiently suppresses fasting-induced adipose factor (Fiaf) expression in the ileum, which inhibits lipoprotein lipase (LPL) activity and fat storage in white adipose tissue. SCFAs-mediated activation of GPR43 results in suppression of insulin signaling in the adipose tissue and subsequent prevention of fat accumulation. SCFAs also activate intestinal gluconeogenesis (IGN) via a gut-brain neural circuit, which can improve glucose metabolism and reduce food intake. VLDL, very low density lipoprotein; FFA, free fatty acid.
Gut permeability and metabolic endotoxemia
Several recent studies have suggested that disruption of the gut barrier function and the gut microbiota-derived endotoxemia could contribute to the pathogenesis of obesity and T2DM [1819]. A HFD dramatically increased gut permeability and reduced the expression of tight junction protein, such as zonula occludens-1 and occludin, in the intestinal epithelial cells of mice (Fig. 2) [20]. Disruption of the gut barrier in genetically or HFD-induced obese mice increased gut permeability, resulting in the leakage of lipopolysaccharide into the portal blood circulation (Fig. 2) [212223]. Consistent with this concept, the modulation of the gut microbiota composition with antibiotics or prebiotics improved gut permeability, reduced metabolic endotoxemia, lowered inflammation, and alleviated glucose intolerance [24].
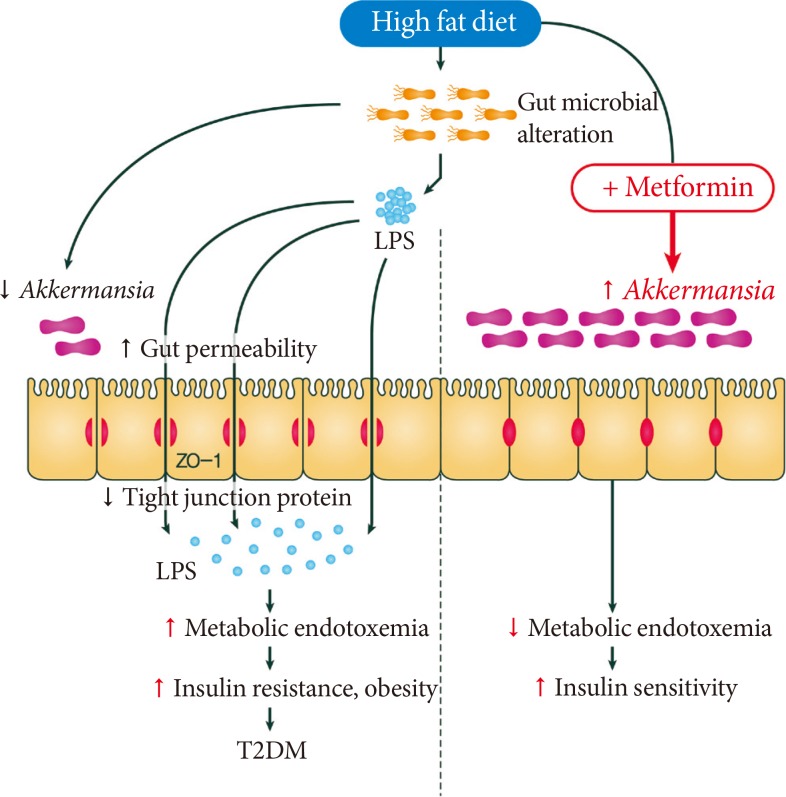
Effect of high fat diet (HFD) and metformin on gut microbiota and intestinal environment. A HFD induces gut microbial alteration, which increases gut permeability and reduces the expression of tight junction protein, such as zonula occludens (ZO)-1 and occludin, in the intestinal epithelial cells, results in the passage of lipopolysaccharide (LPS) into the portal blood circulation. The disruption of the gut barrier function and the gut microbiota-derived endotoxemia could contribute to the pathogenesis of obesity, insulin resistance, and type 2 diabetes mellitus (T2DM). Metformin is able to affect the mouse microbiota and restored the decreased abundance of Akkermansia muciniphila, a mucin-degrading G (-) anaerobes, in the gut of mice fed a HFD to that of mice fed normal chow diet. A. muciniphila had similar beneficial metabolic effects to that of metformin administration.
INTESTINAL MICROBIOTA MODULATION AS NOVEL THERAPEUTIC STRATEGIES FOR OBESITY AND DIABETES
Prebiotics
Prebiotics are non-digestible but fermentable polysaccharides, such as inulin, fructo-, oligosaccharides, galato-oligosaccharides, or lactulose. Foods artificially enriched with these fibers are defined as prebiotics, which promote SCFA production and the growth of beneficial bacteria, especially Bifidobacterium and Lactobacillus [25]. Studies in healthy humans and rodents have demonstrated that prebiotic consumption reduces hunger and enhances satiety [26]. As discussed above, this modulation of ingestive behavior is mediated, in part, by SCFA-induced changes in gut peptide secretion. Additionally, by promoting Bifidobacterium populations, prebiotics improve gut barrier function [27].
Probiotics
Probiotics are defined as live microorganisms that confer a beneficial health effect on the host when administered in proper amounts. Some probiotic strains, especially those of the genera Lactobacillus and Bifidobacterium, have been shown to ameliorate obesity and metabolic disorders. The suggested mechanisms include inhibition of the pathogen adhesion to gut mucosa, stabilization of the microbial community, or improvement of the mucosal integrity and barrier function [2728293031]. The improvements of gut barrier function may reflect the actions of the SCFA products of bacterial fermentation. A recent study reported the direct beneficial actions of Lactobacilli on the epithelial cells and on the enteric nervous system regulating gut contractility [32].
Drugs
A recent study showed that metformin, widely used for treatment of T2DM, is able to slow aging in Caenorhabditis elegans by metabolically altering Escherichia coli with which it is cocultured [33]. This effect was found to be due to the alteration of folate and the methionine metabolism of E. coli by metformin. Metformin decreased the methionine cycle and increased the levels of both S-adenosylmethionine (SAMe) and S-adenosylhomocystein. SAMe acts a co-repressor of methionine synthesis genes and also inhibits the folate cycle, resulting in decelerated aging in the worm [33].
Metformin can also affect the intestinal microbiota of mammals. We have demonstrated that metformin is able to affect the mouse microbiota and increase the abundance of Akkermansia muciniphila, a mucin-degrading G (-) anaerobes, in the gut of experimental mice fed a HFD (Fig. 2) [34]. We also observed that the administration of A. muciniphila had similar beneficial metabolic effects to that of metformin administration: (1) increased the number of mucin-producing goblet cells was similarly found after the administration of metformin or A. muciniphila; (2) diminished regulatory T (Treg) cell numbers and elevated interleukin 1β (IL-1β) or IL-6 mRNA expression in the visceral adipose tissue of mice fed a HFD were similarly reversed after the administration of metformin or A. muciniphila. Mucin has recently been shown to enhance delivery of tolerogenic immunoregulatory signal to the intestinal epithelium by forming galectin-3-dectin-1-FcγRIIB complex besides its classical role as a physical barrier [35] This study therefore underscores that drugs, such as metformin, might exert therapeutic effects, at least in part by modulating the gut micriobiota.
Fecal microbiota transplantation
Recently, articles in the literature regarding fecal microbiota transplantation (FMT) have aroused strong interest. FMT is reported to be a highly successful therapy for recurrent Clostridium difficile infection [36]. These results also suggested a potential therapeutic effect of FMT in metabolic syndrome or T2DM. A recent study showed that FMT via a gastroduodenal tube from lean donors into obese subjects with metabolic syndrome induced a significant improvement of insulin sensitivity in the recipients [37]. FMT resulted in an increase of the microbial diversity and a 2.5-fold increase in the proportion of butyrate producer Roseburia intestinalis after 6 weeks of treatment, whereas, fecal SCFA levels were decreased. Despite this first evidence that such as approach could be attractive, more information is needed with larger, well-designed studies to prove whether such approaches are overall beneficial for patients with metabolic syndrome or T2DM.
CONCLUSIONS
Intestinal microbiota may play an important role in the pathogenesis of T2DM by influencing body weight, proinflammatory activity, and insulin resistance (Fig. 1). Future studies are required to increase our understanding of the complex interplay between intestinal microbiota and the host with T2DM and to enable the development of innovative and effective treatments for T2DM.
ACKNOWLEDGMENTS
This study was supported by a Global Research Laboratory Grant (K21004000003-12A0500-00310) from the National Research Foundation of Korea and the Ulsan National Institute of Science and Technology Research Fund (2014M3A9D8034459).
Notes
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.