
- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Articles
- Page Path
- HOME > Diabetes Metab J > Volume 46(2); 2022 > Article
-
ReviewOthers Links between Thyroid Disorders and Glucose Homeostasis
-
Young Sil Eom1
 , Jessica R. Wilson2, Victor J. Bernet2
, Jessica R. Wilson2, Victor J. Bernet2
-
Diabetes & Metabolism Journal 2022;46(2):239-256.
DOI: https://doi.org/10.4093/dmj.2022.0013
Published online: March 24, 2022
1Division of Endocrinology, Department of Internal Medicine, Gachon University Gil Medical Center, Gachon University College of Medicine, Incheon, Korea
2Division of Endocrinology, Diabetes and Metabolism, Mayo Clinic, Jacksonville, FL, USA
-
Corresponding author: Victor J. Bernet
 Division of Endocrinology, Diabetes and Metabolism, Mayo Clinic, 4500 San Pablo Road, Jacksonville, FL 32224, USA E-mail: Bernet.Victor@mayo.edu
Division of Endocrinology, Diabetes and Metabolism, Mayo Clinic, 4500 San Pablo Road, Jacksonville, FL 32224, USA E-mail: Bernet.Victor@mayo.edu
Copyright © 2022 Korean Diabetes Association
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
- Thyroid disorders and diabetes mellitus often coexist and are closely related. Several studies have shown a higher prevalence of thyroid disorders in patients with diabetes mellitus and vice versa. Thyroid hormone affects glucose homeostasis by impacting pancreatic β-cell development and glucose metabolism through several organs such as the liver, gastrointestinal tract, pancreas, adipose tissue, skeletal muscles, and the central nervous system. The present review discusses the effect of thyroid hormone on glucose homeostasis. We also review the relationship between thyroid disease and diabetes mellitus: type 1, type 2, and gestational diabetes, as well as guidelines for screening thyroid function with each disorder. Finally, we provide an overview of the effects of antidiabetic drugs on thyroid hormone and thyroid disorders.
- Thyroid disorders and diabetes mellitus (DM) are common chronic endocrine disorders that often coexist. A higher prevalence of thyroid disorders is seen in patients with type 1 diabetes mellitus (T1DM) [1] and type 2 diabetes mellitus (T2DM) compared to the general population [2,3]. In addition, a higher prevalence of DM is noted in patients with thyroid disorders [4-6]. While increased medical surveillance in patients with DM or thyroid disorders may account for this trend, potential pathophysiological mechanisms do exist that might explain the development of thyroid disorders in patients with DM and also the increased risk for development of DM in individuals with thyroid disorders. Autoimmunity is an important element for understanding the linkage between T1DM and auto-immune thyroid disease (AITD) while the relationship between T2DM and thyroid disorders is more complex. The aim of this article is to review the links between alterations in glucose homeostasis, including diabetes, as related to the presence of thyroid dysfunction.
INTRODUCTION
- Glucose homeostasis
- Plasma glucose levels are balanced by glucose entry and removal from the circulation. Glucose entering the circulation is derived from intestinal absorption during the fed state, glycogenolysis and gluconeogenesis. The rate of gastric emptying mainly determines the rate of glucose appearance in the circulation while hepatic breakdown of glycogen (glycogenolysis) and formation of glucose from non-carbohydrate substrates such as lactate, amino acids, and glycerol (gluconeogenesis) are other important sources of circulating glucose [7]. Glucose homeostasis is the tight regulation of blood glucose levels critical to maintain life in mammals. This is achieved by a balance of hormone and neuropeptides released from the brain, pancreas, liver, intestine, adipose, and muscle tissue [8]. Insulin and glucagon are the most important hormones for glucose homeostasis. Insulin is a pancreatic β-cell hormone that lowers blood glucose concentration in three ways: (1) promoting glucose uptake by the cells of insulin-sensitive peripheral tissues, including skeletal muscle and adipocytes; (2) promoting storage of glucose as glycogen, or conversion to fatty acids in the liver; and (3) suppression of postprandial glucagon secretion [7]. Glucagon is secreted from pancreatic α-cells and plays a major role in sustaining plasma glucose during the fasting state. When plasma glucose level falls below the normal level, glucagon secretion rises, leading to hepatic glucose production and return of plasma glucose to normal range [9]. Glucose homeostasis is also regulated by other glucoregulatory hormones like amylin, glucagon-like peptide-1 (GLP-1), gastric inhibitory polypeptide (GIP), epinephrine, cortisol, and growth hormone (GH). Amylin is isolated from pancreatic amyloid deposits and co-secreted with insulin by pancreatic β-cells in response to nutrient stimuli [10]. Amylin suppresses postprandial glucagon secretion, slows gastric emptying and reduces food intake and body weight [7]. GLP-1 and GIP, incretin hormones are secreted by intestinal L-cells. GIP stimulates insulin secretion but does not inhibit glucagon secretion or gastric emptying. GLP-1 not only stimulates glucose-dependent insulin secretion but also suppresses postprandial glucagon secretion. It also slows gastric emptying and reduces food intake and thereby body weight [11]. Blood glucose levels can also be sensed by the central nervous system [12]. In response to low plasma glucose, the ventromedial hypothalamus triggers release of counterregulatory hormones [13]. After a meal, glucose is absorbed and plasma glucose levels rise. Increasing glucose levels stimulate pancreatic β-cells to secrete insulin. Insulin increases glucose disposal by glycogen synthesis and lipogenesis in the liver, and promotes the uptake of glucose and conversion to glycogen or triglycerides by skeletal muscle and adipose tissue. In contrast, during a fasting state, glucagon is released to increase blood glucose levels through glycogenolysis. When fasting is prolonged, glucose is produced by hepatic gluconeogenesis (Fig. 1) [7].
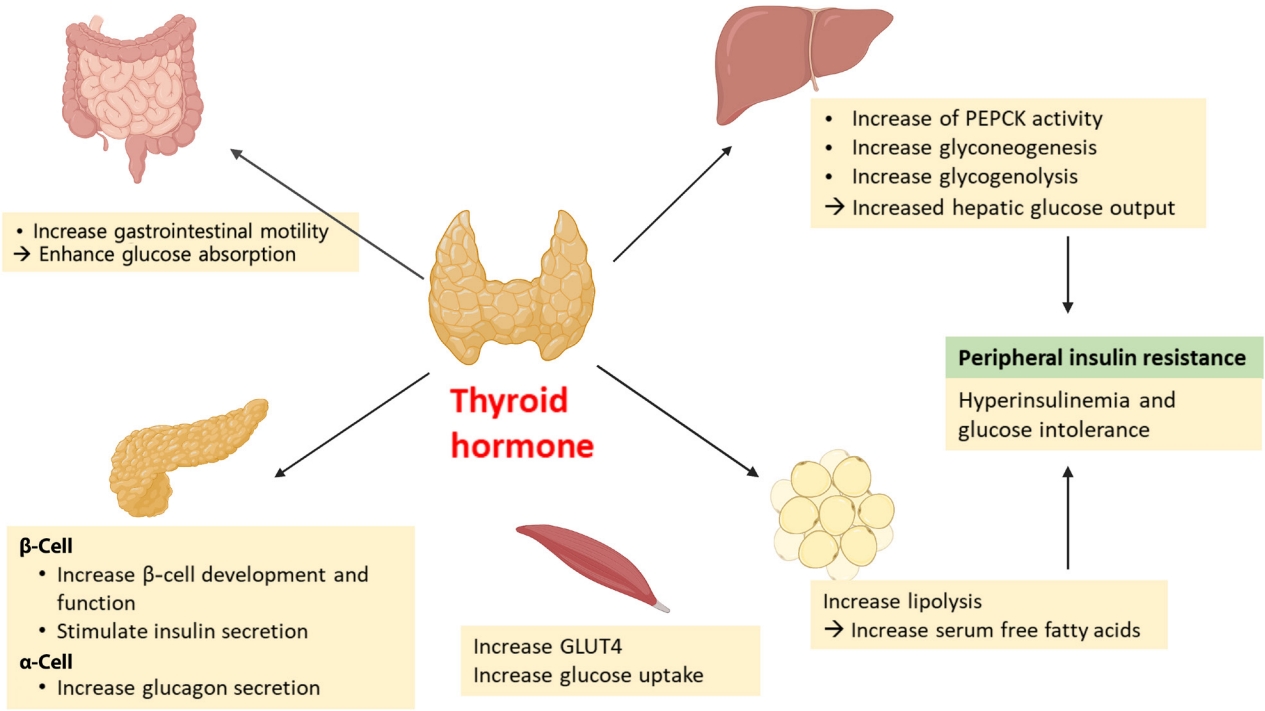
- The effects of thyroid hormone on glucose homeostasis
- Thyroid hormone (TH) has long been known to affect glucose homeostasis. TH has been reported to be related to pancreatic β-cell development and influence glucose metabolism through several organs such as the liver, gastrointestinal tract, pancreas, adipose tissue, skeletal muscles, and the central nervous system (Fig. 2).
- Several studies have shown links between TH action and pancreatic β-cell development and function [14,15]. Neonatal β-cells have thyroid hormone receptors (THRs). Aguayo-Mazzucato et al. [14] have described the role of TH and THRs in postnatal rat pancreatic β-cell development. Specifically, triiodothyronine (T3) stimulates β-cell proliferation from birth through the first week of life. THR also has a direct receptor-ligand interaction with the MAF bZIP transcription factor A (MAFA) promotor. In addition, T3 increases glucose-stimulated insulin secretion in vitro. In vivo, T3 administration acts on a pro-survival, anti-apoptotic factor for β-cells and improves glucose intolerance in streptozotocin-induced diabetic mice [15].
- TH impacts insulin secretion and glucose uptake via differing effects in the gastrointestinal tract, liver, skeletal muscles, and adipose tissue. TH enhances glucose absorption by increasing gastrointestinal motility [16]. In liver, it increases hepatic glucose output through increased hepatic expression of glucose transporter 2 (GLUT2) which stimulates the endogenous production of glucose through an increase in gluconeogenesis and glycogenolysis. T3 also increases hepatic gluconeogenesis by increasing activity of phosphoenolpyruvate carboxykinase (PEPCK), an enzyme that enhances gluconeogenesis [17,18]. Furthermore, stimulated glycogenolysis and gluconeogenesis induces hyperinsulinemia and glucose intolerance, resulting in peripheral insulin resistance [16]. In adipose tissue, TH increases lipolysis, resulting in an increase in free fatty acid that stimulates hepatic gluconeogenesis [19]. TH also directly stimulates insulin secretion by pancreatic β-cells and increases glucagon release by pancreatic α-cells [16]. Hyperthyroidism increases glucose transporter type 4 (GLUT4) gene expression and glucose uptake in skeletal muscles [16].
- T3 can centrally modulate glucose production through a sympathetic pathway from the hypothalamic paraventricular nucleus (PVN) to liver. T3 in the hypothalamic PVN increases hepatic glucose production, independent of glucoregulatory hormones [20].
GLUCOSE HOMEOSTASIS AND THYROID HORMONE
Thyroid hormone effects on the pancreatic β-cell
Peripheral effects of thyroid hormone on insulin secretion and resistance
Central interactions of thyroid hormones on glucose and lipid regulation
- Thyroid disorders and DM are closely linked. Many studies have demonstrated the higher prevalence of thyroid dysfunction in both T1DM and T2DM. The prevalence of thyroid disorders in a patient with DM is variable among the population studied. A large clinical study of 1,310 adult patients with diabetes reported a 13.4% overall prevalence of thyroid diseases. The prevalence was 31.4% in T1DM and 6.9% in T2DM, and 8.8% in men and 16.8% in women [21]. A meta-analysis of all available data in 10,920 patients with DM revealed an 11% prevalence of thyroid diseases. In this study, there was no difference between T1DM and T2DM, but the prevalence in women was more than twofold than that in men [22]. The fact that thyroid disorders increase the risk of DM has been reported in nationwide Danish health register study as follows. In an observational cohort study of 2,631 hyperthyroid singletons and 375 twin pairs discordant for hyperthyroidism, hyperthyroid individuals were 43% more likely to be diagnosed with DM [4]. In separate observational cohort study of 2,822 individuals with hypothyroidism, the group with hypothyroidism had a 40% higher prevalence of DM compared to controls [6].
- Thyroid disorders and T1DM
- T1DM accounts for 5% to 10% of diabetes cases and stems from cell-mediated autoimmune destruction of pancreatic β-cells [23]. Related autoimmune markers include autoantibodies to insulin, glutamic acid decarboxylase 65 (GAD65), the tyrosine phosphatase-related islet antigen-2 (IA-2) and IA2b, and zinc transporter 8 (ZnT8). Patients with T1DM are also at increased risk for development of other autoimmune disorders, such as: Hashimoto thyroiditis, Graves’ disease, celiac disease, Addison disease, autoimmune hepatitis, vitiligo, myasthenia gravis, and pernicious anemia [1]. AITD and T1DM are related target-organ autoimmune diseases with AITD being noted in 17% to 30% of adults with T1DM and with increased risk being observed in both autoimmune hypothyroidism and hyperthyroidism [24]. This association is linked to the expression of thyroid autoantibodies like thyroid peroxidase antibody (TPO Ab) and thyroglobulin antibody (TG Ab) [25,26]. A study that evaluated the prevalence of various autoantibodies in a population of 814 individuals with T1DM revealed that TPO Ab and Tg Ab were the most common autoantibodies with a proportion of 29% [27].
- There is significant evidence for a genetic association between the two diseases [28]. These two diseases frequently occur within the same family and even within the same individual. AITD and T1DM often occur in the same individual or more than one member of the same family, as the phenotype classified as one of the autoimmune polyglandular syndrome 3 variant (APS3), especially APS type 3A [29,30]. Both T1DM and AITD are multifactorial autoimmune endocrine diseases with several susceptibility genes and environmental factors contributing to disease etiology. The human leukocyte antigen (HLA) class II, cytotoxic T lymphocyte antigen-4 (CTLA-4) (on chromosome 2q33), protein tyrosine phosphatase non-receptor type 22 (PTPN22) (1p13), forkhead box P3 (FOXP3) (Xp 11), and interleukin 2 receptor α (IL-2Rα) (10p15) are major genes found to contribute to a joint susceptibility for T1DM and AITD [29,31,32]. These genes are immunologically linked, influencing T cell activity. The HLA-DR molecules present autoantigens to T cells, and CTLA-4 is expressed on T cells and acts as a costimulatory receptor which downregulates T cells. PTPN22 is a negative regulator of T cell receptor signaling pathway, and FOXP3 regulates the differentiation of regulatory T cells, while IL-2Rα actively suppresses autoreactive T cells via CD25 (ɑ chain of the high-affinity IL-2 receptor) [29,32].
- Thyroid dysfunction is especially common in T1DM, particularly those with positive TPO Abs [25]. In these patients, thyroid diseases may be asymptomatic or the symptoms may be masked by symptoms related to poor diabetic control. Thus, a symptom-based approach to thyroid disease can miss the diagnosis in T1DM. Routine screening may help detect thyroid disease in such scenarios. Currently, screening for thyroid dysfunction is recommended in children, adolescents, and adults with T1DM by the American Thyroid Association (ATA) [33], the British Thyroid Association (BTA) [34], the American Diabetes Association (ADA) [35], the American Association of Clinical Endocrinologist (AACE) [36], National Institute for Health and Care Excellence (NICE) [37], and the International Society for Pediatric and Adolescent Diabetes (ISPAD) [38] but not by the United States Preventive Service Task Force (USPSTF) (Table 1) [39].
- Thyroid disorders and T2DM
- The glucose homeostasis disorder, insulin resistance, is defined as the decreased metabolic response to insulin in the peripheral tissues such as muscle, liver, and adipose [40]. To maintain glucose homeostasis, pancreatic β-cells compensate for insulin resistance by augmenting insulin secretion, leading to a state of chronic hyperinsulinemia. Insulin resistance plays a role in the pathogenesis of T2DM. Thyroid function is associated with insulin resistance not only in clinically diagnosed DM but also in subjects with a normal glucose tolerance [41,42]. Both hyperthyroidism and hypothyroidism can affect insulin resistance, although by different mechanisms.
- The prevalence of hyperthyroidism is higher in patients with diabetes than in the general population. Approximately 4.4% of adults with T2DM also had hyperthyroidism compared to an average of 1.3% in the general United States population [2,21]. Thyrotoxicosis has been recognized to lead to hyperglycemia via several mechanism [43]. Excess TH can increase glucose absorption by the gastrointestinal tract, glucose production in the liver via increasing gluconeogenesis and glycogenolysis, and the concentration of free fatty acids via promoting lipolysis [16]. TH stimulates insulin secretion and induces hyperinsulinemia, although thyrotoxicosis also accelerates insulin degradation and decreases the half-life of insulin [44]. Furthermore, the increased hepatic glucose output induces hyperinsulinemia, glucose intolerance, and peripheral insulin resistance. Thus, hyperthyroidism precipitates impaired fasting glucose and/or diabetes and worsens glycemic control in pre-existing T2DM. Moreover, thyrotoxicosis may rarely precipitate diabetic ketoacidosis not only in patients with T1DM, but also in patients with T2DM when accompanied by insulin deficiency which can enhance the risk for lipolysis [16,45,46].
- The prevalence of hypothyroidism is higher in patients with diabetes than in the general population. The prevalence of hypothyroidism in T2DM has been reported to be between 5.7% and 25.3% [47-49]. These wide range could be explained by differences in age, sex, and the degree of iodine intake of the populations surveyed [50]. The prevalence of hypothyroidism increased in older age, female gender, with family history of thyroid disease and positive TPO Ab [51].
- Hypothyroidism is associated with insulin resistance and glucose intolerance with treatment of hypothyroidism and a return to a euthyroid states being associated with improved insulin sensitivity [41,52,53]. The pathogenesis of insulin resistance in a hypothyroid state is different from that seen with thyrotoxicosis. Hypothyroidism results in impaired glucose absorption from the gastrointestinal tract, delayed peripheral glucose assimilation, and gluconeogenesis [41]. Insulin resistance is thought to be associated with decreased glucose disposal, a result of decreased skeletal muscle and adipose tissue sensitivity to insulin [54,55]. This deterioration of glucose metabolism is also seen in subclinical hypothyroidism. One meta-analysis study revealed that the risk of developing subclinical hypothyroidism increased by 1.93-fold in T2DM patients compared to non-diabetics and that subclinical hypothyroidism may also be associated with an increase in diabetic complications [56]. Free thyroxine (T4) levels in the lower end of reference range are also associated with higher glycosylated hemoglobin (HbA1c) levels in euthyroid state [57].
- Contrary to the guideline for T1DM, there is a lack of recommendations for screening for thyroid disease in patients with T2DM. The ATA recommended that adults who are at least 35 years old should be screened for thyroid disorders by measuring a thyroid-stimulating hormone (TSH) concentration and then every 5 years in the 2010 guidelines. Although there is no mention of DM type, the guideline recommends more frequent tests in those with high risk factors like diabetes [33]. The United Kingdom 2006 guidelines recommended a thyroid function test at the time of diagnosis for patients with T2DM but not annual testing [34]. Conversely, USPSTF reports that there is insufficient evidence to recommend thyroid dysfunction screening in non-pregnant or asymptomatic adults in 2015 [39]. There is no mention of monitoring thyroid function in T2DM in the 2015 NICE guidelines [58], the ADA guideline [59], the ATA/AACE guideline [60], and the ISPAD guideline (Table 2) [61,62]. Therefore, the necessity of a screening for thyroid dysfunction in patients with T2DM is debated at this time.
- Thyroid disorders in pregnant women with diabetes
- During pregnancy, numerous hormonal and metabolic changes occur, which affect glucose metabolism and thyroid function [63]. In a normal pregnancy, a 50% to 60% decrease in insulin sensitivity occurs in both pregnant women with normal glucose tolerance and women with gestational diabetes. In women with normal glucose tolerance, the decrease of insulin sensitivity is overcome by increased insulin secretion from pancreatic β-cells, whereas gestational diabetes occurs when insulin secretion is not sufficient to match this higher demand [64].
- During pregnancy, the maternal thyroid gland increases in size by 10% in iodine replete countries but by 20% to 40% in iodine deficient countries [65]. Production of THs, T4, and T3 increases by almost 50% and for this reason, iodine daily intake should also be increased by 50%. The World Health Organization recommends daily iodine intake of 250 μg for pregnant and lactating women [66]. In early pregnancy, the placenta makes human chorionic gonadotropin (hCG) which is a member of the glycoprotein hormone family that is composed of common α-subunits and a hormone-specific β-subunits. The hCG α-subunit sequence is identical to the sequences of the α-subunits of TSH, follicle-stimulating hormone and luteinizing hormone [67]. Increased hCG concentration during the first trimester of pregnancy stimulates the thyroid gland thereby increasing production and release of THs resulting in decrease of TSH [66]. Hyperemesis gravidarum is extreme, persistent nausea and vomiting during pregnancy which can lead to dehydration, weight loss of at least 5% from pre-pregnancy baseline, ketonuria, and electrolyte imbalances. The exact cause is not fully understood, but it is believed to be caused by a rapid rising blood level of hCG [68,69]. hCG is a thyroid stimulator and most transient non-immune hyperthyroidism in early pregnancy is related to increased hCG levels while rare cases of familial recurrent gestational hyperthyroidism are caused by a mutant thyrotropin receptor that is hypersensitive to hGC as has been reported with even normal range serum hCG [70].
- Thyroid dysfunction and DM are the common endocrinopathies during pregnancy and can affect both maternal and fetal health [71,72]. Thyroid function abnormalities are more common in pregnant women in the first trimester [73] with hypothyroidism, hyperthyroidism, and thyroid autoimmunity being common conditions in pregnancy, with prevalence rates of 2%–3%, 0.1%–0.4%, and up to 17% [74-76]. A higher prevalence of hypothyroidism in women with gestational diabetes mellitus (GDM) has been reported [77] and this association is also seen in postpartum thyroiditis (PPT), which is an autoimmune-mediated destructive thyroiditis in the 1st year postpartum. According to the studies, PPT is also three to four times more common in women with T1DM than in healthy women [78-80].
- Considering that THs affect glucose homeostasis, thyroid disorders have been thought to affect the development of gestational diabetes or glucose control of pregnant women with diabetes. Pregnant women with either overt hypothyroidism or subclinical hypothyroidism have an increased risk of GDM [77,81]. There are also reports that maternal isolated hypothyroxinaemia (normal TSH concentration in conjunction with a low free T4) is associated with adverse metabolic parameters including increased maternal body mass index, higher fasting and postprandial glucose, higher HbA1c, higher triglycerides and increased insulin resistance (homeostasis model assessment of insulin resistance) in pregnancy [82,83]. Recently, there is a report that higher free T3 levels in early pregnancy may correlate with a greater risk for GDM developing compared to women who have normal levels of this hormone [84].
- Untreated overt hypothyroidism and hyperthyroidism are associated with adverse obstetrical and fetal outcomes such as spontaneous abortion, preeclampsia, preterm birth, abruptio placentae, poor fetal growth, and stillbirth [85,86]. Subclinical hypothyroidism has been related to adverse obstetrical outcomes such as abruptio placentae, preterm birth, and impaired neurodevelopment in offspring [87,88]. However, detection and treatment of maternal subclinical hypothyroidism has also not been shown to cause improved pregnancy outcome and neurocognitive function in offspring [89,90]. Therefore, clinical guidelines do not recommend universal thyroid function screening in pregnant women, but instead limited to women who have signs and symptoms of possible thyroid dysfunction and high-risk groups including those with T1DM or family history of thyroid disease. Similarly, screening for thyroid dysfunction is recommended in pregnant women with T1DM by the ATA [66], the BTA [34], the Endocrine Society [91], the AACE [60], the Korean Thyroid Association (KTA) [92], and the American College of Obstetricians and Gynecologists (ACOG) (Table 3) [93].
THYROID DISORDERS AND DIABETES MELLITUS
Association of AITD and T1DM
Screening recommendation for thyroid dysfunctions in patients with T1DM
Thyroid disorders and insulin resistance
Thyrotoxicosis and T2DM
Hypothyroidism and T2DM
Screening recommendation for thyroid dysfunction in patients with T2DM
Changes in thyroid function during pregnancy and effect on glucose homeostasis
Screening recommendation for thyroid dysfunction in pregnant patients
- The use of certain antidiabetic drugs in patients with T2DM may influence thyroid function. We explore common oral antidiabetic agents and their impact on TH levels here (Table 4).
- Metformin
- Metformin is a wildly used oral antidiabetic drug for treatment of T2DM. Metformin suppresses hepatic gluconeogenesis via stimulating adenosine monophosphate-activated kinase (AMPK) in the liver, but has the opposite effect inhibiting AMPK in the hypothalamus [94]. Metformin has been shown to cross the blood-brain barrier and its concentrations in the hypothalamus match those in plasma in rat experiments, and substantially metformin levels in the pituitary gland increase as well [95]. These effects may fit with some clinical results, indicating that metformin may inhibit pituitary TSH secretion. It was first reported that metformin therapy decreases serum TSH levels in patients with T2DM with primary hypothyroidism in 2006 [96]. Several studies have shown that metformin administration in diabetic patients with primary hypothyroidism is associated with a reduction in serum TSH levels and this effect was observed in both patients under TH replacement therapy and in untreated patients [97,98]. The TSH-lowering effect of metformin was observed in patients with overt hypothyroidism and subclinical hypothyroidism, but not in euthyroid patients [98,99]. Despite the reduction in TSH, there is no change in [96] and plasma free T4 and free T3 concentrations, and the effect was not associated with clinical hyperthyroidism. In addition, this effect was reversible and usually resolved by 3 months after discontinuation [97,98].
- Metformin has been shown to have beneficial effects in various types of cancers such as colon, rectum, pancreas, breast and prostate cancer [100]. Metformin inhibits the mammalian target of rapamycin (mTOR) activity by activating liver kinase B1 (LKB1) and AMPK, and thus prevents protein synthesis and cell growth. Metformin also can activate p53 by activating AMPK and thereby ultimate stop the cell cycle [101,102]. One study showed that metformin significantly decreased thyroid nodule size by 30%–50% in patients with insulin resistance [103]. Studies on the anti-tumor effect of metformin in thyroid cancer have been conducted, and some studies have reported that metformin inhibits thyroid cancer cell growth and induces cell-cycle arrest and apoptosis through AMPK activation and mTOR inhibition in vivo and in vitro [104-107]. A retrospective cohort study of 128,453 Korean adult diabetic patients reported that thyroid cancer decreased significantly in metformin users compared to metformin nonusers [108]. These findings suggest that metformin could be of potential benefit in thyroid cancer, especially in diabetic patients, but additional studies are required.
- Sulfonylureas
- Sulfonylurea drugs have been used in the treatment of T2DM since 1950’s. These drugs have been reported to have both antithyroid and goitrogenic activity. Early studies showed that large doses of sulfonylureas increase the weight of the thyroid gland and reduced iodine content and fixation of radioiodine uptake in animal models [109,110]. It has been reported that hypothyroidism occurs more frequently in diabetic patients taking first-generation sulfonylureas than in patients treated with diet alone or insulin [111]. However, studies on the second-generation sulfonylureas, glibenclamide, and gliclazide have shown that the use of these agents appear to have no influence on TH metabolism [112,113].
- Thiazolidinediones
- Thiazolidinediones (TZDs) are an important class of insulin sensitizers used in the treatment of T2DM. TZDs were known to act by increasing the transactivation activity of peroxisome proliferator-activated receptors (PPARs). TZDs are agonists of PPAR-γ, can reduce hepatic glucose production, increase peripheral glucose utilization and lipid metabolism [114]. PPAR-γ is found predominantly in adipose tissue and plays a dominant role in adipocyte differentiation [115]. PPAR-γ is also strongly expressed in thyroid tissue of patients with thyroiditis and Graves’ disease and in the orbital tissue of patients with thyroid eye disease (TED) [116]. TED is characterized by swelling of extraocular muscles and an extension of adipose tissue volume within the confines of the bony orbit [117]. TSH receptor expression in TED orbital preadipocyte fibroblasts is linked to adipogenesis, and stimulation of the PPAR-γ receptor results in differentiation of these cells. Rosiglitazone treatment of orbital preadipocyte fibroblasts from patients with TED revealed stimulation of both adipocyte differentiation and TSH-dependent cyclic adenosine monophosphage production in these cells [117]. In some patients with T2DM, it has been reported that eye protrusion can worsen while taking TZDs [118,119]. Thus, TZDs should be used carefully in patients with T2DM with clinically active TED.
- The result from one study suggests that rosiglitazone administration in T2DM may reduce the risk of thyroid cancer [120]. The relationship between TZDs and thyroid cancer, however, has not been clearly elucidated.
- Incretin mimetics: GLP-1 receptor agonists and dipeptidyl peptidase-4 inhibitors
- Incretin mimetics are agents that act like or increase endogenous incretin hormones, including glucagon-like peptide-1 receptor (GLP-1R) agonists and dipeptidyl peptidase 4 (DPP4) inhibitors, respectively. GLP-1R agonists have shown association with C-cell proliferation and potential for increased risk of medullary thyroid cancer (MTC) in preclinical studies in rats [121-123]. Long-term dosing with liraglutide causes C-cell hyperplasia, calcitonin production in a dose dependent manner, and tumor formation in rodents [121-123]. This effect, however, was not seen in monkeys and humans. Prolonged use of liraglutide at very high doses did not produce C-cell proliferation in monkeys and did not induce significant calcitonin level changes in human clinical studies [124,125]. Exenatide also did not increase calcitonin in follow-up [126]. Similarly, in the Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes (LEADER) Trial, liraglutide did not increase calcitonin or cause C-cell malignancies [127]. This may be due to C-cells in monkeys and humans having lower expression of GLP-1R and decreased responsiveness to GLP-1R agonists compared to rodents [125]. The Food and Drug Administration (FDA) does not recommend monitoring for the occurrence of MTC in patients taking GLP-1R agonists; however, the use of GLP-1 R agonists in patients with a personal of family history of MTC or multiple endocrine neoplasia (MEN) type 2 is not recommended.
- The relationship between DPP4 inhibitors and thyroid cancer has not been well reported. One study with sitagliptin among Taiwanese T2DM patients showed an increased prevalence of thyroid cancer. This was during the first year of treatment; however, and the impact of DPP4 inhibitor to this finding is thought to not necessarily be directly related given this short time interval and lack of additional supportive data [128].
- Incretin mimetics have been shown to alter TSH concentration; however, this does not appear to be clinically significant. The GLP-1R agonist, exenatide, decreased TSH concentrations in diabetics after 6 months. Levels remained in the detectable range, and there were no significant changes in free T4, free T3, or thyroid volume [126]. A small study reported that diabetics followed for over three years taking a DPP4 inhibitor compared to diabetics on alternative therapy had higher TSH concentrations. TSH was still within the normal range and free T4 concentration was not significantly different between the two groups [129]. Larger, longer-term studies are warranted.
- Colesevelam
- Bile acid sequestrants (BASs) were developed as lipid-lowering agents for the treatment of hypercholesterolemia and include cholestyramine, colesevelam, colestilan, colestimide, and colestipol. BASs significantly reduce low-density lipoprotein cholesterol levels and improve cardiovascular outcomes in a population of asymptomatic middle-aged men with primary hypercholesterolemia [130]. BASs also lower glucose levels in patients with T2DM [131-134]. Among BAS, colesevelam was approved in 2008 by the U.S. FDA to improve glycemic control in adults T2DM patients [135]. TH including T4 and T3 are secreted into the bile. BASs can sequester T4 in the intestine and increase its fecal excretion, thus, restricting the enterohepatic reabsorption into the systemic circulation. Cholesytramine for example, is used for the treatment of thyrotoxicosis including thyroid storm because of this effect [136]. BSA agents can enhance the clearance of THs and may reduce the blood levels and effects of levothyroxine by interfering with the absorption of the drug. Cholestyramine and colestipol, inhibit absorption of levothyroxine through the binding of T4 to the basic anion copolymer resins in the gastrointestinal tract. Colesevelam is a nonabsorbed polymer that binds to bile acids in the small intestine, thereby preventing their reabsorption. Although it has been proposed that the binding of colesevelam to other drugs should be less problematic than that seen with the older BASs, it is probable that it too binds levothyroxine and decreases its absorption [137]. Therefore, caution should be taken when using colesevelam and levothyroxine together.
- Other agents
- Limited data are available on the potential impact of acarbose on thyroid function and thyroid cancer risk/benefit. Acarbose increased levels of TH in dexamethasone-induced hyperglycemic mice, although the mechanism of this is not well understood [138]. In a retrospective study, acarbose was associated with lower rate of papillary thyroid carcinoma growth [139].
- Similarly, limited data exist on the impact of sodium glucose transporter type 2 (SGLT2) inhibitors and TH. In animal models SGLT2 inhibitors have been theorized to potentially increase risk of MTC; however, this has not been shown in humans [140,141].
ANTIDIABETIC AGENTS AND THYROID DISORDERS
- Glucose is an essential energy resource for the survival and activity of the brain. As the brain is unable to store glucose itself, it is very important that adequate plasma glucose levels be maintained and available to the brain at all times. There are several protective mechanisms to prevent or remediate hypoglycemia. A decrease in insulin secretion and activation of the counterregulatory response are the main response mechanisms for avoidance or correction of hypoglycemia. Counterregulatory hormones that act to prevent hypoglycemia include: glucagon, epinephrine, GH, cortisol, and neurotransmitters [142]. Hypoglycemia is common in T1DM and T2DM, in patients using insulin or some oral hypoglycemia agents. Hypothyroidism is one of the most common endocrine disorders, and frequently coexists with T1DM or T2DM [25,143]. Hypothyroidism may contribute to hypoglycemia via affecting various hormones and nervous function. In secondary hypothyroidism caused by hypopituitarism, hypoglycemia may be induced by adrenocorticotropic hormone and GH deficiency, but the risk of hypoglycemia is also increased in patients with primary hypothyroidism. TH affects hypothalamic-pituitary-adrenal (HPA) function and reduces GH and cortisol secretion, thereby interfering with the appropriate counterregulatory response to insulin induced hypoglycemia. Primary hypothyroidism reduces basal and stimulated GH, by acting on both the hypothalamus and pituitary [144]. In addition, hypothyroid patients may have co-existing relative adrenal insufficiency, thus weakening HPA response to hypoglycemia [145]. Hypothyroidism also affects glucose homeostasis, and these changes may be related to hypoglycemia. In hypothyroid patients, gluconeogenesis is reduced in skeletal muscle and in adipose tissue [146] and glycogenolysis is impaired [147]. These changes induce a delayed recovery from hypoglycemia. There are also reports that hypothyroidism may contribute to hypoglycemia through mechanisms including potentially impaired glucagon response and slowed gastric emptying [148,149].
THYROID DISORDERS AND HYPOGLYCEMIA
- TH affects glucose homeostasis, and thyroid disorders and DM are associated with each other. Autoimmunity is an important element in the relationship between T1DM and AITD. Thyroid dysfunction, both hyperthyroidism and hypothyroidism, is associated with insulin resistance and T2DM. Hypothyroidism can also increase risk of hypoglycemia in some setting. The association between TH and DM is also seen in pregnant women with gestational or pre-existing T1DM or T2DM. Screening for thyroid dysfunction is usually recommended in patients with T1DM, but there is a lack of recommendations in the screening for thyroid disease in patient with T2DM. Moreover, most guidelines do not recommend universal thyroid function screening in pregnant women, but instead limited to high-risk groups including T1DM. The use of certain antidiabetic agents influences thyroid function, TED, and thyroid cancer risk. Therefore, caution is required not only in interpreting TH levels when using these drugs in patients with T2DM, but also in using the drugs in patients with TED or risk of thyroid cancer. In conclusion, future research is warranted to further investigate the connections between DM and thyroid disorders and clinicians should take special consideration when evaluation patients with these conditions.
CONCLUSIONS
-
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
-
FUNDING
None
NOTES
-
Acknowledgements
- None
| Guideline | Screening recommendation | Comments |
|---|---|---|
| 2010 Guideline of the ATA for detection of thyroid dysfunction [33] | Patients with diabetes may require more frequent TSH monitoring. | Recommend TSH beginning at age 35 years and every 5 years thereafter, with more frequent monitoring if high risk factors such as diabetes. |
| BTA, the UK guidelines for the use of thyroid function tests, 2006 [34] | Annually | They note a high frequency of asymptomatic thyroid dysfunction in patients with T1DM and that screening is cost-effective. |
| ADA, standards of medical care in diabetes, 2008 [35] | Screen for TPO Ab and Tg Ab at diagnosis | |
| Check TSH after metabolic control established. If normal, recheck every 1–2 years, or sooner if signs/symptoms. Check FT4 if TSH is abnormal. | ||
| AACE, medical guidelines for clinical practice for the evaluation and treatment of hyperthyroidism and hypothyroidism, 2002 [36] | Examine for goiter. Check TSH regularly, especially if a goiter develops or if evidence is found of other autoimmune disorders. | They note that approximately 10% of patients with T1DM will develop chronic thyroiditis +/– subclinical hypothyroidism. |
| NICE guideline for type 1 diabetes in adults: diagnosis and management, 2015 [37] | Annually | |
| ISPAD, 2018 [38] | Check TSH and TPO Abs at diagnosis. If normal and asymptomatic, screen every other year. | |
| 2015 Recommendation statement of USP- STF: screening for thyroid dysfunction [39] | No specific recommendation | They conclude that the current evidence is insufficient to recommend screening for thyroid dysfunction in non-pregnant, asymptomatic adults. |
T1DM, type 1 diabetes mellitus; ATA, American Thyroid Association; TSH, thyroid-stimulating hormone (thyrotropin); BTA, British Thyroid Association; UK, United Kingdom; ADA, American Diabetes Association; TPO Ab, thyroid peroxidase antibody; Tg Ab, thyroglobulin antibody; FT4, free thyroxine; AACE, American Association of Clinical Endocrinologist; NICE, National Institute for Health and Care Excellence; ISPAD, International Society for Pediatric and Adolescent Diabetes guidelines; USPSTF, United States Preventive Service Task Force.
| Guideline | Screening recommendation | Comments |
|---|---|---|
| 2010 Guideline of the ATA for detection of thyroid dysfunction [33] | Patients with diabetes may require more frequent TSH measurement. | Recommend TSH beginning at age 35 years and every 5 years thereafter, with more frequent monitoring if high risk factors such as diabetes (does not distinguish between T1DM and T2DM) |
| BTA, the UK guidelines for the use of thyroid function tests, 2006 [34] | Check TSH at diagnosis. | They do not recommend routine annual screening. |
| 2015 Recommendation statement of USP- STF: screening for thyroid dysfunction [39] | No specific recommendation | They conclude that the current evidence is insufficient to recommend screening for thyroid dysfunction in non-pregnant, asymptomatic adults. |
| NICE guideline for type 2 diabetes in adults: management, 2015 [58] | No specific recommendation | |
| ADA, guideline 2018 [59] | No specific recommendation | |
| ATA/AACE, clinical practice guidelines for hypothyroidism in adults, 2012 [60] | No specific recommendation | |
| ISPAD, 2009 [61,62] | No specific recommendation |
T2DM, type 2 diabetes mellitus; ATA, American Thyroid Association; TSH, thyroid-stimulating hormone (thyrotropin); T1DM, type 1 diabetes mellitus; BTA, British Thyroid Association; UK, United Kingdom; USPSTF, United States Preventive Service Task Force; NICE, National Institute for Health and Care Excellence; ADA, American Diabetes Association; AACE, American Association of Clinical Endocrinologist; ISPAD, International Society for Pediatric and Adolescent Diabetes guidelines.
| Guideline | Screening recommendation | Comment |
|---|---|---|
| 2017 Guidelines of the ATA for the diagnosis and management of thyroid disease during pregnancy and the postpartum [66] | Check TSH in pregnant women with T1DM or other autoimmune disorders. | They do not recommend universal screening for patients who are pregnant or are planning pregnancy. Screening is recommended if one of the following risk factors: (1) A history of or current symptoms/signs of thyroid dysfunction, (2) thyroid antibody positivity or goiter, (3) prior head and neck radiation or thyroid surgery, (4) age >30 years, (5) T1DM or other autoimmune disorders, (6) prior pregnancy loss, preterm delivery, or infertility, (7) prior pregnancies (≥2), (8) family history of AITD or thyroid dysfunction, (9) morbid obesity (BMI ≥40 kg/m2), (10) use of amiodarone or lithium, or recent administration of iodinated radiologic contrast, (11) residing in an area of known iodine insufficiency. |
| BTA, 2006 UK guidelines for the use of thyroid function test [34] | Check TSH, FT4, and TPO Abs in women with T1DM prior to conception. Monitor thyroid function during pregnancy and 3 months post-partum. | They note that women with T1DM are three times more likely to develop post-partum thyroid dysfunction. |
| 2014 Endocrine Society Recommendation [91] | Check TSH in pregnant women with T1DM. | T1DM is considered a significant risk factor as are: current thyroid therapy, family history of AITD, goiter, history of autoimmune disorder, high-dose neck radiation, postpartum thyroid dysfunction, and previous delivery of infant with thyroid disease. |
| ATA/AACE, clinical practice guidelines for hypothyroidism in adults, 2012 [60] | They do not recommend universal screening for patients who are pregnant or are planning pregnancy. | |
| 2014 KTA guideline for the diagnosis and management of thyroid disease during pregnancy and postpartum [92] | Check TSH in pregnant women with T1DM. | They recommend screening early in pregnancy in the setting of T1DM or if other high risk factors. |
| ACOG practice bulletin, clinical management guideline for obstetrician-gynecologists: thyroid disease in pregnancy [93] | Check TSH in pregnant women with T1DM. | They do not recommend universal screening for thyroid disease in pregnancy. Indications include personal or family history of thyroid disease, T1DM, or clinical suspicion of thyroid disease. |
ATA, American Thyroid Association; TSH, thyroid-stimulating hormone (thyrotropin); T1DM, type 1 diabetes mellitus; AITD, autoimmune thyroid disease; BMI, body mass index; BTA, British Thyroid Association; UK, United Kingdom; FT4, free thyroxine; TPO Ab, thyroid peroxidase antibody; AACE, American Association of Clinical Endocrinologist; KTA, Korean Thyroid Association; ACOG, American College of Obstetricians and Gynecologists.
TSH, thyroid-stimulating hormone (thyrotropin); FT4, free thyroxine; FT3, free triiodothyronine; TZD, thiazolidinedione; TED, thyroid eye disease; T2DM, type 2 diabetes mellitus; GLP-1RA, glucagon-like peptide-1 receptor agonist; MTC, medullary thyroid cancer; MEN, multiple endocrine neoplasia; T4, thyroxine.
- 1. Nederstigt C, Corssmit EP, de Koning EJ, Dekkers OM. Incidence and prevalence of thyroid dysfunction in type 1 diabetes. J Diabetes Complications 2016;30:420-5.ArticlePubMed
- 2. Hollowell JG, Staehling NW, Flanders WD, Hannon WH, Gunter EW, Spencer CA, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab 2002;87:489-99.ArticlePubMed
- 3. Gu Y, Li H, Bao X, Zhang Q, Liu L, Meng G, et al. The relationship between thyroid function and the prevalence of type 2 diabetes mellitus in euthyroid subjects. J Clin Endocrinol Metab 2017;102:434-42.ArticlePubMed
- 4. Brandt F, Thvilum M, Almind D, Christensen K, Green A, Hegedus L, et al. Morbidity before and after the diagnosis of hyperthyroidism: a nationwide register-based study. PLoS One 2013;8:e66711.ArticlePubMedPMC
- 5. Gronich N, Deftereos SN, Lavi I, Persidis AS, Abernethy DR, Rennert G. Hypothyroidism is a risk factor for new-onset diabetes: a cohort study. Diabetes Care 2015;38:1657-64.ArticlePubMedPDF
- 6. Thvilum M, Brandt F, Almind D, Christensen K, Brix TH, Hegedus L. Type and extent of somatic morbidity before and after the diagnosis of hypothyroidism: a nationwide register study. PLoS One 2013;8:e75789.ArticlePubMedPMC
- 7. Aronoff SL, Berkowitz K, Shreiner B, Want L. Glucose metabolism and regulation: beyond insulin and glucagon. Diabetes Spectr 2004;17:183-90.ArticlePDF
- 8. Roder PV, Wu B, Liu Y, Han W. Pancreatic regulation of glucose homeostasis. Exp Mol Med 2016;48:e219.ArticlePubMedPMCPDF
- 9. Gerich J, Davis J, Lorenzi M, Rizza R, Bohannon N, Karam J, et al. Hormonal mechanisms of recovery from insulin-induced hypoglycemia in man. Am J Physiol 1979;236:E380-5.ArticlePubMed
- 10. Moore CX, Cooper GJ. Co-secretion of amylin and insulin from cultured islet beta-cells: modulation by nutrient secretagogues, islet hormones and hypoglycemic agents. Biochem Biophys Res Commun 1991;179:1-9.PubMed
- 11. Nauck MA, Holst JJ, Willms B, Schmiegel W. Glucagon-like peptide 1 (GLP-1) as a new therapeutic approach for type 2-diabetes. Exp Clin Endocrinol Diabetes 1997;105:187-95.ArticlePubMed
- 12. Levin BE, Routh VH, Kang L, Sanders NM, Dunn-Meynell AA. Neuronal glucosensing: what do we know after 50 years? Diabetes 2004;53:2521-8.PubMed
- 13. Borg WP, Sherwin RS, During MJ, Borg MA, Shulman GI. Local ventromedial hypothalamus glucopenia triggers counterregulatory hormone release. Diabetes 1995;44:180-4.ArticlePubMed
- 14. Aguayo-Mazzucato C, Zavacki AM, Marinelarena A, Hollister-Lock J, El Khattabi I, Marsili A, et al. Thyroid hormone promotes postnatal rat pancreatic β-cell development and glucose-responsive insulin secretion through MAFA. Diabetes 2013;62:1569-80.ArticlePubMedPMCPDF
- 15. Verga Falzacappa C, Mangialardo C, Madaro L, Ranieri D, Lupoi L, Stigliano A, et al. Thyroid hormone T3 counteracts STZ induced diabetes in mouse. PLoS One 2011;6:e19839.ArticlePubMedPMC
- 16. Nishi M. Diabetes mellitus and thyroid diseases. Diabetol Int 2018;9:108-12.ArticlePubMedPMCPDF
- 17. Park EA, Jerden DC, Bahouth SW. Regulation of phosphoenolpyruvate carboxykinase gene transcription by thyroid hormone involves two distinct binding sites in the promoter. Biochem J 1995;309(Pt 3):913-9.ArticlePubMedPMCPDF
- 18. Park EA, Song S, Vinson C, Roesler WJ. Role of CCAAT enhancer-binding protein beta in the thyroid hormone and cAMP induction of phosphoenolpyruvate carboxykinase gene transcription. J Biol Chem 1999;274:211-7.PubMed
- 19. Hage M, Zantout MS, Azar ST. Thyroid disorders and diabetes mellitus. J Thyroid Res 2011;2011:439463.ArticlePubMedPMCPDF
- 20. Klieverik LP, Janssen SF, van Riel A, Foppen E, Bisschop PH, Serlie MJ, et al. Thyroid hormone modulates glucose production via a sympathetic pathway from the hypothalamic paraventricular nucleus to the liver. Proc Natl Acad Sci U S A 2009;106:5966-71.ArticlePubMedPMC
- 21. Perros P, McCrimmon RJ, Shaw G, Frier BM. Frequency of thyroid dysfunction in diabetic patients: value of annual screening. Diabet Med 1995;12:622-7.ArticlePubMed
- 22. Kadiyala R, Peter R, Okosieme OE. Thyroid dysfunction in patients with diabetes: clinical implications and screening strategies. Int J Clin Pract 2010;64:1130-9.ArticlePubMed
- 23. Orzan A, Novac C, Mihu M, Tirgoviste CI, Balgradean M. Type 1 diabetes and thyroid autoimmunity in children. Maedica (Bucur) 2016;11:308-12.PubMedPMC
- 24. Roldan MB, Alonso M, Barrio R. Thyroid autoimmunity in children and adolescents with type 1 diabetes mellitus. Diabetes Nutr Metab 1999;12:27-31.PubMed
- 25. Umpierrez GE, Latif KA, Murphy MB, Lambeth HC, Stentz F, Bush A, et al. Thyroid dysfunction in patients with type 1 diabetes: a longitudinal study. Diabetes Care 2003;26:1181-5.PubMed
- 26. Kordonouri O, Klinghammer A, Lang EB, Gruters-Kieslich A, Grabert M, Holl RW. Thyroid autoimmunity in children and adolescents with type 1 diabetes: a multicenter survey. Diabetes Care 2002;25:1346-50.PubMed
- 27. Barker JM, Yu J, Yu L, Wang J, Miao D, Bao F, et al. Autoantibody “subspecificity” in type 1 diabetes: risk for organ-specific autoimmunity clusters in distinct groups. Diabetes Care 2005;28:850-5.PubMed
- 28. Huber A, Menconi F, Corathers S, Jacobson EM, Tomer Y. Joint genetic susceptibility to type 1 diabetes and autoimmune thyroiditis: from epidemiology to mechanisms. Endocr Rev 2008;29:697-725.ArticlePubMedPMC
- 29. Biondi B, Kahaly GJ, Robertson RP. Thyroid dysfunction and diabetes mellitus: two closely associated disorders. Endocr Rev 2019;40:789-824.ArticlePubMedPMCPDF
- 30. Villano MJ, Huber AK, Greenberg DA, Golden BK, Concepcion E, Tomer Y. Autoimmune thyroiditis and diabetes: dissecting the joint genetic susceptibility in a large cohort of multiplex families. J Clin Endocrinol Metab 2009;94:1458-66.ArticlePubMedPMC
- 31. Tomer Y, Menconi F. Type 1 diabetes and autoimmune thyroiditis: the genetic connection. Thyroid 2009;19:99-102.ArticlePubMed
- 32. Frommer L, Kahaly GJ. Type 1 diabetes and autoimmune thyroid disease: the genetic link. Front Endocrinol (Lausanne) 2021;12:618213.ArticlePubMedPMC
- 33. Ladenson PW, Singer PA, Ain KB, Bagchi N, Bigos ST, Levy EG, et al. American Thyroid Association guidelines for detection of thyroid dysfunction. Arch Intern Med 2000;160:1573-5.ArticlePubMed
- 34. British Thyroid Association, The Association for Clinical Biochemistry: UK guidelines for the use of thyroid function tests. Available from: http://www.british-thyroid-association.org/sandbox/bta2016/uk_guidelines_for_the_use_of_thyroid_function_tests.pdf (cited 2022 Feb 22).
- 35. American Diabetes Association. Standards of medical care in diabetes: 2008. Diabetes Care 2008;31 Suppl 1:S12-54.ArticlePubMedPDF
- 36. Baskin HJ, Cobin RH, Duick DS, Gharib H, Guttler RB, Kaplan MM, et al. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the evaluation and treatment of hyperthyroidism and hypothyroidism. Endocr Pract 2002;8:457-69.Article
- 37. National Institute for Health and Care Excellence: Type 1 diabetes in adults: diagnosis and management. Available from: https://www.nice.org.uk/guidance/ng17/resources/type-1-diabetesin-adults-diagnosis-and-management-pdf-1837276469701 (cited 2022 Feb 22).
- 38. Mahmud FH, Elbarbary NS, Frohlich-Reiterer E, Holl RW, Kordonouri O, Knip M, et al. ISPAD Clinical Practice Consensus Guidelines 2018: other complications and associated conditions in children and adolescents with type 1 diabetes. Pediatr Diabetes 2018;19(Suppl 27):275-86.ArticlePubMedPMCPDF
- 39. LeFevre ML; U.S. Preventive Services Task Force. Screening for thyroid dysfunction: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2015;162:641-50.ArticlePubMed
- 40. Gierach M, Gierach J, Junik R. Insulin resistance and thyroid disorders. Endokrynol Pol 2014;65:70-6.PubMed
- 41. Duntas LH, Orgiazzi J, Brabant G. The interface between thyroid and diabetes mellitus. Clin Endocrinol (Oxf) 2011;75:1-9.ArticlePubMed
- 42. Roos A, Bakker SJ, Links TP, Gans RO, Wolffenbuttel BH. Thyroid function is associated with components of the metabolic syndrome in euthyroid subjects. J Clin Endocrinol Metab 2007;92:491-6.ArticlePubMed
- 43. Maxon HR, Kreines KW, Goldsmith RE, Knowles HC Jr. Long-term observations of glucose tolerance in thyrotoxic patients. Arch Intern Med 1975;135:1477-80.ArticlePubMed
- 44. O’Meara NM, Blackman JD, Sturis J, Polonsky KS. Alterations in the kinetics of C-peptide and insulin secretion in hyperthyroidism. J Clin Endocrinol Metab 1993;76:79-84.ArticlePubMed
- 45. Eledrisi MS, Alshanti MS, Shah MF, Brolosy B, Jaha N. Overview of the diagnosis and management of diabetic ketoacidosis. Am J Med Sci 2006;331:243-51.ArticlePubMed
- 46. Potenza M, Via MA, Yanagisawa RT. Excess thyroid hormone and carbohydrate metabolism. Endocr Pract 2009;15:254-62.ArticlePubMed
- 47. Tamez-Perez HE, Martinez E, Quintanilla-Flores DL, TamezPena AL, Gutierrez-Hermosillo H, Diaz de Leon-Gonzalez E. The rate of primary hypothyroidism in diabetic patients is greater than in the non-diabetic population: an observational study. Med Clin (Barc) 2012;138:475-7.ArticlePubMed
- 48. Distiller LA, Polakow ES, Joffe BI. Type 2 diabetes mellitus and hypothyroidism: the possible influence of metformin therapy. Diabet Med 2014;31:172-5.ArticlePubMed
- 49. Al-Geffari M, Ahmad NA, Al-Sharqawi AH, Youssef AM, Alnaqeb D, Al-Rubeaan K. Risk factors for thyroid dysfunction among type 2 diabetic patients in a highly diabetes mellitus prevalent society. Int J Endocrinol 2013;2013:417920.ArticlePubMedPMCPDF
- 50. Chen G, Wu J, Lin Y, Huang B, Yao J, Jiang Q, et al. Associations between cardiovascular risk, insulin resistance, beta-cell function and thyroid dysfunction: a cross-sectional study in She ethnic minority group of Fujian Province in China. Eur J Endocrinol 2010;163:775-82.PubMed
- 51. Song F, Bao C, Deng M, Xu H, Fan M, Paillard-Borg S, et al. The prevalence and determinants of hypothyroidism in hospitalized patients with type 2 diabetes mellitus. Endocrine 2017;55:179-85.ArticlePubMedPDF
- 52. Chaker L, Ligthart S, Korevaar TI, Hofman A, Franco OH, Peeters RP, et al. Thyroid function and risk of type 2 diabetes: a population-based prospective cohort study. BMC Med 2016;14:150.ArticlePubMedPMCPDF
- 53. Joffe BI, Distiller LA. Diabetes mellitus and hypothyroidism: strange bedfellows or mutual companions? World J Diabetes 2014;5:901-4.ArticlePubMedPMC
- 54. Dubaniewicz A, Kaciuba-Uscilko H, Nazar K, Budohoski L. Sensitivity of the soleus muscle to insulin in resting and exercising rats with experimental hypo- and hyper-thyroidism. Biochem J 1989;263:243-7.ArticlePubMedPMCPDF
- 55. Rochon C, Tauveron I, Dejax C, Benoit P, Capitan P, Fabricio A, et al. Response of glucose disposal to hyperinsulinaemia in human hypothyroidism and hyperthyroidism. Clin Sci (Lond) 2003;104:7-15.ArticlePubMedPDF
- 56. Han C, He X, Xia X, Li Y, Shi X, Shan Z, et al. Subclinical hypothyroidism and type 2 diabetes: a systematic review and meta-analysis. PLoS One 2015;10:e0135233.ArticlePubMedPMC
- 57. Ha J, Lee J, Lim DJ, Lee JM, Chang SA, Kang MI, et al. Association of serum free thyroxine and glucose homeostasis: Korea National Health and Nutrition Examination Survey. Korean J Intern Med 2021;36(Suppl 1):S170-9.ArticlePubMedPDF
- 58. National Institute for Health and Care Excellence: Type 2 diabetes in adults: management. Available from: https://www.nice.org.uk/guidance/ng28/resources/type-2-diabetes-inadults-management-1837338615493 (cited 2022 Feb 22).
- 59. American Diabetes Association. 3. Comprehensive medical evaluation and assessment of comorbidities: standards of medical care in diabetes-2018. Diabetes Care 2018;41:S28-37.ArticlePubMedPDF
- 60. Garber JR, Cobin RH, Gharib H, Hennessey JV, Klein I, Mechanick JI, et al. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Endocr Pract 2012;18:988-1028.ArticlePubMed
- 61. Kordonouri O, Maguire AM, Knip M, Schober E, Lorini R, Holl RW, et al. Other complications and associated conditions with diabetes in children and adolescents. Pediatr Diabetes 2009;10 Suppl 12:204-10.ArticlePubMed
- 62. Rosenbloom AL, Silverstein JH, Amemiya S, Zeitler P, Klingensmith GJ. Type 2 diabetes in children and adolescents. Pediatr Diabetes 2009;10 Suppl 12:17-32.ArticlePubMed
- 63. Konar H, Sarkar M, Roy M. Association of thyroid dysfunction and autoimmunity in pregnant women with diabetes mellitus. J Obstet Gynaecol India 2018;68:283-8.ArticlePubMedPDF
- 64. Kampmann U, Knorr S, Fuglsang J, Ovesen P. Determinants of maternal insulin resistance during pregnancy: an updated overview. J Diabetes Res 2019;2019:5320156.ArticlePubMedPMCPDF
- 65. Vannucchi G, Covelli D, Vigo B, Perrino M, Mondina L, Fugazzola L. Thyroid volume and serum calcitonin changes during pregnancy. J Endocrinol Invest 2017;40:727-32.ArticlePubMedPDF
- 66. Alexander EK, Pearce EN, Brent GA, Brown RS, Chen H, Dosiou C, et al. 2017 Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. Thyroid 2017;27:315-89.ArticlePubMed
- 67. Hershman JM. Physiological and pathological aspects of the effect of human chorionic gonadotropin on the thyroid. Best Pract Res Clin Endocrinol Metab 2004;18:249-65.ArticlePubMed
- 68. Goodwin TM, Montoro M, Mestman JH, Pekary AE, Hershman JM. The role of chorionic gonadotropin in transient hyperthyroidism of hyperemesis gravidarum. J Clin Endocrinol Metab 1992;75:1333-7.ArticlePubMed
- 69. McCarthy FP, Lutomski JE, Greene RA. Hyperemesis gravidarum: current perspectives. Int J Womens Health 2014;6:719-25.PubMedPMC
- 70. Rodien P, Bremont C, Sanson ML, Parma J, Van Sande J, Costagliola S, et al. Familial gestational hyperthyroidism caused by a mutant thyrotropin receptor hypersensitive to human chorionic gonadotropin. N Engl J Med 1998;339:1823-6.ArticlePubMed
- 71. Johns EC, Denison FC, Norman JE, Reynolds RM. Gestational diabetes mellitus: mechanisms, treatment, and complications. Trends Endocrinol Metab 2018;29:743-54.ArticlePubMed
- 72. Korevaar TIM, Medici M, Visser TJ, Peeters RP. Thyroid disease in pregnancy: new insights in diagnosis and clinical management. Nat Rev Endocrinol 2017;13:610-22.ArticlePubMedPDF
- 73. Dieguez M, Herrero A, Avello N, Suarez P, Delgado E, Menendez E. Prevalence of thyroid dysfunction in women in early pregnancy: does it increase with maternal age? Clin Endocrinol (Oxf) 2016;84:121-6.ArticlePubMed
- 74. Klein RZ, Haddow JE, Faix JD, Brown RS, Hermos RJ, Pulkkinen A, et al. Prevalence of thyroid deficiency in pregnant women. Clin Endocrinol (Oxf) 1991;35:41-6.ArticlePubMed
- 75. Glinoer D. Thyroid hyperfunction during pregnancy. Thyroid 1998;8:859-64.ArticlePubMed
- 76. Ashoor G, Maiz N, Rotas M, Jawdat F, Nicolaides KH. Maternal thyroid function at 11 to 13 weeks of gestation and subsequent fetal death. Thyroid 2010;20:989-93.ArticlePubMed
- 77. Gong LL, Liu H, Liu LH. Relationship between hypothyroidism and the incidence of gestational diabetes: a meta-analysis. Taiwan J Obstet Gynecol 2016;55:171-5.ArticlePubMed
- 78. Gallas PR, Stolk RP, Bakker K, Endert E, Wiersinga WM. Thyroid dysfunction during pregnancy and in the first postpartum year in women with diabetes mellitus type 1. Eur J Endocrinol 2002;147:443-51.ArticlePubMed
- 79. Gerstein HC. Incidence of postpartum thyroid dysfunction in patients with type I diabetes mellitus. Ann Intern Med 1993;118:419-23.ArticlePubMed
- 80. Maleki N, Tavosi Z. Evaluation of thyroid dysfunction and autoimmunity in gestational diabetes mellitus and its relationship with postpartum thyroiditis. Diabet Med 2015;32:206-12.ArticlePubMed
- 81. Toulis KA, Stagnaro-Green A, Negro R. Maternal subclinical hypothyroidsm and gestational diabetes mellitus: a metaanalysis. Endocr Pract 2014;20:703-14.ArticlePubMed
- 82. Knight BA, Shields BM, Hattersley AT, Vaidya B. Maternal hypothyroxinaemia in pregnancy is associated with obesity and adverse maternal metabolic parameters. Eur J Endocrinol 2016;174:51-7.ArticlePubMedPMC
- 83. Bassols J, Prats-Puig A, Soriano-Rodriguez P, Garcia-Gonzalez MM, Reid J, Martinez-Pascual M, et al. Lower free thyroxin associates with a less favorable metabolic phenotype in healthy pregnant women. J Clin Endocrinol Metab 2011;96:3717-23.ArticlePubMedPDF
- 84. Rawal S, Tsai MY, Hinkle SN, Zhu Y, Bao W, Lin Y, et al. A longitudinal study of thyroid markers across pregnancy and the risk of gestational diabetes. J Clin Endocrinol Metab 2018;103:2447-56.ArticlePubMedPMCPDF
- 85. Casey BM, Leveno KJ. Thyroid disease in pregnancy. Obstet Gynecol 2006;108:1283-92.ArticlePubMed
- 86. Yazbeck CF, Sullivan SD. Thyroid disorders during pregnancy. Med Clin North Am 2012;96:235-56.ArticlePubMed
- 87. Casey BM, Dashe JS, Wells CE, McIntire DD, Byrd W, Leveno KJ, et al. Subclinical hypothyroidism and pregnancy outcomes. Obstet Gynecol 2005;105:239-45.ArticlePubMed
- 88. Haddow JE, Palomaki GE, Allan WC, Williams JR, Knight GJ, Gagnon J, et al. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N Engl J Med 1999;341:549-55.ArticlePubMed
- 89. Casey BM, Thom EA, Peaceman AM, Varner MW, Sorokin Y, Hirtz DG, et al. Treatment of subclinical hypothyroidism or hypothyroxinemia in pregnancy. N Engl J Med 2017;376:815-25.ArticlePubMedPMC
- 90. Lazarus JH, Bestwick JP, Channon S, Paradice R, Maina A, Rees R, et al. Antenatal thyroid screening and childhood cognitive function. N Engl J Med 2012;366:493-501.ArticlePubMed
- 91. Carney LA, Quinlan JD, West JM. Thyroid disease in pregnancy. Am Fam Physician 2014;89:273-8.PubMed
- 92. Yi KH, Kim KW, Yim CH, Jung ED, Chung JH, Chung HK, et al. Guidelines for the diagnosis and management of thyroid disease during pregnancy and postpartum. J Korean Thyroid Assoc 2014;7:7-39.Article
- 93. Thyroid disease in pregnancy: ACOG practice bulletin, number 223. Obstet Gynecol 2020;135:e261-74.PubMed
- 94. Lim CT, Kola B, Korbonits M. AMPK as a mediator of hormonal signalling. J Mol Endocrinol 2010;44:87-97.ArticlePubMed
- 95. Labuzek K, Suchy D, Gabryel B, Bielecka A, Liber S, Okopien B. Quantification of metformin by the HPLC method in brain regions, cerebrospinal fluid and plasma of rats treated with lipopolysaccharide. Pharmacol Rep 2010;62:956-65.ArticlePubMed
- 96. Vigersky RA, Filmore-Nassar A, Glass AR. Thyrotropin suppression by metformin. J Clin Endocrinol Metab 2006;91:225-7.ArticlePubMedPDF
- 97. Isidro ML, Penin MA, Nemina R, Cordido F. Metformin reduces thyrotropin levels in obese, diabetic women with primary hypothyroidism on thyroxine replacement therapy. Endocrine 2007;32:79-82.ArticlePubMedPDF
- 98. Cappelli C, Rotondi M, Pirola I, Agosti B, Gandossi E, Valentini U, et al. TSH-lowering effect of metformin in type 2 diabetic patients: differences between euthyroid, untreated hypothyroid, and euthyroid on L-T4 therapy patients. Diabetes Care 2009;32:1589-90.PubMedPMC
- 99. Lupoli R, Di Minno A, Tortora A, Ambrosino P, Lupoli GA, Di Minno MN. Effects of treatment with metformin on TSH levels: a meta-analysis of literature studies. J Clin Endocrinol Metab 2014;99:E143-8.ArticlePubMed
- 100. Saraei P, Asadi I, Kakar MA, Moradi-Kor N. The beneficial effects of metformin on cancer prevention and therapy: a comprehensive review of recent advances. Cancer Manag Res 2019;11:3295-313.PubMedPMC
- 101. Foretz M, Guigas B, Bertrand L, Pollak M, Viollet B. Metformin: from mechanisms of action to therapies. Cell Metab 2014;20:953-66.ArticlePubMed
- 102. Muaddi H, Chowdhury S, Vellanki R, Zamiara P, Koritzinsky M. Contributions of AMPK and p53 dependent signaling to radiation response in the presence of metformin. Radiother Oncol 2013;108:446-50.ArticlePubMed
- 103. Rezzonico J, Rezzonico M, Pusiol E, Pitoia F, Niepomniszcze H. Metformin treatment for small benign thyroid nodules in patients with insulin resistance. Metab Syndr Relat Disord 2011;9:69-75.ArticlePubMed
- 104. Klubo-Gwiezdzinska J, Jensen K, Costello J, Patel A, Hoperia V, Bauer A, et al. Metformin inhibits growth and decreases resistance to anoikis in medullary thyroid cancer cells. Endocr Relat Cancer 2012;19:447-56.ArticlePubMed
- 105. Chen G, Xu S, Renko K, Derwahl M. Metformin inhibits growth of thyroid carcinoma cells, suppresses self-renewal of derived cancer stem cells, and potentiates the effect of chemotherapeutic agents. J Clin Endocrinol Metab 2012;97:E510-20.ArticlePubMed
- 106. Klubo-Gwiezdzinska J, Costello J Jr, Patel A, Bauer A, Jensen K, Mete M, et al. Treatment with metformin is associated with higher remission rate in diabetic patients with thyroid cancer. J Clin Endocrinol Metab 2013;98:3269-79.ArticlePubMed
- 107. Cho SW, Yi KH, Han SK, Sun HJ, Kim YA, Oh BC, et al. Therapeutic potential of metformin in papillary thyroid cancer in vitro and in vivo. Mol Cell Endocrinol 2014;393:24-9.ArticlePubMed
- 108. Cho YY, Kang MJ, Kim SK, Jung JH, Hahm JR, Kim TH, et al. Protective effect of metformin against thyroid cancer development: a population-based study in Korea. Thyroid 2018;28:864-70.ArticlePubMed
- 109. Brown J, Solomon DH. Mechanism of antithyroid effects of a sulfonylurea in the rat. Endocrinology 1958;63:473-80.ArticlePubMed
- 110. Nikkila EA, Jakobson T, Jokipii SG, Karlsson K. Thyroid function in diabetic patients under long-term sulfonylurea treatment. Acta Endocrinol (Copenh) 1960;33:623-9.PubMed
- 111. Tranquada RE, Solomon DH, Brown J, Greene R. The effect of oral hypoglycemic agents on thyroid function in the rat. Endocrinology 1960;67:293-7.ArticlePubMed
- 112. England ML, Hartnell JM, Hershman JM, Levin SR. Glyburide does not alter thyroid function. Diabetes Res 1986;3:471-4.PubMed
- 113. Ikeda T, Ito Y, Murakami I, Mokuda O, Tokumori Y, Tominaga M, et al. Effect of glibenclamide on thyroid hormone metabolism in rats. Horm Metab Res 1986;18:517-20.ArticlePubMed
- 114. Nanjan MJ, Mohammed M, Prashantha Kumar BR, Chandrasekar MJ. Thiazolidinediones as antidiabetic agents: a critical review. Bioorg Chem 2018;77:548-67.ArticlePubMed
- 115. Yki-Jarvinen H. Thiazolidinediones. N Engl J Med 2004;351:1106-18.ArticlePubMed
- 116. Ferrari SM, Fallahi P, Vita R, Antonelli A, Benvenga S. Peroxisome proliferator-activated receptor-γ in thyroid autoimmunity. PPAR Res 2015;2015:232818.ArticlePubMedPMCPDF
- 117. Valyasevi RW, Harteneck DA, Dutton CM, Bahn RS. Stimulation of adipogenesis, peroxisome proliferator-activated receptor-gamma (PPARgamma), and thyrotropin receptor by PPARgamma agonist in human orbital preadipocyte fibroblasts. J Clin Endocrinol Metab 2002;87:2352-8.PubMed
- 118. Lee S, Tsirbas A, Goldberg RA, McCann JD. Thiazolidinedione induced thyroid associated orbitopathy. BMC Ophthalmol 2007;7:8.ArticlePubMedPMCPDF
- 119. Dorkhan M, Lantz M, Frid A, Groop L, Hallengren B. Treatment with a thiazolidinedione increases eye protrusion in a subgroup of patients with type 2 diabetes. Clin Endocrinol (Oxf) 2006;65:35-9.ArticlePubMed
- 120. Tseng CH. Rosiglitazone may reduce thyroid cancer risk in patients with type 2 diabetes. Ann Med 2013;45:539-44.ArticlePubMed
- 121. Lamari Y, Boissard C, Moukhtar MS, Jullienne A, Rosselin G, Garel JM. Expression of glucagon-like peptide 1 receptor in a murine C cell line: regulation of calcitonin gene by glucagonlike peptide 1. FEBS Lett 1996;393:248-52.ArticlePubMedPDF
- 122. Crespel A, De Boisvilliers F, Gros L, Kervran A. Effects of glucagon and glucagon-like peptide-1-(7-36) amide on C cells from rat thyroid and medullary thyroid carcinoma CA-77 cell line. Endocrinology 1996;137:3674-80.ArticlePubMed
- 123. Bjerre Knudsen L, Madsen LW, Andersen S, Almholt K, de Boer AS, Drucker DJ, et al. Glucagon-like peptide-1 receptor agonists activate rodent thyroid C-cells causing calcitonin release and C-cell proliferation. Endocrinology 2010;151:1473-86.ArticlePubMedPDF
- 124. Drucker DJ, Sherman SI, Bergenstal RM, Buse JB. The safety of incretin-based therapies: review of the scientific evidence. J Clin Endocrinol Metab 2011;96:2027-31.ArticlePubMed
- 125. Hegedus L, Moses AC, Zdravkovic M, Le Thi T, Daniels GH. GLP-1 and calcitonin concentration in humans: lack of evidence of calcitonin release from sequential screening in over 5000 subjects with type 2 diabetes or nondiabetic obese subjects treated with the human GLP-1 analog, liraglutide. J Clin Endocrinol Metab 2011;96:853-60.ArticlePubMedPDF
- 126. Sencar ME, Sakiz D, Calapkulu M, Hepsen S, Kizilgul M, Ozturk IU, et al. The effect of exenatide on thyroid-stimulating hormone and thyroid volume. Eur Thyroid J 2019;8:307-11.ArticlePubMedPMCPDF
- 127. Hegedus L, Sherman SI, Tuttle RM, von Scholten BJ, Rasmussen S, Karsbol JD, et al. No evidence of increase in calcitonin concentrations or development of c-cell malignancy in response to liraglutide for up to 5 years in the LEADER trial. Diabetes Care 2018;41:620-2.ArticlePubMedPDF
- 128. Tseng CH. Sitagliptin use and thyroid cancer risk in patients with type 2 diabetes. Oncotarget 2016;7:24871-9.ArticlePubMedPMC
- 129. Okada J, Yamada E, Niijima Y, Okada S, Yamada M. Dipeptidyl peptidase-4 inhibitors attenuate thyroid-stimulating hormone concentrations. J Diabetes 2019;11:497.ArticlePubMedPDF
- 130. Bays HE. Lowering low-density lipoprotein cholesterol levels in patients with type 2 diabetes mellitus. Int J Gen Med 2014;7:355-64.ArticlePubMedPMC
- 131. Goldberg RB, Fonseca VA, Truitt KE, Jones MR. Efficacy and safety of colesevelam in patients with type 2 diabetes mellitus and inadequate glycemic control receiving insulin-based therapy. Arch Intern Med 2008;168:1531-40.ArticlePubMed
- 132. Jialal I, Abby SL, Misir S, Nagendran S. Concomitant reduction in low-density lipoprotein cholesterol and glycated hemoglobin with colesevelam hydrochloride in patients with type 2 diabetes: a pooled analysis. Metab Syndr Relat Disord 2009;7:255-8.ArticlePubMedPMC
- 133. Yamakawa T, Takano T, Utsunomiya H, Kadonosono K, Okamura A. Effect of colestimide therapy for glycemic control in type 2 diabetes mellitus with hypercholesterolemia. Endocr J 2007;54:53-8.ArticlePubMed
- 134. Handelsman Y. Role of bile acid sequestrants in the treatment of type 2 diabetes. Diabetes Care 2011;34(Suppl 2):S244-50.ArticlePubMedPMCPDF
- 135. Fonseca VA, Handelsman Y, Staels B. Colesevelam lowers glucose and lipid levels in type 2 diabetes: the clinical evidence. Diabetes Obes Metab 2010;12:384-92.ArticlePubMedPMC
- 136. Solomon BL, Wartofsky L, Burman KD. Adjunctive cholestyramine therapy for thyrotoxicosis. Clin Endocrinol (Oxf) 1993;38:39-43.ArticlePubMed
- 137. Steinmetz KL. Colesevelam hydrochloride. Am J Health Syst Pharm 2002;59:932-9.ArticlePubMed
- 138. Rameshwar J, Anand K. Antihyperglycaemic and antiperoxidative roles of acarbose in type 2 diabetes mellitus are possibly mediated through changes in thyroid function. Clin Exp Pharmacol Physiol 2006;33:1104-6.ArticlePubMed
- 139. Li C, Kuang J, Zhao Y, Sun H, Guan H. Effect of type 2 diabetes and antihyperglycemic drug therapy on signs of tumor invasion in papillary thyroid cancer. Endocrine 2020;69:92-9.ArticlePubMedPDF
- 140. De Jonghe S, Proctor J, Vinken P, Feyen B, Wynant I, Marien D, et al. Carcinogenicity in rats of the SGLT2 inhibitor canagliflozin. Chem Biol Interact 2014;224:1-12.ArticlePubMed
- 141. Taub ME, Ludwig-Schwellinger E, Ishiguro N, Kishimoto W, Yu H, Wagner K, et al. Sex-, species-, and tissue-specific metabolism of empagliflozin in male mouse kidney forms an unstable hemiacetal metabolite (M466/2) that degrades to 4-hydroxycrotonaldehyde, a reactive and cytotoxic species. Chem Res Toxicol 2015;28:103-15.ArticlePubMed
- 142. Cryer PE. Glucose counterregulation: prevention and correction of hypoglycemia in humans. Am J Physiol 1993;264(2 Pt 1):E149-55.ArticlePubMed
- 143. Demitrost L, Ranabir S. Thyroid dysfunction in type 2 diabetes mellitus: a retrospective study. Indian J Endocrinol Metab 2012;16(Suppl 2):S334-5.PubMedPMC
- 144. Katz HP, Youlton R, Kaplan SL, Grumbach MM. Growth and growth hormone. 3. Growth hormone release in children with primary hypothyroidism and thyrotoxicosis. J Clin Endocrinol Metab 1969;29:346-51.PubMed
- 145. Kamilaris TC, DeBold CR, Pavlou SN, Island DP, Hoursanidis A, Orth DN. Effect of altered thyroid hormone levels on hypothalamic-pituitary-adrenal function. J Clin Endocrinol Metab 1987;65:994-9.ArticlePubMed
- 146. McCulloch AJ, Johnston DG, Baylis PH, Kendall-Taylor P, Clark F, Young ET, et al. Evidence that thyroid hormones regulate gluconeogenesis from glycerol in man. Clin Endocrinol (Oxf) 1983;19:67-76.ArticlePubMed
- 147. McDaniel HG, Pittman CS, Oh SJ, DiMauro S. Carbohydrate metabolism in hypothyroid myopathy. Metabolism 1977;26:867-73.ArticlePubMed
- 148. Clausen N, Lins PE, Adamson U, Hamberger B, Efendic S. Counterregulation of insulin-induced hypoglycaemia in primary hypothyroidism. Acta Endocrinol (Copenh) 1986;111:516-21.ArticlePubMed
- 149. Muller MJ, Seitz HJ. Interrelation between thyroid state and the effect of glucagon on gluconeogenesis in perfused rat livers. Biochem Pharmacol 1987;36:1623-7.ArticlePubMed
REFERENCES
Figure & Data
References
Citations

- Linkage and association of rs3110045 and rs28499085 variants in the thyrotropin-releasing hormone receptor (TRHR) gene with the risk of familial type 2 diabetes
Rongling Wu, Claudia Gragnoli
Aspects of Molecular Medicine.2024; 3: 100037. CrossRef - Obesity and Obesity-Related Thyroid Dysfunction: Any Potential Role for the Very Low-Calorie Ketogenic Diet (VLCKD)?
Sebastián Pablo Chapela, Alison Simancas-Racines, Florencia Ceriani, Andrés Luciano Nicolas Martinuzzi, María Paula Russo, Ana Karina Zambrano, Daniel Simancas-Racines, Ludovica Verde, Giovanna Muscogiuri, Christos S. Katsanos, Evelyn Frias-Toral, Luigi B
Current Nutrition Reports.2024;[Epub] CrossRef - View on Metformin: Antidiabetic and Pleiotropic Effects, Pharmacokinetics, Side Effects, and Sex-Related Differences
Guglielmina Froldi
Pharmaceuticals.2024; 17(4): 478. CrossRef - Thyroid Hormones and Diabetes in Euthyroid Hispanic/Latino Adults of Diverse Backgrounds: HCHS/SOL
Victoria Persky, Chibuzor Abasilim, Konstantina Tsintsifas, Tessa Day, Robert M Sargis, Martha Daviglus, Jianwen Cai, Sally Freels, Robert Kaplan, Carmen R Isasi, Amber Pirzada, Michelle L Meyer, Gregory A Talavera, Bharat Thyagarajan, Shivani Agarwal, No
Journal of the Endocrine Society.2024;[Epub] CrossRef - Managing Adults With Hypoglycemia
Christopher James Watson, Jonathan A. Edlow
Annals of Emergency Medicine.2023; 82(6): 705. CrossRef - Relationship of Glucose, C-peptide, Leptin, and BDNF in Maternal and Umbilical Vein Blood in Type-1 Diabetes
Josip Delmis, Slavko Oreskovic, Vesna Elvedji Gasparovic, Mirta Starcevic, Mislav Herman, Nada Dessardo, Vito Starcevic, Marina Ivanisevic
Nutrients.2023; 15(3): 600. CrossRef - Isolated Maternal Hypothyroxinemia May be Associated with Insulin
Requirement in Gestational Diabetes Mellitus
Ömercan Topaloğlu, Mehmet Uzun, Seda Nur Topaloğlu, Ibrahim Sahin
Hormone and Metabolic Research.2023; 55(04): 245. CrossRef - Association of urinary iodine concentration with prediabetes/diabetes in adults: Analysis of the NHANES 2005–2016
Jingmin Chen, Huanzhu Liang, Yuxuan Tan, Lin Wen, Ziang Guo, Jiyu Nie, Xiaoxiao Lin, Feng Huang, Jie Wang, Puyi Xing, Lihong Nie, Lihong Wang, Chunxia Jing
Journal of Trace Elements in Medicine and Biology.2023; 77: 127144. CrossRef - Central sensitivity to thyroid hormones is reduced in youths with overweight or obesity and impaired glucose tolerance
Procolo Di Bonito, Domenico Corica, Maria Rosaria Licenziati, Anna Di Sessa, Emanuele Miraglia del Giudice, Maria Felicia Faienza, Valeria Calcaterra, Francesca Franco, Giulio Maltoni, Giuliana Valerio, Malgorzata Wasniewska
Frontiers in Endocrinology.2023;[Epub] CrossRef - Association of thyroid stimulating hormone and time in range with risk of diabetic retinopathy in euthyroid type 2 diabetes
Yaxin Wang, Jingyi Lu, Jiaying Ni, Ming Wang, Yun Shen, Wei Lu, Wei Zhu, Yuqian Bao, Jian Zhou
Diabetes/Metabolism Research and Reviews.2023;[Epub] CrossRef - The circadian rhythm: an influential soundtrack in the diabetes story
Amirali Hariri, Mina Mirian, Ali Zarrabi, Mohammad Kohandel, Maryam Amini-Pozveh, Amir Reza Aref, Aliye Tabatabaee, Pranav Kumar Prabhakar, Ponnurengam Malliappan Sivakumar
Frontiers in Endocrinology.2023;[Epub] CrossRef - Folate deficiency may increase the risk for elevated TSH in patients with type 2 diabetes mellitus
Lin Lin, Yushan Du, Guanyu Niu, Shuangbo Xia, Jufen Liu
BMC Endocrine Disorders.2023;[Epub] CrossRef - L- Thyroxine ameliorates renal function in thyroidectomized diabetic nephropathy rats through downregulation of TGF- β1, Ang II and ET-1 expression
Zeinab H. El-Said, Sherihan I. Gouda, Hebatallah A. Mahgoub, S El_desouky, Neven A. Ebrahim
Egyptian Journal of Basic and Applied Sciences.2023; 10(1): 632. CrossRef - Thyroid dysfunction in children and adolescents affected by undernourished and overnourished eating disorders
Valeria Calcaterra, Vittoria Carlotta Magenes, Francesca Siccardo, Chiara Hruby, Martina Basso, Veronica Conte, Giulia Maggioni, Valentina Fabiano, Susanna Russo, Pierangelo Veggiotti, Gianvincenzo Zuccotti
Frontiers in Nutrition.2023;[Epub] CrossRef - Preventive Effect of Molecular Iodine in Pancreatic Disorders from Hypothyroid Rabbits
Julia Rodríguez-Castelán, Evangelina Delgado-González, Esteban Rodríguez-Benítez, Francisco Castelán, Estela Cuevas-Romero, Brenda Anguiano, Michael C. Jeziorski, Carmen Aceves
International Journal of Molecular Sciences.2023; 24(19): 14903. CrossRef - Hypothyroidism increases angiotensinogen gene expression associated with vascular smooth muscle cells cholesterol metabolism dysfunction and aorta remodeling in Psammomys obesus
Samia Neggazi, Nadjiba Hamlat, Sihem Berdja, Saliha Boumaza, Leila Smail, Michel Beylot, Souhila Aouichat-Bouguerra
Scientific Reports.2023;[Epub] CrossRef - The Correlation Between Thyroid Parameters and the Ratios of Neutrophil/Lymphocyte and Platelet/Lymphocyte in Euthyroid Type 2 Diabetic Patients
Hui Chen, Jun-Qiang Ju, Xiao-Wu Qian, Zheng-Tai Zhu, Chun-Zhi Zhao, Zhe Liu
Diabetes, Metabolic Syndrome and Obesity.2023; Volume 16: 3763. CrossRef - Effects of high-intensity interval training program on pituartry function in basketball players: a randomized controlled trial
Recep Soslu, Abdullah Uysal, Meltem Devrilmez, İsmail Can Çuvalcıoğlu, Ali Ahmet Doğan, Sülbiye Karaburgu, Murat Taş
Frontiers in Physiology.2023;[Epub] CrossRef - Selenium and Selenoproteins at the Intersection of Type 2 Diabetes and Thyroid Pathophysiology
Francesca Gorini, Cristina Vassalle
Antioxidants.2022; 11(6): 1188. CrossRef - TSH levels within the normal range and risk of cardiovascular and all-cause mortality among individuals with diabetes
Ping Zhu, Guojuan Lao, Chuping Chen, Lihui Luo, Jing Gu, Jianmin Ran
Cardiovascular Diabetology.2022;[Epub] CrossRef - Metabolite Changes during the Transition from Hyperthyroidism to Euthyroidism in Patients with Graves’ Disease
Ho Yeop Lee, Byeong Chang Sim, Ha Thi Nga, Ji Sun Moon, Jingwen Tian, Nguyen Thi Linh, Sang Hyeon Ju, Dong Wook Choi, Daiki Setoyama, Hyon-Seung Yi
Endocrinology and Metabolism.2022; 37(6): 891. CrossRef

 KDA
KDA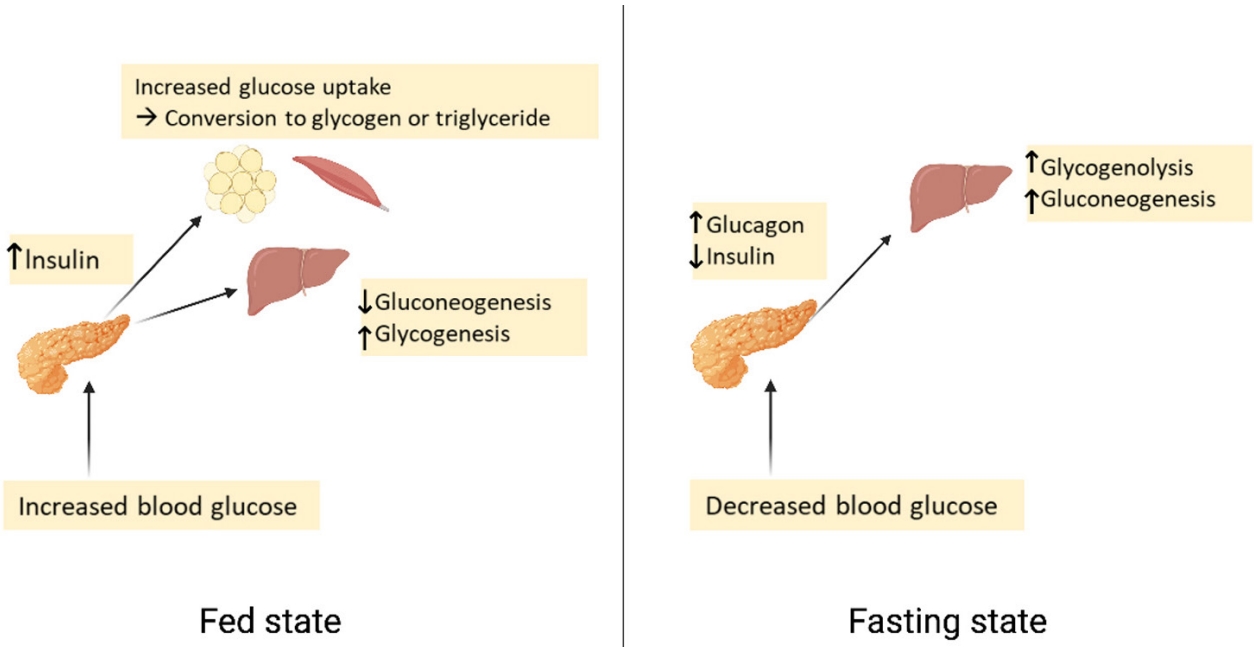
 PubReader
PubReader ePub Link
ePub Link Cite
Cite





