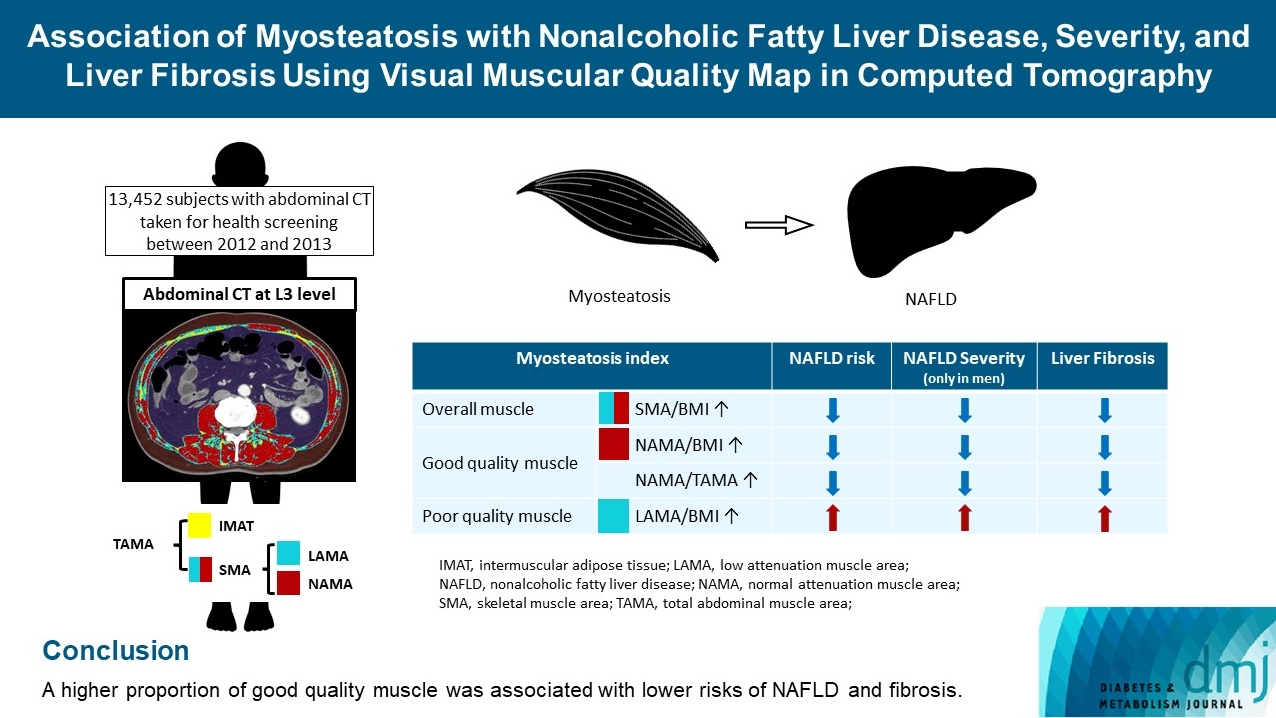
Association of Myosteatosis with Nonalcoholic Fatty Liver Disease, Severity, and Liver Fibrosis Using Visual Muscular Quality Map in Computed Tomography
Article information
Abstract
Background
The association of myosteatosis measured using visual muscular quality map in computed tomography (CT) with nonalcoholic fatty liver disease (NAFLD), its severity, and fibrosis was analyzed in a large population.
Methods
Subjects (n=13,452) with abdominal CT between 2012 and 2013 were measured total abdominal muscle area (TAMA) at L3 level. TAMA was segmented into intramuscular adipose tissue and skeletal muscle area (SMA), which was further classified into normal attenuation muscle area (NAMA) and low attenuation muscle area (LAMA). The following variables were adopted as indicators of myosteatosis: SMA/body mass index (BMI), NAMA/BMI, NAMA/TAMA, and LAMA/BMI. NAFLD and its severity were assessed by ultrasonography, and liver fibrosis was measured by calculating the NAFLD fibrosis score (NFS) and fibrosis-4 index (FIB-4) scores.
Results
According to multiple logistic regression analyses, as quartiles of SMA/BMI, NAMA/BMI, and NAMA/TAMA increased, the odds ratios (ORs) for NAFLD decreased in each sex (P for trend <0.001 for all). The ORs of moderate/severe NAFLD were significantly higher in the Q1 group than in the Q4 group for SMA/BMI, NAMA/BMI, and NAMA/TAMA in men. The ORs of intermediate/high liver fibrosis scores assessed by NFS and FIB-4 scores increased linearly with decreasing quartiles for SMA/BMI, NAMA/BMI, and NAMA/TAMA in each sex (P for trend <0.001 for all). Conversely, the risk for NAFLD and fibrosis were positively associated with LAMA/BMI quartiles in each sex (P for trend <0.001 for all).
Conclusion
A higher proportion of good quality muscle was associated with lower risks of NAFLD and fibrosis.
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) is the most common liver disease worldwide [1]. NAFLD includes progressive conditions, ranging from simple hepatic steatosis to nonalcoholic steatohepatitis (NASH) with or without fibrosis [1]. NAFLD can also develop into liver cirrhosis and hepatocellular carcinoma [1]. Moreover, NAFLD is associated with cardio-metabolic disease, which is the leading cause of mortality in patients with NAFLD [1]. Therefore, significant effort has been focused on developing an effective screening tool for modifiable risk factors to reduce the burden of NAFLD.
Sarcopenia, a muscular disease characterized by the gradual loss of muscle mass and strength, is also suggested to be one of the risk factors of NAFLD [2,3]. Primarily, most studies about sarcopenia have relied on bioelectrical impedance analysis or dual energy X-ray absorptiometry to measure skeletal muscle mass [4-6]. Similarly, the association between NAFLD and sarcopenia has mostly been evaluated using these modalities to measure sarcopenia [2,3].
However, sarcopenia is a more complex condition which cannot be fully explained by the loss of muscle mass and strength [6]. As muscle mass and functioning decline with age, several changes occur locally within individual muscles, which affect the muscle quality, the physiological functional capacity of muscle tissue [7]. Accordingly, the updated guidelines of the European Working Group on Sarcopenia in Older People highlighted low muscle strength and poor muscle quality as the primary characteristics of sarcopenia [6]. As most previous studies measured the muscle mass, not the muscle quality to define sarcopenia [6,7], the association between sarcopenia and NAFLD should be revisited by incorporating the concept of muscle quality.
Regarding the muscle quality, the redistribution of adipose tissue where subcutaneous adipose tissue relocates to more detrimental locations such as intramuscular and intermuscular adipose tissue (IMAT), is one of the contributing factors to the poor muscle quality [7,8]. This is called myosteatosis and it negatively affects muscle strength by changing muscle fiber disorientation [7]. Muscle biopsy is the gold standard for diagnosing myosteatosis [9]. However, this procedure is not practical because of its invasiveness. Recently, muscle attenuation measured by computed tomography (CT) scanning has been reported to identify fat infiltration and indirectly estimate muscle strength and physical function [9,10]. Specifically, low radiation attenuation indicates a high proportion of myosteatosis (i.e., poor quality muscle), whereas high attenuation indicates low muscle fat infiltration (i.e., good quality muscle) [9,10].
Hence, we aimed to investigate whether indices of myosteatosis determined by abdominal CT are associated with the risk of NAFLD. If so, we aimed to further examine whether myosteatosis is a significant contributor to NAFLD severity and liver fibrosis. For this purpose, we analyzed abdominal CT scans obtained from healthy Korean populations and constructed a visual muscular quality map in CT.
METHODS
Study population
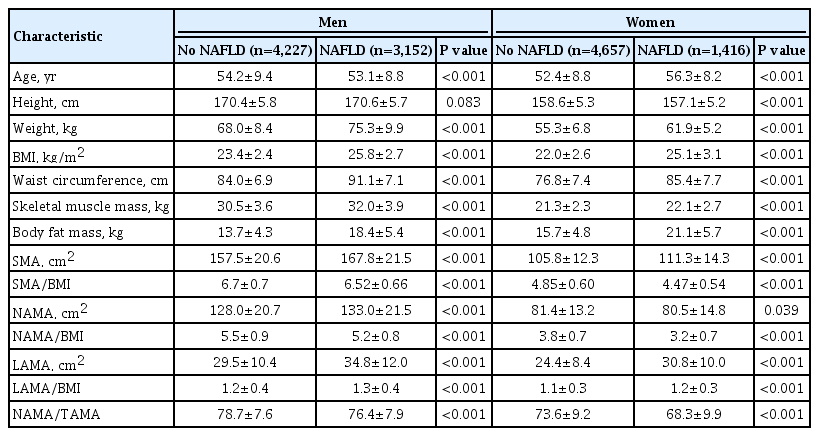
This cross-sectional study was performed on 23,311 subjects who underwent abdominal CT scans during routine health check-ups at the Health Screening and Promotion Center of the Asan Medical Center (Seoul, Korea) between January 2012 and December 2013. We excluded 9,859 subjects lacking abdominal ultrasonography data and with excess alcohol intake (>30 g/day in men; >20 g/day in women) as well as those who had systemic disorders including liver cirrhosis, hepatitis B or C, overt thyroid dysfunction (free thyroxine >1.9 ng/dL or <0.8 ng/dL, thyroid stimulating hormone <0.4 mU/L, or >5.0 mU/L), chronic renal insufficiency (<60 mL/min/1.73 m2), cancer, a history of cardiovascular disease (CVD) or cerebrovascular disease, or those who were currently taking glucocorticoids or hormone replacement. Several subjects met ≥2 exclusion criteria. Finally, 13,452 subjects were eligible for analysis (Supplementary Fig. 1).
Following the ethical guidelines of the Declaration of Helsinki and Korea Good Clinical Practice, all subjects provided written informed consent, and this study was approved by the Institutional Review Board of Asan Medical Center (No. 2020-0343).
Definitions of NAFLD and liver fibrosis
NAFLD was diagnosed with hepatic ultrasonography [11]. The severity of liver fibrosis was determined the NAFLD fibrosis score (NFS) and the fibrosis-4 (FIB-4) score [12,13]. Detailed definitions of NAFLD and liver fibrosis are described in the Supplementary Methods.
CT image collection
Methods used to collect CT images are presented in the Supplementary Methods.
Assessment of abdominal skeletal muscle area and myosteatosis
For each CT scan, the axial CT slice number of the L3 vertebra inferior endplate was annotated, and the lumbar vertebral anatomic variant was identified by two board-certified radiologists. The CT images were automatically segmented to generate the boundary of total abdominal muscle area (TAMA), visceral fat area, and subcutaneous fat area. The TAMA included all muscles on the selected axial images (i.e., psoas, para-spinal, transversus abdominis, rectus abdominis, quadratus lumborum, and internal and external obliques). To evaluate myosteatosis, the TAMA was divided into three areas according to the CT density as follows: (1) normal attenuation muscle area (NAMA, +30 to +150 HU), representing nonfatty muscle with little intramuscular fat; (2) low attenuation muscle area (LAMA, −29 to +29 HU), representing fatty muscles with intramuscular lipid pool; and (3) IMAT (−190 to −30 HU), representing the apparent fat tissue between muscle groups and muscle fibers [9]. The skeletal muscle area (SMA, −29 to +150 HU) referred to the combined areas of the NAMA and LAMA, as illustrated in Supplementary Fig. 2. All measurements were divided by body mass index (BMI) to adjust the body size of the patient. The NAMA/TAMA ratio was calculated as NAMA divided by TAMA and multiplied by 100. The following variables were used to define myosteatosis: SMA/BMI, NAMA/BMI, NAMA/TAMA, and LAMA/BMI.
Statistical analysis
We analyzed the sex-specific quartiles for SMA/BMI, NAMA/BMI, NAMA/TAMA, and LAMA/BMI and applied this quartile classification throughout our analyses. Logistic regression analyses were used to analyze the odds ratios (ORs) and 95% confidence intervals (CIs) of the myosteatosis indices for NAFLD status (US findings and surrogate markers), the severity of NAFLD (mild vs. moderate to severe), and liver fibrosis stages (low vs. intermediate to high) based on the NFS and FIB-4 score. The ORs were adjusted for age, smoking status, alcohol consumption, regular exercise, hypertension, diabetes, triglyceride, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, and alanine transferase. Correlations between the BMI-based myosteatosis indices and the NFS or FIB-4 scores were assessed using Pearson’s correlation coefficients. We also analyzed the receiver operating characteristic (ROC) curves and areas under the curve (AUC) and compared the AUCs to evaluate the ability of myosteatosis indices for predicting NAFLD risk and severity. The AUCs were conducted using MedCalc version 11.20.0 for Windows (MedCalc Software, Mariakerke, Belgium). All statistical analyses but ROC curves were performed with SPSS software version 21.0 for Windows (IBM Inc., Armonk, NY, USA). Values of P<0.05 were considered statistically significant.
RESULTS
Baseline characteristics according to NAFLD status
A total of 13,452 subjects (7,379 men and 6,073 women) were included in this analysis. The men and women had mean ages of 53.7±9.2 and 53.3±8.8 years, respectively. Among the study population, 1,082 (8.0%) subjects were diagnosed with type 2 diabetes mellitus, and 79 (0.6%) subjects with CVD. The anthropometric characteristics and CT measurements of the study subjects are presented in Table 1. The subjects were categorized into four subgroups based on sex and the presence or absence of NAFLD. Of the total subjects, 4,568 (34.0%) had NAFLD whereas 8,884 (66.0%) did not. Men and women differed significantly in all variables, including anthropometric measurements, body composition, and lifestyles; thus, the statistical analyses were conducted separately for each sex. All myosteatosis indices measured by CT scanning showed significant differences according to NAFLD status in each sex. Subgroup comparisons of laboratory baseline characteristics according to sex and NAFLD status are shown in Supplementary Table 1. Subjects with NAFLD showed worse metabolic profiles such as higher blood pressure, elevated fasting glucose, and worse lipid profiles than subjects without NAFLD in both males and females.
Association of myosteatosis indices with the presence of NAFLD
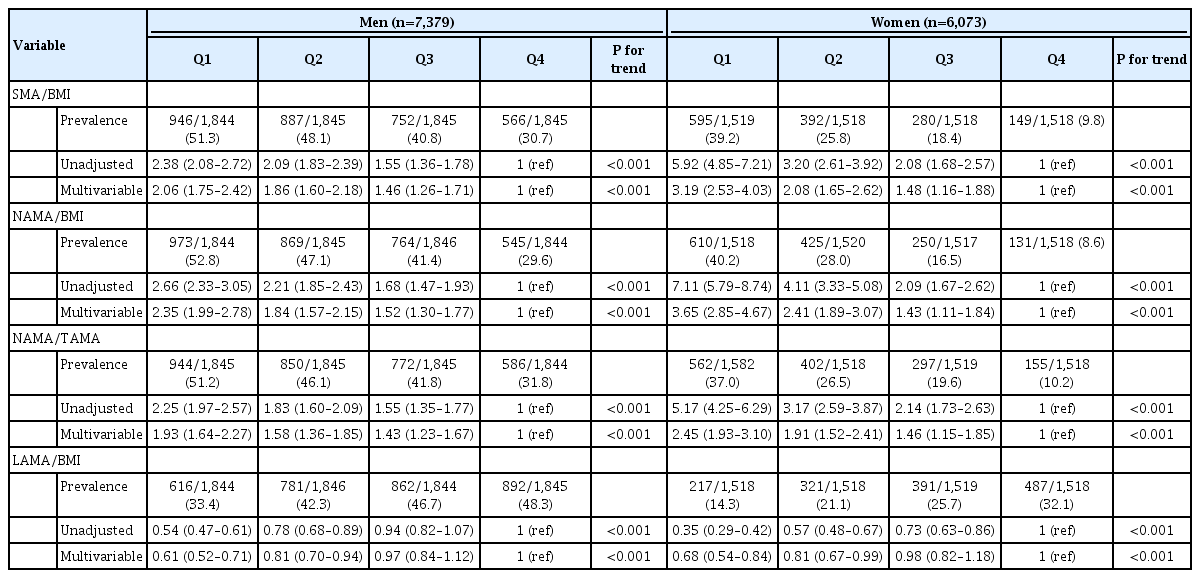
To evaluate the role of myosteatosis on the risk of NAFLD, we calculated the ORs using multiple logistic regression analyses (Table 2 and Supplementary Table 2). The adjusted ORs (95% CIs) for NAFLD in Q1, Q2, and Q3 compared with Q4 showed graded associations of SMA/BMI, NAMA/BMI, NAMA/TAMA, and LAMA/BMI with the presence of NAFLD in each sex (Table 2) and both combined (Supplementary Table 2) (P for trend <0.001 for all). The risk of NAFLD increased linearly with decreasing quartiles of SMA/BMI, NAMA/BMI, and NAMA/TAMA in each sex. The adjusted ORs for NAFLD in Q1, Q2, and Q3 for SMA/BMI compared with Q4 were 2.06 (95% CI, 1.75 to 2.42), 1.86 (95% CI, 1.60 to 2.18), and 1.46 (95% CI, 1.26 to 1.71) in men and 3.19 (95% CI, 2.53 to 4.03), 2.08 (95% CI, 1.65 to 2.62), and 1.48 (95% CI, 1.16 to 1.88) in women, respectively. The adjusted ORs for NAFLD in Q1, Q2, and Q3 for NAMA/BMI compared with Q4 were 2.35 (95% CI, 1.99 to 2.78), 1.84 (95% CI, 1.57 to 2.15), and 1.52 (95% CI, 1.30 to 1.77) in men and 3.65 (95% CI, 2.85 to 4.67), 2.41 (95% CI, 1.89 to 3.07), and 1.43 (95% CI, 1.11 to 1.84) in women, respectively. The adjusted ORs for NAFLD in Q1, Q2, and Q3 for NAMA/TAMA compared with Q4 were 1.93 (95% CI, 1.64 to 2.27), 1.58 (95% CI, 1.36 to 1.85), and 1.43 (95% CI, 1.23 to 1.67) in men and 2.45 (95% CI, 1.93 to 3.10), 1.91 (95% CI, 1.52 to 2.41), and 1.46 (95% CI, 1.15 to 1.85) in women, respectively.
Conversely, the risk of NAFLD decreased in the sequence of the decreasing quartiles in LAMA/BMI in each sex. The adjusted ORs for NAFLD in Q1, Q2, and Q3 for LAMA/BMI compared with Q4 were 0.61 (95% CI, 0.52 to 0.71), 0.81 (95% CI, 0.70 to 0.94), and 0.97 (95% CI, 0.84 to 1.12) in men and 0.68 (95% CI, 0.54 to 0.84), 0.81 (95% CI, 0.67 to 0.99), and 0.98 (95% CI, 0.82 to 1.18) in women, respectively.
Additionally, when NAFLD was defined according to the surrogate markers such as Hepatic Steatosis Index and Simple NAFLD score, the risk of NAFLD showed statistically significant associations with myosteatosis indices (Supplementary Table 3). NAFLD risk was also analyzed in the subgroups of age (<65 years vs. ≥65 years), BMI (<25 kg/m2 vs. ≥25 kg/m2), and underlying diabetes mellitus or not. The muscle parameters in the subgroups showed similar trends as the sex subgroup although not all of the ORs were statistically significant (data not shown).
Association of myosteatosis indices with NAFLD severity
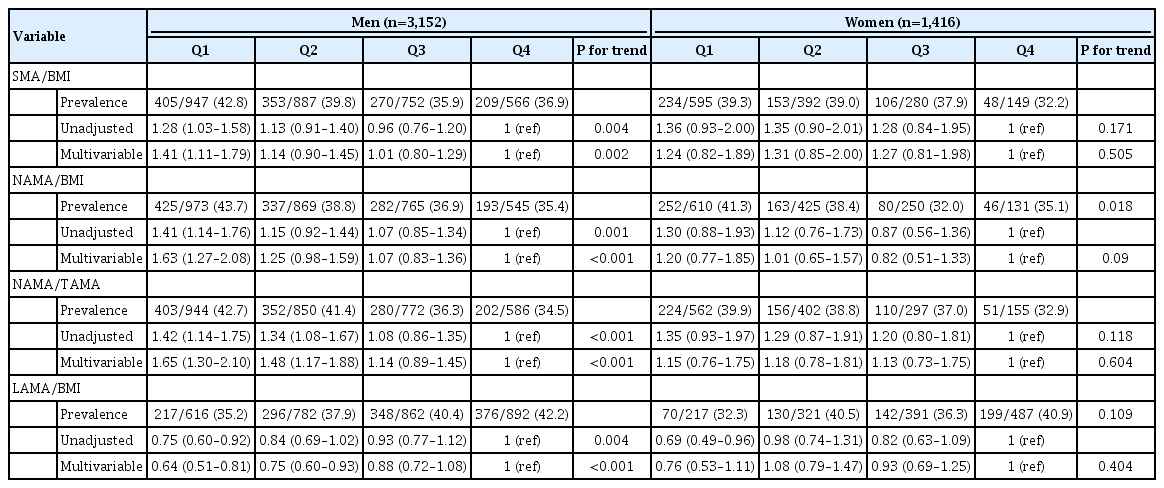
Among the 4,568 subjects with NAFLD, hepatic ultrasonography revealed that 2,790 (61.1%) had mild NAFLD, 1,449 (31.7%) had moderate NAFLD, and 329 (7.2%) had severe NAFLD. Compared with subjects with mild NAFLD, the risk of moderate/severe NAFLD was higher in the Q1 group than in the Q4 group for SMA/BMI, NAMA/BMI, and NAMA/TAMA in men (Table 3). The risk of moderate/severe NAFLD was lower in the Q1 group than in the Q4 group for LAMA/BMI in men. Conversely, in women, the risk of moderate/severe NAFLD compared with mild NAFLD did not show statistical significance for SMA/BMI, NAMA/BMI, NAMA/TAMA, and LAMA/BMI in Q1, Q2, and Q3 compared with Q4 (Table 3).
Association of myosteatosis indices with liver fibrosis based on the NFS and FIB‑4 score
Among the subjects with NAFLD, 2,593 (56.8%) had a low NFS, 1,898 (41.5%) had an intermediate NFS, and 76 (1.7%) had a high NFS. Additionally, 2,847 (62.3%) had a low FIB-4 score, 1,640 (35.9%) had an intermediate FIB-4 score, and 81 (1.8%) had a high FIB-4 score.
Multiple logistic regression analyses showed a graded association between quartiles of myosteatosis indices and liver fibrosis stage as assessed by NFS (P for trend <0.001 for all) (Table 4). Compared with subjects with a low NFS, the adjusted ORs for an intermediate/high NFS were negatively associated with SMA/BMI, NAMA/BMI, and NAMA/TAMA in each sex. The adjusted ORs for an intermediate/high NFS in Q1, Q2, and Q3 for SMA/BMI compared with Q4 were 1.95 (95% CI, 1.56 to 2.43), 1.31 (95% CI, 1.05 to 1.64), and 1.15 (95% CI, 0.91 to 1.45) in men and 2.72 (95% CI, 1.79 to 4.14), 1.45 (95% CI, 0.94 to 2.22), and 1.97 (95% CI, 1.26 to 3.10) in women, respectively. The adjusted ORs for an intermediate/high NFS in Q1, Q2, and Q3 for NAMA/BMI compared with Q4 were 2.55 (95% CI, 2.03 to 3.21), 1.70 (95% CI, 1.35 to 2.14), and 1.11 (95% CI, 0.87 to 1.41) in men and 4.10 (95% CI, 2.53 to 6.64), 2.28 (95% CI, 1.39 to 3.74), and 2.53 (95% CI, 1.50 to 4.26) in women, respectively. The adjusted ORs for an intermediate/high NFS in Q1, Q2, and Q3 for NAMA/TAMA compared with Q4 were 2.88 (95% CI, 2.30 to 3.61), 1.79 (95% CI, 1.42 to 2.24), and 1.31 (95% CI, 1.04 to 1.66) in men and 3.61 (95% CI, 2.36 to 5.50), 1.97 (95% CI, 1.27 to 3.05), and 1.82 (95% CI, 1.15 to 2.87) in women, respectively.

The risk of intermediate/high NFS compared with low NFS according to myosteatosis indices in patients with NAFLD
Conversely, the risk of intermediate/high NFS was positively associated with quartiles of LAMA/BMI in each sex. The adjusted ORs for an intermediate/high NFS in Q1, Q2, and Q3 for LAMA/BMI compared with Q4 were 0.34 (95% CI, 0.27 to 0.42), 0.49 (95% CI, 0.40 to 0.60), and 0.65 (95% CI, 0.54 to 0.79) in men and 0.42 (95% CI, 0.30 to 0.60), 0.48 (95% CI, 0.36 to 0.65), and 0.59 (95% CI, 0.44 to 0.78) in women, respectively.
Multiple logistic regression analyses using the FIB-4 score are shown in Table 5. Compared with subjects with a low FIB-4 score, similar linear trends for intermediate/high FIB-4 scores as those for the NFS were observed for SMA/BMI, NAMA/BMI, NAMA/TAMA, and LAMA/BMI in each sex (P for trend <0.001 for all) (Table 5).
Correlation between myosteatosis indices and liver fibrosis based on the NFS and FIB‑4 score
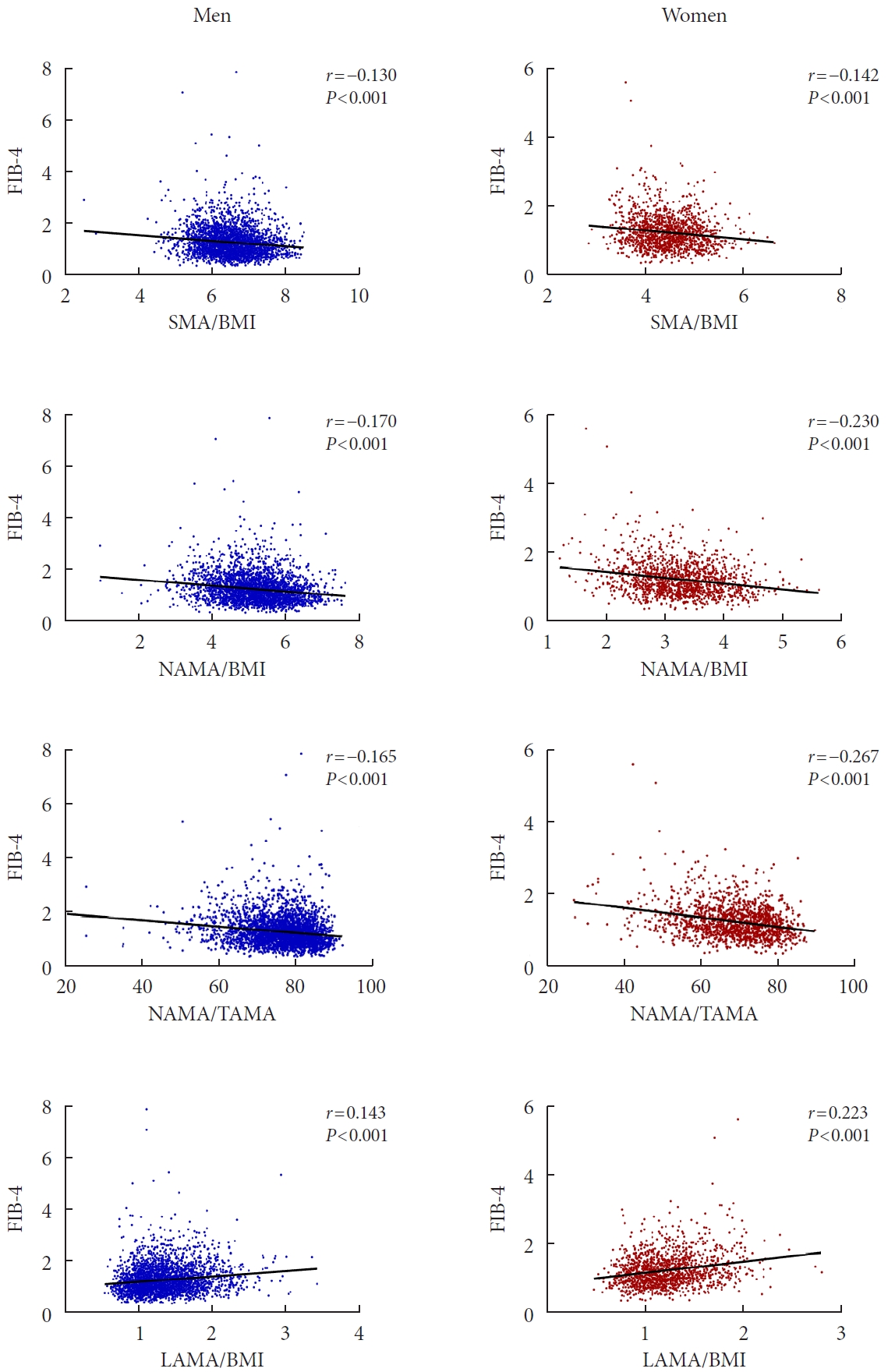
The relationships between SMA/BMI, NAMA/BMI, NAMA/TAMA, LAMA/BMI, and the NFS were analyzed by Pearson correlation coefficients in each sex (Supplementary Fig. 3). All four myosteatosis indices and the NFS showed statistically significant correlations in each sex (P<0.001 for all). Decreasing NFS values were observed for SMA/BMI (r=−0.161 in men, r=−0.195 in women), NAMA/BMI (r=−0.242 in men, r=−0.297 in women), and NAMA/TAMA (r=−0.260 in men, r=−0.322 in women). Conversely, increasing NFS values were found for LAMA/BMI (r=0.245 in men, r=0.270 in women). A similar relationship was observed between SMA/BMI, NAMA/BMI, NAMA/TAMA, LAMA/BMI, and the FIB-4 score (P<0.001 for all) (Fig. 1).
Area under ROC curves of myosteatosis indices for NAFLD risk and severity
The myosteatosis indices each showed a moderate predictive ability for NAFLD risk and severity. LAMA/BMI showed the highest AUC for NAFLD risk (AUC, 0.608), followed by NAMA/TAMA (AUC, 0.568), SMA/BMI (AUC, 0.536), and NAMA/BMI (AUC, 0.509) (Supplementary Table 4). The highest AUC for NAFLD severity was demonstrated by NAMA/TAMA and LAMA/BMI (both AUC, 0.532).
DISCUSSION
Our study showed that muscle quality, as assessed by the degree of myosteatosis (increased proportion of muscle fat infiltration), was associated with the risk of NAFLD, its severity, and liver fibrosis. As the proportion of good quality muscle (i.e., NAMA/BMI and NAMA/TAMA) increased, the risk of NAFLD decreased. Among the subjects with NAFLD, a higher proportion of good quality muscle was also significantly associated with a decreased risk of moderate to severe NAFLD in men and intermediate to high levels of liver fibrosis in both sexes. These relationships remained significant after adjusting for other risk factors of NAFLD. To our best knowledge, our study is the first to present the association between muscle quality measured by CT and the risk of NAFLD, its severity, and fibrosis in a large population.
NAFLD is not merely a disease of the liver; its spectrum also extends to an elevated risk of CVD, cerebrovascular disease, and chronic kidney disease [14]. NAFLD is heterogeneous in its pathophysiology, which is often neglected because its diagnosis is focused on liver histology [14]. Despite its heterogeneity, the underlying pathophysiology of NAFLD involves inflammation and insulin resistance [14]. Therefore, NAFLD is closely related to metabolic diseases such as obesity, type 2 diabetes mellitus, hypertension, and dyslipidemia [14,15]. Our results also showed that subjects with NAFLD had worse metabolic profiles compared with subjects without NAFLD (Table 1 and Supplementary Table 1).
With NAFLD considered a metabolic disorder of the liver, it is becoming more critical to identify its risk factors and provide proper management for people at risk. Among the various risk factors, sarcopenia has been suggested to play a role in the pathophysiology of NAFLD [16,17]. For example, decreased muscle mass measured by dual energy X-ray absorptiometry was associated with an increased risk of NAFLD, independent of insulin resistance [16,17]. Additionally, NAFLD patients with sarcopenia, as measured by dual energy X-ray absorptiometry, were more likely to have significant liver fibrosis, regardless of obesity and insulin resistance [2]. The risk of NASH was also increased in biopsy-proven NAFLD patients with sarcopenia (measured by bioelectrical impedance analysis) [3]. However, because these tools do not consider muscle quality, there has been the need for a novel approach to access the degree of myosteatosis which could determine the muscle quality.
CT has emerged as the standard diagnostic tool for quantitatively and qualitatively evaluating muscle and fat [10]. In addition to muscle mass, muscle quality can be measured through CT by identifying the low attenuation areas of fat within the muscle [9,10]. Most previous studies measured IMAT by CT and showed that IMAT was associated with inflammation, insulin resistance, carotid atherosclerosis, and subclinical coronary artery calcification [18-20]. Most recently, our group separately evaluated LAMA and NAMA and suggested that LAMA was associated with an increased risk of subclinical coronary atherosclerosis [21]. Collectively, increasing evidence has shown the importance of muscle quality in the pathogenesis of various cardio-metabolic disorders and NAFLD.
Thus far, a few studies have addressed the association between muscle quality by CT imaging of the L3 level and NAFLD [22,23]. Hsieh et al. [23] used pre-defined cutoff values of skeletal muscle index (SMA divided by the square of the height in meters) and muscle attenuation to determine the presence of low skeletal muscle mass and myosteatosis; they determined that the prevalence of significant liver fibrosis was associated with low skeletal muscle mass and myosteatosis. Tanaka et al. [22] quantified SMA, NAMA, and LAMA, divided these values by BMI, and calculated SMA, NAMA, and LAMA indices; however, only the SMA index was significantly associated with the prevalence of NAFLD, whereas the NAMA and LAMA indices were not significant. In our study with a greater number of subjects, not only the SMA index but also the NAMA and LAMA indices were significantly associated with the prevalence of NAFLD in both sexes. Furthermore, we analyzed the relationship between these indices and the severity of NAFLD and liver fibrosis. The NAMA/TAMA index, which also considers IMAT in TAMA, was applied in the analysis, which demonstrated that a higher NAMA/TAMA index (higher proportion of good quality muscle out of TAMA with the consideration of IMAT) was associated with a possible protective effect against NAFLD and liver fibrosis. Our results further support the crucial role of good muscle quality and muscle mass in the pathogenesis of NAFLD.
Muscle areas were divided by BMI to adjust for the body size. Determining the ideal adjustment method among height, weight, and BMI has been a long debate in the field of sarcopenia, especially in Asian populations [24]. In previous studies about age-related changes in muscle mass or quality of lumbar SMA, authors compared the prevalence of sarcopenia or myosteatosis with height, weight, or BMI-adjusted indices and showed that BMI-adjusted index may be an ideal index for diagnosing sarcopenia and myosteatosis [24,25]. Moreover, the Foundation for the National Institutes of Health Sarcopenia Project proposed a consensus and recommended using BMI-adjusted appendicular skeletal muscle mass for diagnosis of sarcopenia reflecting muscle weakness [26].
Insulin resistance is suggested as a shared pathophysiology because myosteatosis increases diacylglycerol (DAG) in muscle, activating DAG-novel protein kinase C, which inhibits insulin signaling [27]. DAG also accumulates in the liver and inhibits insulin-mediated glycogen synthesis [27]. Moreover, glucose uptake by insulin-dependent glucose transporter 4 in muscles is decreased, causing excess glucose to be converted into triacylglycerol in the liver [28]. Decreased physical activity and resistance to anabolic hormones impair protein homeostasis in muscle and reduce muscle mass, which is commonly observed in NAFLD [28]. Muscle loss decreases basal metabolic rate and mitochondrial capacity, resulting in more muscle wasting [28]. A higher NAMA/TAMA index was previously associated with greater insulin sensitivity [21]; therefore, good muscle quality may have a preventive effect against steatotic and fibrotic liver. In our study, subjects with a higher NAMA/TAMA index showed favorable metabolic characteristics, including lower blood pressure, fasting glucose, and homeostatic model assessment for insulin resistance values, thereby supporting this hypothesis (data not shown).
Chronic inflammation is another possible pathophysiologic mechanism linking NAFLD and myosteatosis [27-29]. In obese individuals, inflammatory cytokines and adipokines are excreted by the excess adipose tissue, stimulating inflammatory cell (macrophage) accumulation [18,29]. These macrophages further release proinflammatory cytokines such as C-reactive protein (CRP), interleukin-6 (IL-6), and tumor necrosis factor α [30]. High CRP and IL-6 levels were associated positively with total fat mass and negatively with appendicular lean mass, suggesting the possible link between inflammation and sarcopenia [31]. In our study, we observed a significant correlation between plasma high-sensitivity CRP levels and myosteatosis indices (Supplementary Table 5), which further supports this inflammation-mediated theory.
Finally, the skeletal muscle has been identified as an active endocrine organ that releases a variety of peptides known as myokines [28]. Various myokines and hepatokines are also involved in the association between myosteatosis and NAFLD [28]. For example, irisin was shown to decrease in obese subjects with NAFLD, whereas myostatin was increased in subjects with muscle wasting and insulin resistance [28,32]. Although we could not measure these organokines in our study populations, these cytokines and organokines interact with one another in metabolic regulation, contributing to the pathophysiology of myosteatosis and NAFLD.
Myosteatosis caused by these complex mechanisms, leads to mitochondrial dysfunction, myocellular death, and abnormal secretion of myokines, disrupting hormone interactions with fat and liver, ultimately developing NAFLD [32]. Evidence suggests that skeletal muscle health is associated with outcome and progression of NAFLD, as one Korean study showed that sarcopenia was more prevalent in NASH group, compared to control and NAFLD groups (35.0% vs. 8.7% and 17.9% respectively, P<0.001) [33]. Additionally, the prevalence of sarcopenia was greater in patients with NAFLD-associated liver fibrosis in Western studies [34,35]. Therefore, effective management stop and reverse the progression of skeletal muscle dysfunction due to myosteatosis are expected to have a beneficial effect on the progression and outcomes in all stages of NAFLD.
The myosteatosis indices in our study did not show a significant association with NAFLD severity in women (Table 3). Previous studies have indicated that NAFLD is more prevalent in men, and estrogen is considered to have a protective effect against NAFLD [36]. The results regarding sex differences in the severity of NAFLD and liver fibrosis are conflicting [36,37]. We believe that the lack of association in women might be due to the relatively smaller number of female patients with moderate to severe NAFLD (541 female patients compared with 1,237 male patients). Additionally, the study population is relatively young, and the premenopausal women are known to be protected against NAFLD as they are from CVD [36]. However, additional studies are necessary to validate the cause of this sex difference in the association between muscle quality and NAFLD severity.
This study has some limitations. First, this study was conducted retrospectively based on cross-sectional data. Second, selection bias may be present, and possible positive and negative predictive values should be considered because the study population was composed of people who voluntarily participated in routine health examinations. Third, muscle strength was only indirectly estimated by muscle quality on CT. Previous studies have shown that grip strength is a simple and reliable tool for assessing muscle strength and predicting adverse outcomes [38]. Unfortunately, grip strength was not included in our center’s routine health examination. Fourth, while subcutaneous fat in the lower part of the body has been suggested to predict cardio-metabolic diseases [39], our study only measured myosteatosis at the L3 level on CT, so lower extremity muscle and fat distribution was not covered. Still, measuring the abdominal skeletal muscles at L3 level were favored in previous studies [6] and were commonly used to evaluate sarcopenia in previous studies [40,41] because this could be measured opportunistically without additional cost or radiation exposure by using the clinical abdominal CT scans obtained during routine care. Lastly, since the study subjects were diagnosed with NAFLD during routine health exams, the diagnosis of NAFLD was made by morphologic changes detected by ultrasound, although the gold standard is a liver biopsy. Although it is widely used due to its low cost, safety and accessibility, its specificity for the diagnosis of NAFLD is relatively low because it can detect hepatic steatosis only when it is greater than 25% to 30% [11]. Similarly, the severity and the extent of hepatic fibrosis were classified by calculating non-invasive markers without performing a liver biopsy. Although NFS and FIB-4 are among the most widely validated and recommended tests given their low cost and accessibility in routine clinical practice, these markers might not be the most adequate measures of hepatic fibrosis in the general population owing to their low sensitivity for the non-advanced fibrosis [42].
Despite these limitations, our study has several strengths. This is the first large population study showing the association between myosteatosis and NAFLD as well as its severity and liver fibrosis. Previous studies were either large population studies measuring muscle mass through bioelectrical impedance analysis [3] and dual energy X-ray absorptiometry [2,4,16,17] or small population studies using abdominal CT [23]. Additionally, we divided the abdominal muscle area according to muscle quality: good quality (NAMA) and poor quality (IMAT and LAMA). This was the first attempt to suggest the NAMA/TAMA index, which is originally developed in our group [25], as a predicting factor for NAFLD and liver fibrosis. The NAMA/TAMA index may help identify subjects at a high risk of NAFLD and liver fibrosis for further liver evaluation (e.g., transient elastography or liver biopsy) and proper treatment.
In conclusion, a higher proportion of good quality muscle was associated with a significantly lower prevalence of NAFLD and liver fibrosis. Conversely, poor muscle quality is suggested to be a potential risk factor for NAFLD and liver fibrosis. Therefore, subjects with previous abdominal CT scans may benefit from measuring muscle area and quality to evaluate the risk of NAFLD. By encouraging intensive lifestyle modification, individuals at high risk may improve their muscle quality and prevent NAFLD progression. Further prospective studies applying the new index to identify the risk status of individuals are necessary for use in clinical practice.
SUPPLEMENTARY MATERIALS
Supplementary materials related to this article can be found online at https://doi.org/10.4093/dmj.2022.0081.
Baseline laboratory and clinical characteristics of the study population
The risk of NAFLD according to myosteatosis indices in the total population
The risk of NAFLD defined by surrogate markers according to myosteatosis indices in the total population
AUC curves of myosteatosis indices for NAFLD risk and severity
Correlation between plasma hsCRP and myostatosis indices
Flow diagram showing the selection of the study population. CT, computed tomography; HBsAg, hepatitis B surface antigen; HCV Ab, hepatitis C antibody; T4, thyroxine; TSH, thyroid stimulating hormone; eGFR, estimated glomerular filtration rate.
Graphical summary of skeletal muscle area and quality according to the computed tomography density. SFat, subcutaneous fat; VFat, visceral fat; IMAT, intermuscular adipose tissue; LAMA, low attenuation muscle area; NAMA, normal attenuation muscle area; SMA, skeletal muscle area; TAMA, total abdominal muscle area.
Scatter plot of the correlation between myosteatosis indices and nonalcoholic fatty liver disease (NAFLD) fibrosis score (NFS). SMA, skeletal muscle area; BMI, body mass index; NAMA, normal attenuation muscle area; TAMA, total abdominal muscle area; LAMA, low attenuation muscle area.
Notes
CONFLICTS OF INTEREST
Chang Hee Jung has been associate editor of the Diabetes & Metabolism Journal since 2022. He was not involved in the review process of this article. Otherwise, there was no conflict of interest.
AUTHOR CONTRIBUTIONS
Conception or design: H.K.K., C.H.J.
Acquisition, analysis, or interpretation of data: H.S.K., J.L., E.H.K., M.J.L., W.J.L., J.Y.P., C.H.J.
Drafting the work or revising: H.S.K., H.K.K., C.H.J.
Final approval of the manuscript: H.S.K., J.L., E.H.K., M.J.L., I.Y.B., W.J.L., J.Y.P., H.K.K., C.H.J.
FUNDING
This work was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education (grant numbers: 2015R1D1A1A01057720, NRF- 2020R1A2C1101977: Chang Hee Jung).
Acknowledgements
The authors thank Editage for the English language review.