Glutamic Acid Decarboxylase and Tyrosine Phosphatase-Related Islet Antigen-2 Positivity among Children and Adolescents with Diabetes in Korea
Article information
Abstract
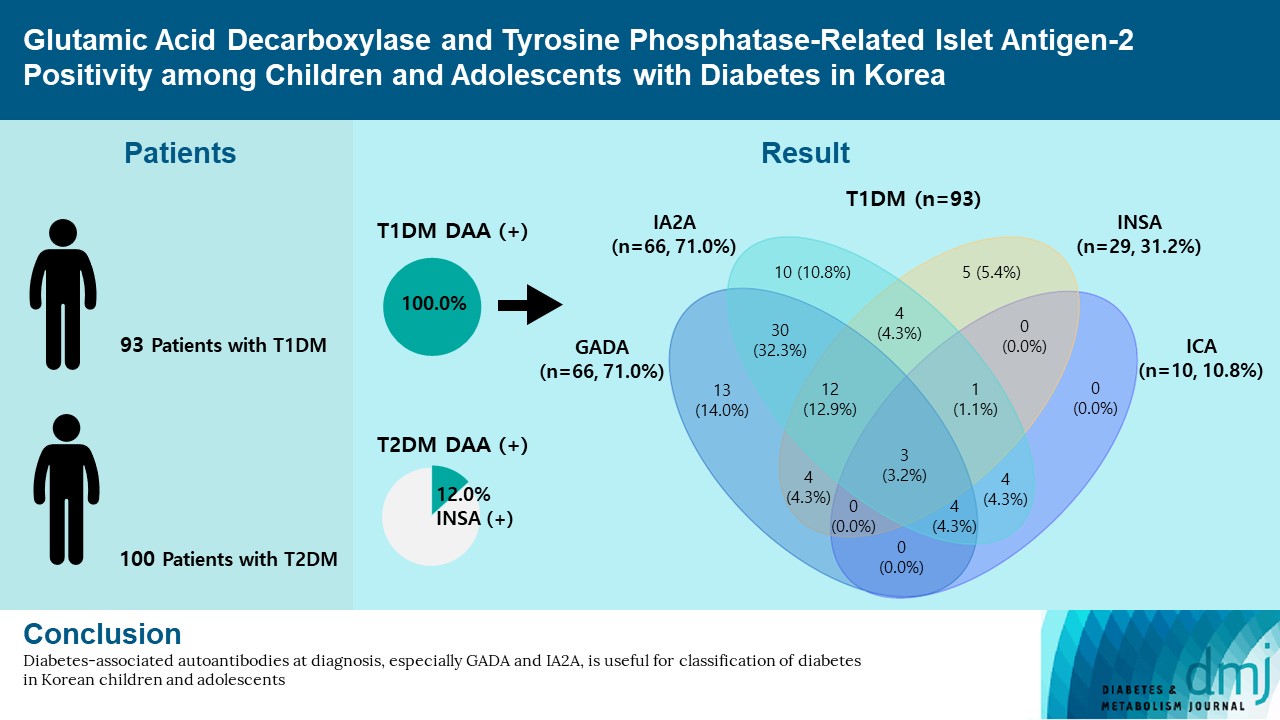
Autoantibodies against glutamic acid decarboxylase (GADA), tyrosine phosphatase-related islet antigen 2 (IA2A), insulin (INSA), and islet cells (ICA) are critical for determining the type of diabetes and management strategy in new-onset diabetes mellitus (NODM), but there have been few reports of all diabetes-associated autoantibody (DAA) in Korea. We retrospectively analyzed 193 patients with NODM aged 0 to 18 years who were followed at two tertiary centers in Korea (2017 to 2021). Patients with type 1 diabetes mellitus (T1DM) and type 2 diabetes mellitus (T2DM) were 93 (48.2%) and 100 (51.8%), respectively. In T1DM patients, the DAA positivity rate was 94.6%; prevalence of GADA, IA2A, INSA, and ICA was 71.0%, 71.0%, 31.2%, and 10.8%, respectively; and IA2A added 10.7% point autoantibody positivity (83.9% for GADA+INSA+ICA and 94.6% for GADA+INSA+ICA+IA2A). Among the patients with T2DM, 12 (12.0%) were positive for DAA, and all were positive for INSA. These findings suggest that DAA at diagnosis, especially GADA and IA2A, is useful for classifying diabetes in Korean children and adolescents.
INTRODUCTION
Diabetes mellitus is a chronic metabolic disease characterized by hyperglycemia resulting from insulin deficiency, insulin resistance, or both. Type 1 diabetes mellitus (T1DM) is characterized by insulin deficiency caused by the autoimmune destruction of pancreatic islet β-cells [1]. In contrast, type 2 diabetes mellitus (T2DM) results from insulin resistance and relative insulin deficiency [2,3]. Compared to T2DM, T1DM involves development of autoantibodies to pancreatic β-cell antigens [4].
In Western countries, the prevalence of diabetes-associated autoantibody (DAA) positivity is more than 90% in patients clinically diagnosed with T1DM at clinical onset [3,5,6]. However, the prevalence of DAA positivity in Asian patients with T1DM is low (Japan 81% [7], China 61% [8], and India 72% [9]). The positivity rate of DAA in Korean patients with T1DM is similar to other Asian countries [10,11]. Additionally, the presence of DAA has been reported in patients clinically diagnosed with T2DM [12].
There have been few reports of DAA positivity in Korean pediatric patients with new-onset diabetes mellitus (NODM). In addition, most previous studies have reported targeting of autoantibody to glutamic acid decarboxylase (GADA) and/or insulin autoantibody (INSA), not autoantibody to tyrosine phosphatase-related islet antigen 2 (IA2A) for DAA testing in Korea. Herein, we demonstrate the positivity rate of DAA, including IA2A, and determine the effectiveness of DAA in classifying the type of diabetes in pediatric patients with NODM in Korea.
METHODS
Study design and subjects
We collected data from 238 children and adolescents (aged 0 to 18 years) with NODM between March 2017 and March 2021 in two tertiary medical centers. We excluded a total of 45 patients with diabetes other than T1DM or T2DM (Supplementary Fig. 1). DAA results were not available for 13 patients within 3 months of diagnosis. Diagnosis of diabetes mellitus was based on the diagnostic criteria of the American Diabetes Association [4]. The patients were classified into T1DM or T2DM on the basis of clinical characteristics, laboratory findings, and disease course. T1DM patients required insulin treatment and had any of the following characteristics: serum C-peptide level of <0.6 ng/mL, 24-hour urine C-peptide level of <30 μg/day, diabetic ketoacidosis (DKA) at diagnosis, or positive DAA. T2DM patients were mostly overweight and obese, had normal or high fasting serum or 24-hour urine C-peptide level, showed signs of insulin resistance. This study was approved by the Institutional Review Board of the Seoul National University Bundang Hospital (B-2105/683-108) and Seoul National University Hospital (2104-218-1215). Informed consent was waived by the Institutional Review Board.
Clinical and laboratory measurements
The data for this study were obtained through the retrieval of medical records. We collected information regarding age at diagnosis, sex, anthropometric measures, duration of diabetes, birth weight, family history of diabetes, acanthosis nigricans, diabetes type, duration of diabetes, and medication status. Body mass index (BMI) z-scores were calculated on the basis of the 2017 Korean National Growth Charts [13]. We collected laboratory data including plasma glucose, insulin, glycosylated hemoglobin (HbA1c), serum C-peptide, 24-hour urine C-peptide, thyroid autoantibodies, and DAA, including GADA, IA2A, INSA, and autoantibody to islet cells (ICA).
Statistical analysis
Data analyses were performed using SPSS Statistics version 26.0 (IBM Corp., Armonk, NY, USA). The Kolmogorov-Smirnov test was used as the test for normality. Continuous variables were compared using the Student’s t-test or Mann-Whitney U test, and categorical variables were compared using the chi-square test or Fisher exact test. For all analyses, a two-sided P<0.05 was considered statistically significant.
RESULTS
Characteristics of study subjects
Table 1 shows the demographic and clinical characteristics of the patients (48.2% of women) according to the type of diabetes. Of the 193 patients, 93 (48.2%) were T1DM, and 100 (51.8%) were T2DM. Compared to T2DM, T1DM patients had a lower age at diagnosis, BMI z-score, serum C-peptide and 24-hour urine C-peptide, and higher serum glucose, HbA1c, DKA at diagnosis, and thyroid autoantibodies.
Diabetes-associated autoantibody positivity at diagnosis
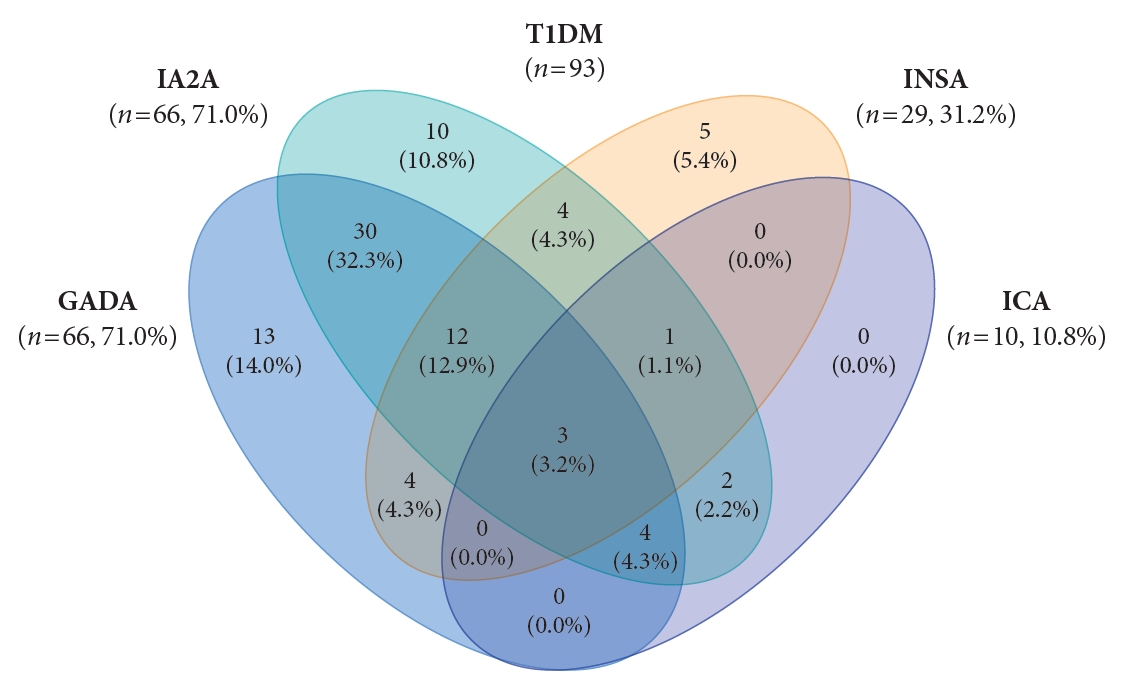
The DAA status in T1DM patients is shown in Fig. 1. In T1DM patients, the positivity rate of DAA was 94.6%; the prevalence of GADA, IA2A, INSA, and ICA was 71.0%, 71.0%, 31.2%, and 10.8%, respectively. The positivity rate of only GADA was 14.0%, and that of only IA2A was 10.8%, respectively. Among the 100 patients with T2DM, 12 (12.0%) were positive for DAA; all cases were positive for INSA.
Clinical characteristics of patients according to autoantibody positivity at diagnosis of diabetes
In T1DM patients, the clinical characteristics at diagnosis were compared by classifying DAA-positive and DAA-negative patients (Supplementary Table 1). Of the 93 patients with T1DM, 88 (94.6%) were positive for DAA. There were no significant differences in age, glucose, HbA1c, DKA, and C-peptide between the DAA-positive and DAA-negative groups at the time of diagnosis and at the last follow-up. The clinical characteristics of T2DM patients were compared with respect to INSA positivity (Supplementary Table 2). The difference in the rate of insulin initiation between the groups was not significant. Among the 12 INSA-positive patients, one was weaned off insulin and five were responsive to metformin without insulin. In the two of the remaining patients, metformin was added late because of liver function abnormalities. There were no significant differences in clinical characteristics between the INSA-positive and INSA-negative groups at the time of diagnosis and at the last follow-up.
DISCUSSION
In this study, we analyzed clinical characteristics by diabetes type and retrospectively investigated the prevalence of DAA positivity among 193 children and adolescents with NODM in Korea. The T1DM patients in our study presented a high prevalence of DAA, and the positivity rate of IA2A (71.0%) was similar to that of GADA. In addition, DAA was detected in the T2DM patients, among which all showed positivity only for INSA.
A high prevalence of DAA in T1DM patients has been reported among children and adolescents in Western countries: 93.8% in the United States [5] and 97.0% in Italy [14]. A high positivity rate of GADA (73.2% to 86.0%) has been reported in American and European patients with T1DM [5,14]. In contrast, a low positivity rate of GADA (29.4% to 72.2%) and IA2A (34.0% to 41.1%) has been reported in Asian countries [8,9]. In previous studies conducted in Korea, the positivity rates of one or more of the GADA and INSA were 66.7% in T1DM patients [10]. And the prevalence of IA2A was 36.0% which was about 20% lower than that of GADA [11]. According to this study, the positivity rate of DAA in Korea was 94.6%, which is as high as that in Western countries. And the positivity rate of IA2A (71.0%) in T1DM patients was as high as that of GADA (71.0%), and considerably higher than previously reported values [10,11]. The high positivity rate of DAA is likely due to the number of DAA tested more or increased involvement of autoimmunity in the pathogenesis of diabetes in Korea. In this study, the autoantibody positivity was 89.2% for GADA+IA2A and 94.6% for GADA+INSA+ICA+IA2A. The inclusion of GADA in T1DM increased the detection rate of DAA from 80.6% for IA2A+INSA+ICA to 94.6% for GADA+INSA+ICA+IA2A. The inclusion of IA2A added 10.7% point autoantibody positivity. Thus, it is especially crucial to measure a combination of GADA and IA2A in classifying the type of diabetes in pediatric patients with NODM in Korea.
In T2DM patients, DAA positivity has been reported recently. The prevalence of DAA in T2DM patients varies by country: 6.4% in the USA [15], 5.7% in Europe, and 2.1% to 28.5% in China [12,15,16]. In a previous smaller study on pediatric patients with T2DM in Korea, the prevalence of one or more of the GADA and INSA was 43.9%, and the positivity rate of INSA was the highest at 31.7% [17]. In this study, the prevalence of DAA positivity in T2DM patients was 12.0%, and all cases were positive for INSA. INSA positivity was observed following insulin injection after 7 to 10 days of insulin therapy [18], which explains the INSA-positivity in two patients, who received insulin therapy before DAA testing. Furthermore, INSA is associated with systemic inflammation promoted by metabolic stress in obesity [19]. In patients with clinically diagnosed T2DM, the presence of DAA has been associated with rapid progression to insulin dependence [2].
This study had some limitations. First, although zinc transporter 8 autoantibody (ZnT8A) was recently identified as an autoantibody marker in younger T1DM patients, it was not included in this study because this testing is not available in Korea [20]. Second, repeated testing for DAA was not performed among patients with DAA-negative T1DM or DAA-positive T2DM.
In summary, the prevalence of DAA was 94.6% in pediatric patients with T1DM in Korea, similar to that in the Western population. In contrast, among pediatric patients with T2DM in Korea, 12.0% were DAA-positive, and all were positive for INSA. We suggest that DAA testing, especially the combination of GADA and IA2A, is useful for the classification of diabetes. Long-term follow-up is needed for the clinical course of DAA-negative T1DM and DAA-positive T2DM. In the future, the addition of ZnT8A to DAA testing is warranted for increasing the diagnosis rate.
SUPPLEMENTARY MATERIALS
Supplementary materials related to this article can be found online at https://doi.org/10.4093/dmj.2021.0332.
Clinical characteristics of patients with type 1 diabetes mellitus by autoantibody positivity
Clinical characteristics of patients with type 2 diabetes mellitus according to INSA positivity
Flowgram of the study. NODM, new-onset diabetes mellitus; T1DM, type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus; DAA, diabetes-associated autoantibody; INSA, insulin autoantibody; DM, diabetes mellitus.
Notes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTIONS
Conception or design: J.H.K.
Acquisition, analysis, or interpretation of data: K.Y.K., M.S.K., Y.J.L., Y.A.L., S.Y.L., C.H.S., J.H.K.
Drafting the work or revising: K.Y.K., Y.J.L., J.H.K.
Final approval of the manuscript: K.Y.K., M.S.K., Y.J.L., Y.A.L., S.Y.L., C.H.S., J.H.K.
FUNDING
None
Acknowledgements
None