Association between Type 2 Diabetes Mellitus and Brain Atrophy: A Meta-Analysis
Article information
Abstract
Background
Type 2 diabetes mellitus (T2DM) is known to be associated with cognitive decline and brain structural changes. This study systematically reviews and estimates human brain volumetric differences and atrophy associated with T2DM.
Methods
PubMed, PsycInfo and Cochrane Library were searched for brain imaging studies reporting on brain volume differences between individuals with T2DM and healthy controls. Data were examined using meta-analysis, and association between age, sex, diabetes characteristics and brain volumes were tested using meta-regression.
Results
A total of 14,605 entries were identified; after title, abstract and full-text screening applying inclusion and exclusion criteria, 64 studies were included and 42 studies with compatible data contributed to the meta-analysis (n=31,630; mean age 71.0 years; 44.4% male; 26,942 control; 4,688 diabetes). Individuals with T2DM had significantly smaller total brain volume, total grey matter volume, total white matter volume and hippocampal volume (approximately 1% to 4%); meta-analyses of smaller samples focusing on other brain regions and brain atrophy rate in longitudinal investigations also indicated smaller brain volumes and greater brain atrophy associated with T2DM. Meta-regression suggests that diabetes-related brain volume differences start occurring in early adulthood, decreases with age and increases with diabetes duration.
Conclusion
T2DM is associated with smaller total and regional brain volume and greater atrophy over time. These effects are substantial and highlight an urgent need to develop interventions to reduce the risk of T2DM for brain health.
INTRODUCTION
Type 2 diabetes mellitus (T2DM) is a common, chronic, and progressive metabolic disorder characterised by abnormally high blood glucose levels for a prolonged period, termed hyperglycaemia, due to insulin resistance and decreased production of insulin. Typical T2DM complications include retinopathy, kidney failure, and peripheral neuropathy [1]. Because of the high prevalence of T2DM among the elderly and the growing concern over cognitive health in older populations, there is a growing interest in how T2DM affects brain functions and related brain structures. T2DM is associated with an approximately 50% increased risk of developing dementia [2]; higher blood glucose levels in non-diabetics, which are known to be associated with increased risk of T2DM, are also associated with elevated risk of dementia [3]. Cognitive domains that may be affected by T2DM include memory, processing speed, and executive function [4]. Although the specific mechanisms that result in cognitive impairment in T2DM are not clear, hyperglycaemia, vascular disorders, hypoglycaemia, and insulin resistance are associated with increased risk; T2DM may also be involved in the pathogenesis of Alzheimer’s disease [5]. Moreover, there is emerging evidence that brain changes that lead to functional deficits may start developing well before T2DM is clinically diagnosed [3].
Many studies have used human brain magnetic resonance imaging (MRI) to measure structural changes in vivo that may be associated with T2DM. Typically, they have used brain volumetry to measure the extent of brain atrophy. Cross-sectional studies have consistently identified associations between T2DM and a decrease in mean total brain volume by 0.2 to 0.6 standard deviation units, which is comparable to 3 to 5 years of normal ageing [6–8]. More frequent brain lesions [8,9] and greater number of white matter hyperintensities [7,8] have also been identified in T2DM, likely due to the increased vascular pathology in this disease. These brain structural differences may also be associated with T2DM duration and blood glucose levels [8]. Studies focusing on specific brain regions have found negative associations between T2DM and volume of sub-regions including the hippocampus, basal ganglia, and many cortical regions among cognitively healthy individuals [6,8,10,11].
Longitudinal case-control and population-based studies have identified brain atrophy three times greater in T2DM than normal ageing [11–14]. Ventricular enlargement has also been observed [11,12,14,15], suggesting vulnerability of subcortical areas surrounding the ventricles. However, we lack robust estimates of atrophy rates attributable to T2DM as well as an understanding of which brain structures are most affected, as well as the timing of these changes across adulthood.
Therefore, the aim of this systematic review is to precisely quantify the volumetric differences and rates of brain atrophy associated with T2DM using a published methodology. We hypothesise that those with T2DM have smaller brain volumes and higher brain atrophy rate than those without T2DM.
METHODS
This systematic review and meta analysis was based on our previously published methodology [16], following predetermined search terms, inclusion and exclusion criteria, and quality assessment at the study level. This review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [17] and was pre-registered in PROSPERO (No.: CRD42021230535).
Search strategy
PubMed, PsycInfo, and Cochrane Library (1950 to January 2020) was searched using the following terms: “(Diabetes or T2D) AND (brain or cerebrum or cerebral or cerebellum or cerebellar or hippocampus or hippocampal or subcortical or (cerebral ventricle) or ventricular or thalamus or thalamic or (basal ganglia) or striatum or (grey matter) or (white matter)) AND ((magnetic resonance imaging) or MRI or (computed tomography) or neuroimaging or morphometry or (diffusion tensor imaging) or volume or volumetric or thickness or atrophy or shrinkage).” Search included all text, all dates, full text papers in English (all article types for PubMed and PsycInfo; trials for Cochrane Library). Both literal and Medical Subject Heading searches were performed when possible. Titles and abstracts of the paper were screened by two reviewers (T.Z. and N.C./M.S.) for full text review. Full text and supplemental material of qualified studies were retrieved and examined by two reviewers against the inclusion and exclusion criteria. Disagreements between two reviewers were resolved by consensus or by a third reviewer. Citation maps of retrieved papers, previous reviews and previously identified journals were examined to identify additional journal articles that meet the criteria.
Inclusion and exclusion criteria
Studies were included if they had: (1) human adult participants; (2) at least a control group consisting of healthy participants without T2DM; (3) at least one comparison between participants diagnosed with T2DM and controls; (4) brain grey matter or white matter volume data from structural MRI or computed tomography (CT) scan data of T2DM and control participants; (5) data acquired using a validated automatic or manual segmentation method; (6) for longitudinal studies, longitudinal measurements at a minimum of two time points. Studies were excluded if they had: (1) participants with only type 1 diabetes mellitus (T1DM) or that did not differentiate T1DM and T2DM; (2) participants with only subclinical diabetes, including impaired fasting glucose, impaired glucose tolerance, insulin resistance, in the disease group or control group; (3) T2DM participants that all have major conditions other than diabetes, e.g., mental illness, behavioural problems, substance abuse, systemic illness or major brain structural abnormalities; (4) T2DM participants that are all under diabetes treatment (including placebo treatments) and being compared with those without such treatment; (5) only case studies and small samples with less than 10 participants in one group; (6) duplicate samples (for identified duplicates, the sample that best fits the study criteria will be included and the other samples will be excluded); (7) review articles, theses, unfinished studies, or entries with abstract only; (8) for longitudinal studies, samples where the total MRI follow-up period is less than 12 months.
Studies meeting the inclusion and exclusion criteria were assessed for quality using the Newcastle-Ottawa scale. Each study was evaluated on eight items classified into three categories including the selection of the study groups, the comparability of the groups, and the ascertainment of outcome of interest. Each quality item was awarded by a star (except two for comparability) and for each study up to nine stars in total.
Data extraction
Two of the authors extracted data (T.Z. and N.C.) and discrepancies were resolved by consensus. Data extracted consisted of (1) study design and number of participants in each group; (2) participants’ demographics including age, sex ratio, diagnostic criteria for T2DM, duration of T2DM, medication status, glycosylated hemoglobin (HbA1c) levels, fasting plasma glucose levels and body mass index; (3) measurement details including MRI parameters, structural measurements and segmentation method; and (4) study results including areas of interest (left and right) and effect sizes (left, right, and total). Data from studies reporting ratios relative to intracranial volume were included if a volumetric difference could be computed based on group statistics.
Multiple reports on the same cohort but on different brain structures were considered independent studies and included. Where a particular sample was reported in multiple studies on the same brain structure, the study that fit the selection criteria and provided data compatible for meta-analysis was included and other studies were excluded. Studies that reported effect sizes (or provided them after contact) were considered and from those the most recent study with the largest sample size was selected. If there was more than one study similar in sample size and time, the one with the highest quality rating was selected.
Statistical analysis
R version 3.1.1 (R Foundation for Statistical Computing, Vienna, Austria) [18] was used for statistical analysis. Meta-analyses were performed using the Metafor version 1.9–4 R package (https://www.metafor-project.org) [19]. Volumes of brain structures and annual percentage mean atrophy rate was considered as the effect size, and calculation of required standard error (SE) for meta-analysis was based on the standard deviation and number of participants in each group for each individual study. Availability of volume of a brain structure, either reported or computed based on other reported results, was the essential requirement for the meta-analysis. Atrophy rate was calculated using the formula: atrophy=[(volume_time1–volume_time2)/volume_time1]/(time1–time2) if not provided. Where insufficient data were available for inclusion in the meta-analysis, authors were contacted directly to seek additional information.
A random-effects model using a restricted maximum likelihood estimator was used for all meta-analyses. A random effects model was chosen based on the assumption that included studies are heterogeneous because they sample populations with different characteristics using a range of methodologies and therefore one cannot assume that there is a single effect size [20]. A random effects meta-analysis estimates the mean of a distribution of effects rather than estimating a unique effect [20]. We assessed heterogeneity across studies with the Q statistic (with P<0.01 being suggestive of significant heterogeneity) and the I2 statistic (values of 25%, 50%, and 75% were indicative of low, medium, and high heterogeneity). Separate meta-analyses were performed for different brain structures.
Meta-regression was used to investigate the impact of demographic and diabetes characteristics on differences in brain volumes between metabolically healthy individuals and individuals with diabetes, if there were at least 10 studies providing information of a covariate, including age (centred at 60 years), sex, diabetes duration, ratio of diabetes patients taking medication, plasma insulin levels, HbA1c levels and fasting plasma glucose levels, using linear mixed-effects models.
Sensitivity analyses were conducted using the leave-one-out method to identify studies contributing excessively to heterogeneity. Visual evaluation of asymmetry of the funnel plots was used to assess the bias in the meta-analyses results toward publication of studies with significant outcomes. The trim-and-fill method was used to estimate the number of missing studies (representative of unreported effect sizes) in the meta-analysis to estimate adjusted effect sizes.
RESULTS
Literature search and study inclusion
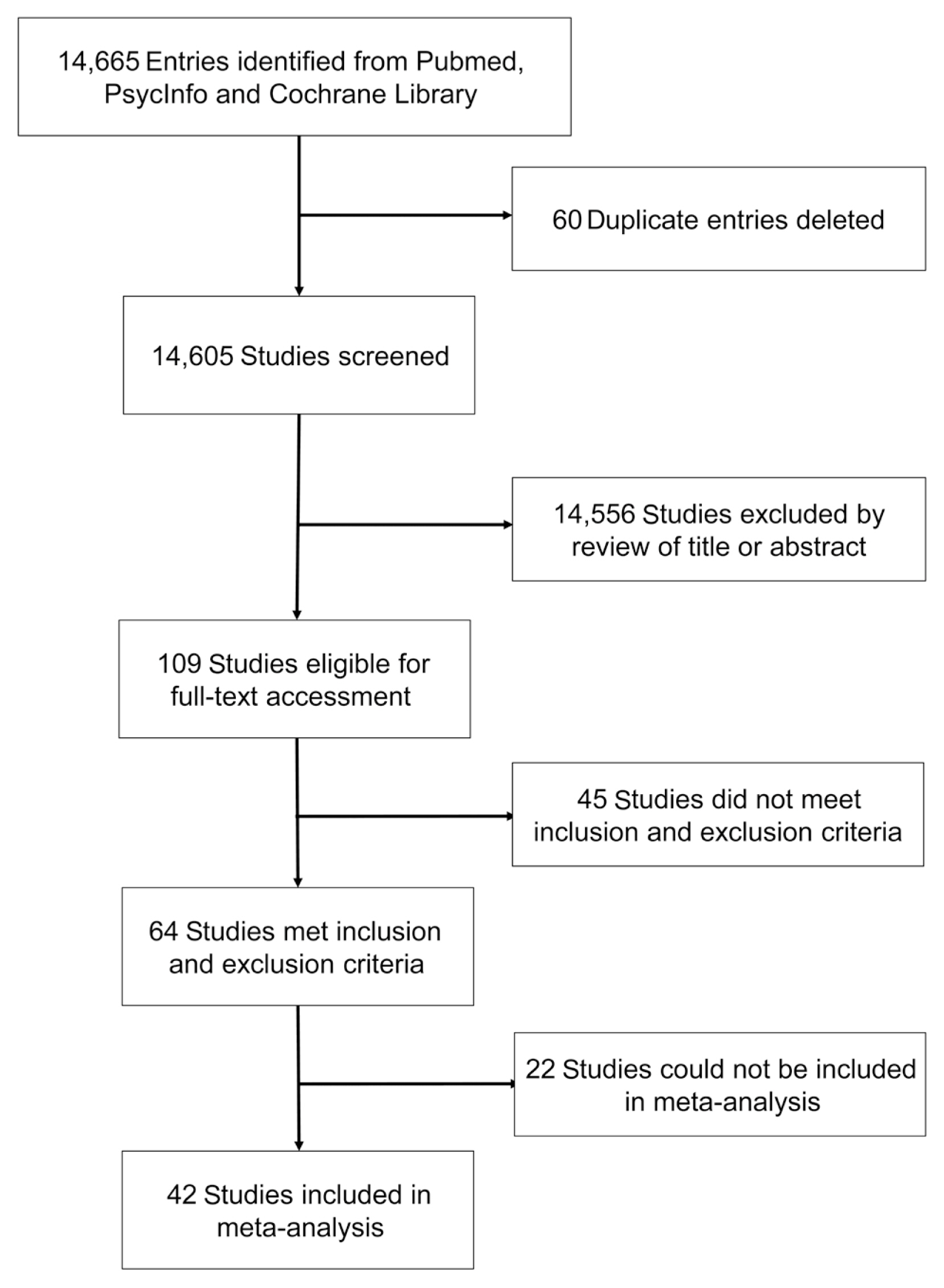
The systematic search identified 10,360 entries from PubMed, 664 from PsycInfo, and 3,641 from Cochrane Library; 60 duplicate entries were identified and excluded. Of these 14,605 studies, 355 studies passed title screening, and 109 studies remained after abstract screening; 64 studies remained after applying inclusion criteria in full-text assessment (Fig. 1).
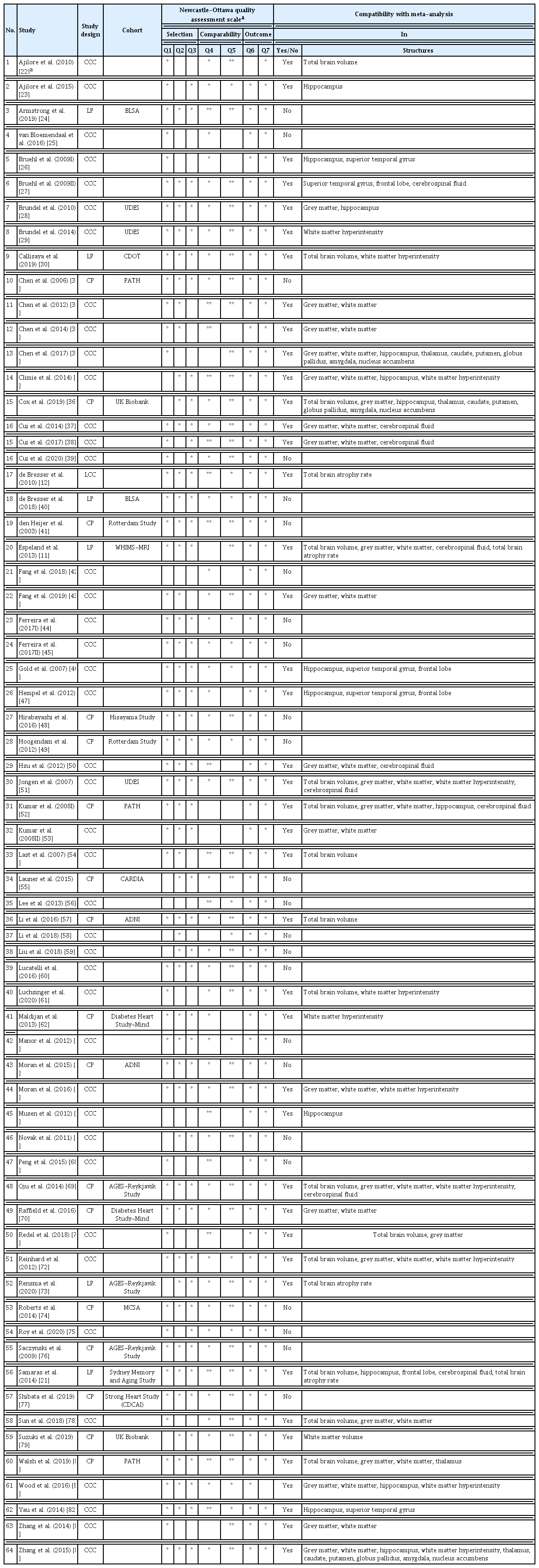
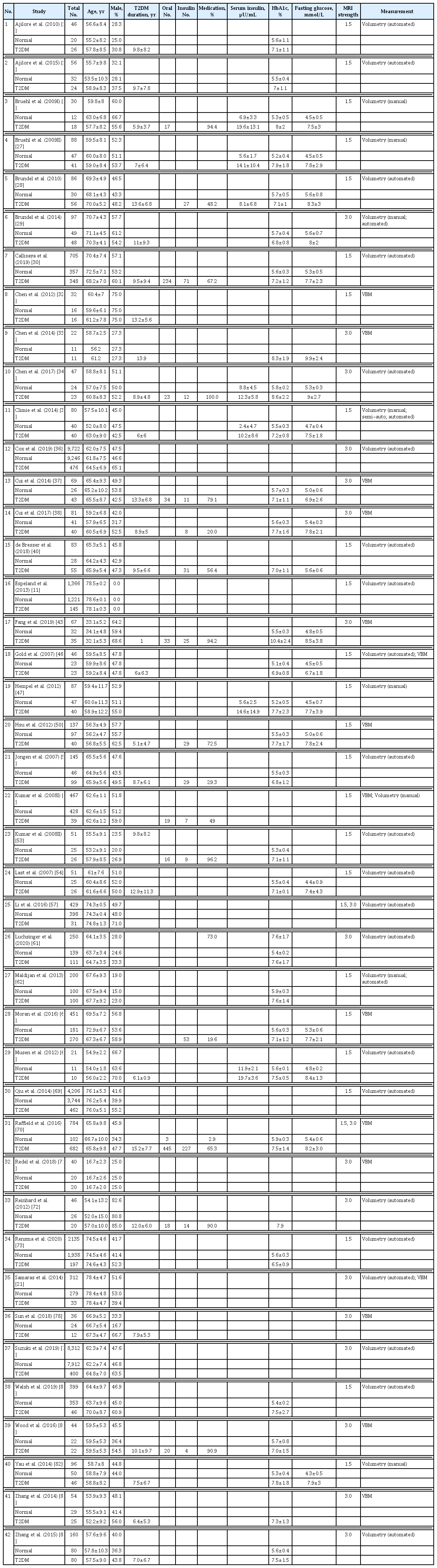
Of the included studies, 40 were cross-sectional case-control, 17 cross-sectional population-based, one longitudinal case-control, and six longitudinal population-based studies. Forty-two studies (n=31,630; mean age 71.0 years; 44.4% male; 26,942 controls; 4,688 diabetes) with enough volumetric data could be considered for meta-analysis. Of these, 25 used automated segmentation, six used manual tracing, and 15 studies applied voxel-based morphometry (Tables 1 and 2) [11,21–84].
Twenty-eight studies reported disease duration, 17 medication status (number of patients taking medication, or specifically taking oral medication or insulin), five plasma insulin levels, 29 HbA1c levels, and 22 fasting plasma glucose levels (for specific demographic and T2DM-related information) (Table 2).
Meta-analysis
Of all the brain regions investigated, only total brain volume, total grey matter, total white matter, hippocampus, thalamus, caudate, putamen, globus pallidus, amygdala, nucleus accumbens, frontal lobe, superior temporal gyrus, total cerebrospinal fluid, and white matter hyperintensity were reported in a sufficient number of studies to be included in meta-analyses. Total brain volume annual atrophy rate was also reported in five longitudinal studies (see Table 1 for details).
Global brain volumes
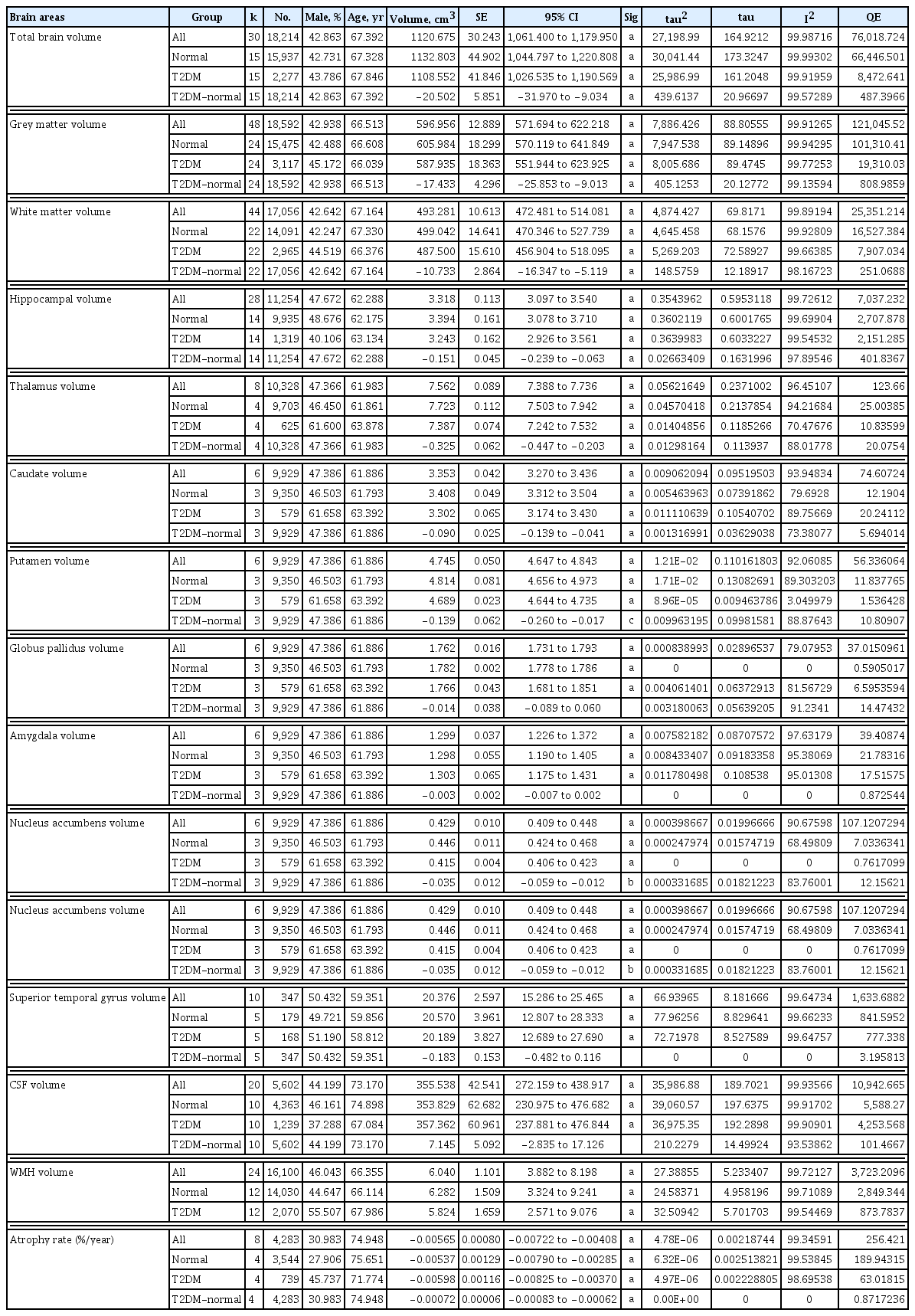
Fifteen studies reported on total brain volume (15,937 normal; 2,277 diabetes), 24 studies on total grey matter volume (15,475 normal; 3,117 diabetes), and 22 studies on total white matter volume (14,091 normal; 2,965 diabetes). Participants with T2DM had significantly smaller total brain volume, with volumetric difference attributable to T2DM (T2DM-normal in Table 3) of 20.50 cm3 (1.81% of normal volume). They also had significantly smaller grey matter volume (−17.43 cm3; 2.88%) and white matter volume (−10.73 cm3; 2.15%) (Table 3, Fig. 2).
Random-effect models of brain volumes and atrophy rates in normal controls and type 2 diabetes mellitus patients, including total volumes and differences between groups

Forest plots of differences in global brain volumes and total brain atrophy rate between participants with and without type 2 diabetes mellitus. (A) Total brain volume difference, (B) grey matter volume difference, (C) white matter volume difference, (D) total brain atrophy difference. CI, confidence interval.
Subcortical volumes
Fourteen studies reported on hippocampal volume (9,935 normal; 1,319 diabetes), four studies on thalamus (9,703 normal; 625 diabetes), and three studies on caudate, putamen, globus pallidus, amygdala, and nucleus accumbens (9,350 normal; 579 diabetes). Participants with T2DM had smaller hippocampus (−0.15 cm3; 4.4%), thalamus (−0.33 cm3; 4.2%), caudate (−0.09 cm3; 2.6%), putamen (−0.14 cm3; 2.9%), globus pallidus (−0.014 cm3; 0.8%), amygdala (−0.003 cm3; 0.2%), and nucleus accumbens volume (−0.035 cm3; 7.8%); these associations were significant in hippocampus, thalamus, caudate, putamen, and nucleus accumbens (Table 3, Supplementary Fig. 1).
Local cortical volumes
Five studies reported on superior temporal gyrus volume (820 normal; 665 diabetes) and five studies on frontal lobe volume (1,617 normal; 282 diabetes). Participants with T2DM had smaller superior temporal gyrus (−0.18 cm3; 0.88%) and smaller frontal lobe volume (−1.04 cm3; 0.71%) but the association was not significant (Table 3, Supplementary Fig. 1).
Total cerebrospinal fluid volume
Ten studies reported on total cerebrospinal fluid volume (4,363 normal; 1,239 diabetes). Participants with T2DM had higher cerebrospinal fluid volume (7.15 cm3; 1%) but the association was not significant (Table 3, Supplementary Fig. 1).
White matter hyperintensities volume
Twelve studies reported on white matter hyperintensities volume (14,030 normal; 2,070 diabetes). Participants with T2DM had smaller white matter hyperintensities volume (−0.006 cm3; 0.001%) but the association was not significant (Table 3, Supplementary Fig. 1).
Total brain atrophy rate
Five longitudinal studies reported on total brain atrophy rate (3,823 normal; 778 diabetes). An initial analysis produced a paradoxical non-significant result given all studies reported a greater atrophy in T2DM, with four being highly significant (Supplementary Fig. 2). A leave-one-out analysis indicated that this perplexing finding was attributable to a single study [21], likely due to it being an outlier in the size of its estimate and having a very large confidence interval, which would unduly inflate the error variance estimate of the analysis. Consequently, a follow-up analysis excluding this study is reported. Participants with T2DM had significantly larger atrophy rate (0.072%; 13.4% larger than normal) (Table 3, Fig. 2).
Inhomogeneity and publication bias
Significant inhomogeneity was observed in Q tests of the meta-analysis, except in thalamus, caudate, putamen, globus pallidus, amygdala, nucleus accumbens, and in T2DM-normal difference in superior temporal gyrus and frontal lobe (Table 3).
Evidence of some publication bias was also detected for most brain regions investigated. Visual inspection of funnel plots revealed that studies were likely missing for total brain volume (13.3% of total), globus pallidus (40%), superior temporal gyrus (16.7%), frontal lobe (28.6%), cerebrospinal fluid (20%), and white matter hyperintensity (20%). Although asymmetry and presence of missing studies suggested some publication bias toward studies reporting higher atrophy rates, trim and fill test indicated that the differences between corrected and reported volumetric differences were generally small and therefore publication bias is unlikely to have significantly influenced the present results (Fig. 3).
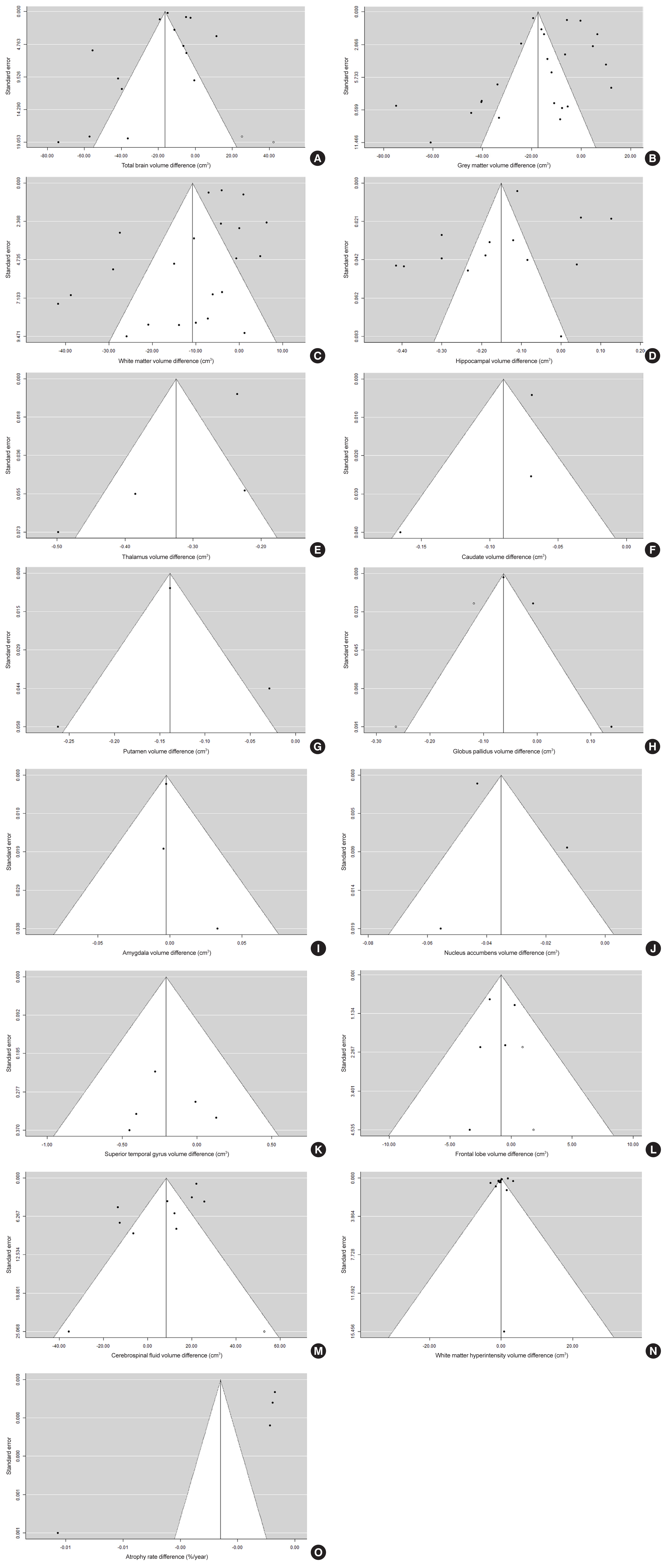
Funnel plots of brain volumes and atrophy rate assessing possible publication bias using the trim and fill method. Filled circles represent studies included in the meta-analysis. Open circles represent possible missing studies. Brain volumes: (A) total brain volume, (B) grey matter, (C) white matter, (D) hippocampus, (E) thalamus, (F) caudate, (G) putamen, (H) globus pallidus, (I) amygdala, (J) nucleus accumbens, (K) superior temporal gyrus, (L) frontal lobe, (M) cerebrospinal fluid, (N) white matter hyperintensity, and (O) total brain atrophy rate._(Continued to the next page)
Meta-regression analyses
The effect of age, sex ratio, diabetes duration, medication, fasting glucose and HbA1c on the association between T2DM and volume of total brain, grey matter, white matter and hippocampus was investigated by meta-regression analysis. A significant negative association between decreasing total brain volume difference and increasing age was detected such that every additional year in age above 60 was associated with a 4.4% smaller volumetric dif ference between individuals with and without diabetes (individuals with diabetes at age 60 years are 28.45cm3 smaller; this difference decreases by 1.24 cm3 per year) (Supplementary Table 1). A significant positive association between decreasing grey matter volume difference and increasing diabetes duration was also detected such that every additional year above mean diabetes duration (10.5 years) in age above 60 was associated with an 8.8% larger volumetric difference between individuals with and without diabetes. No significant effects were observed in other analyses (Supplementary Table 1, Supplementary Fig. 3).
DISCUSSION
The aim of this study was to synthesise the evidence on quantitative differences in brain volumes and rates of brain atrophy associated with T2DM via systematic review and meta-analysis of the published literature. In total, 42 studies including 31,630 participants were included. The main findings indicated that individuals with T2DM had significantly smaller brain volumes compared to those without T2DM, as well as larger atrophy rates. Moreover, T2DM-related volumetric differences appeared to decrease with age and increase with diabetes duration but did not differ between men and women.
Global and local brain volumes
This study’s results are consistent with the findings of previous reviews reporting that brain volumes are smaller in those who live with T2DM, but it also substantially extends our understanding of the scope of this effect. The present study was able to convincingly demonstrate volumetric differences in four brain structures while also precisely summarising their magnitude. It demonstrated that diabetes-related volumetric brain differences were substantial (total brain: 1.88%; grey matter: 2.81%; white matter: 2.15%; hippocampus: 4.4%). Indeed, in normal ageing, total brain volume shrinks by about 0.5% every year from the 40s onwards with further acceleration after age 70 [21]. Similarly, the hippocampus shrinks by about 0.3%/year before 55, 0.85%/year between 55 and 70, and 1.1%/year thereafter in those cognitively intact [16]. Thus, the differences observed in T2DM correspond to about 4 to 5 years of normal ageing, and possibly more. It is also worth noting that while these effects were relatively large across these brain regions, they were particularly strong in the hippocampus. This is noteworthy because subcortical atrophy in the hippocampus is a hallmark of Alzheimer’s disease and is one of the strongest predictors of conversion from mild cognitive impairment to Alzheimer’s disease [85,86]. Importantly, the rate of hippocampal atrophy in mild cognitive impairment has been estimated in a recent meta-analysis to be approximately 2.5%/year [87]. This may suggest that the hippocampal volumetric difference observed in T2DM might lead to an earlier conversion to Alzheimer’s disease by almost 2 years.
Furthermore, the rate of total brain atrophy in T2DM was significantly higher than in metabolically healthy individuals by 13.4%. This is consistent with cross-sectional results, and that cross-sectional volumetric differences may increase with diabetes duration according to meta-regression analysis. However, meta-regression results also revealed that the difference in total brain volume between those with T2DM and metabolically healthy individuals decreased with age (4.4% for every year above 60). This implies that a disease onset before age 60 years may have a greater impact on brain volumes. Moreover, similar consistent trends were observed for grey matter, white matter, and hippocampal volumes. Together, these may suggest that diabetes-related neurodegeneration occurs before onset of diabetes and slows down with increasing age. Hyperglycaemia is the main characteristic of T2DM pathology, and studies on metabolically healthy individuals and individuals with prediabetes have found association between higher blood glucose levels, smaller brain volumes and poorer cognitive functions [21,88,89]. Known mechanisms that may contribute to T2DM-related brain changes, such as hyperglycaemia, vascular disorders and insulin resistance, were likely present before clinical diagnosis [5]. An implication of these findings is that it will be important for future research to investigate brain changes leading to T2DM diagnosis, and to conduct more longitudinal studies following individuals with T2DM over longer periods of time to clarify these issues. From a health policy perspective, it may also suggest that more resources should be directed towards risk reduction interventions in those at risk before the disease develops, rather than mitigate the effects of T2DM when much of the damage has already taken place.
Clinical implications and moderators of T2DM-related brain atrophy
T2DM is a known risk factor of dementia, with increased risk of dementia by two-to-three fold [90]. The current study not only shows substantial brain volume differences that might result in earlier conversion to mild cognitive impairment and Alzheimer’s disease, but also indicates potential predictors and structural basis of cognitive deficit among individuals with T2DM. Previous studies showed evidence of cognitive decline in important functions that may affect self-caring ability of diabetes patients, such as executive function [91] and processing speed [89]. Our study has also found significant associations between brain volume of some subcortical structures and diabetes, even though studies on local volumes were fewer. Indeed, studies on specific cognitive deficits in T2DM are relatively few with inconsistent results, and even fewer studies on both brain volumes and cognition. Further studies with larger sample size are needed to understand how changes at local brain structures are related to cognitive functions of diabetes patients.
We conducted meta-regression analyses to investigate moderators of volumetric differences between metabolically healthy individuals and individuals with T2DM. In this study, meta-regression analyses to examine the moderating effects of age, sex ratio, diabetes duration, ratio of medication, fasting glucose and HbA1c were only possible for some brain structures. These showed that volumetric differences decreased with age, which suggests early pre-clinical occurrence of diabetes-related brain changes. However, an increase in volumetric differences was also observed with diabetes duration in grey matter, and a similar trend in white matter. A likely explanation may be that effects related to T2DM are attributable to pathogenic mechanisms but that they become obscured by the increasingly prevalent, and often related, effects of other risk factors for neurodegeneration including cardiovascular diseases, hyperlipidemia, obesity, and others. The cause of the brain differences reported in the present study may be a combination and interaction of pathogenic mechanisms of T2DM and genetic factors, environmental exposures, age, sex, comorbidities, and medication. Some anti-diabetes oral medications, such as dipeptidyl peptidase 4 inhibitors, metformin, thiazolidinediones and sulfonylurea have potential neuroprotective effects for individuals with diabetes, whereas insulin may be associated with increased risk of dementia [92]. Although lifestyle factors such as high cholesterol diet, smoking, etc. are known risk factors for cognitive decline common among people with T2DM, no conclusive evidence is available on whether cardiovascular risk factor management via lifestyle change, controlling blood pressure and cholesterol levels may reduce the risk of cognitive dysfunction in people with diabetes compared with those without diabetes [93]. While these factors are sometimes reported by studies included in our meta-analysis, usually only some of these factors were reported in one study; reports on medication were often unspecific. This limited the covariates we could control for in our meta-analysis, the types of meta-regression we could conduct and the number of studies that could be included.
Strengths and limitations
The main strengths of this review were an extensive search of the literature using a wide range of search terms across multiple databases, and inclusion of both cross-sectional and longitudinal studies across many brain structures. The main limitation was the relatively small number of studies which could be included in meta-analyses of regional volumes and particularly longitudinal atrophy. This also limited the number of moderators that could be tested in meta-regressions.
CONCLUSIONS
To our knowledge, this is the first meta-analysis that synthesises and precisely quantifies findings from both cross-sectional and longitudinal studies on the association between T2DM and brain atrophy. Results showed that T2DM is associated with smaller total and regional brain volumes with this difference decreasing at older ages. These effects are important and highlight an urgent need for the development of interventions to prevent them. How T2DM-related brain atrophy changes over time, and in the pre-clinical stages of the disease is unclear based on the available evidence and requires further investigation.
SUPPLEMENTARY MATERIALS
Supplementary materials related to this article can be found online at https://doi.org/10.4093/dmj.2021.0189.
Results from meta-regression analyses for association between T2DM-normal brain volume differences and age, sex ratio, T2DM duration, fasting glucose, and HbA1c
Forest plots of differences in local brain volumes between participants with and without type 2 diabetes mellitus. (A) Hippocampus, (B) caudate, (C) thalamus, (D) putamen, (E) globus pallidus, (F) amygdala, (G) nucleus accumbens, (H) superior temporal gyrus, (I) frontal lobe, (J) cerebrospinal fluid, and (K) white matter hyperintensity. CI, confidence interval.
Forest plot of difference in brain atrophy rates between participants with and without type 2 diabetes mellitus, including the excluded study [21]. CI, confidence interval.
Association between type 2 diabetes mellitus-normal brain volume differences and age, sex ratio, diabetes duration, medication, fasting glucose, and glycosylated hemoglobin. (A) Age and total brain volume, (B) age and grey matter volume, (C) age and white matter volume, (D) age and hippocampal volume, (E) age and cerebrospinal fluid volume, (F) age and white matter hyperintensity volume, (G) sex ratio and total brain volume, (H) sex ratio and grey matter volume, (I) sex ratio and white matter volume, (J) sex ratio and hippocampal volume, (K) sex ratio and cerebrospinal fluid volume, (L) sex ratio and white matter hyperintensity volume, (M) diabetes duration and grey matter volume, (N) diabetes duration and white matter volume, (O) diabetes duration and hippocampal volume, (P) fasting glucose and grey matter volume, (Q) fasting glucose and white matter volume, (R) HbA1c and grey matter volume, and (S) HbA1c and white matter volume.
ACKNOWLEDGMENTS
The authors are grateful to Dr. Simon Cox of University of Edinburgh, Prof. Jose Luchsinger and Mr. Brady Rippon of University of Colombia (support for the reported work was provided by United States National Institutes of Health grants R01AG050440, RF1AG051556, and K24AG045334), and Assis. Prof. Hideaki Suzuki of Tohoku University for providing additional data for meta-analysis.
Notes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
FUNDING
The study was supported by Australian Research Council grant No. 120100227, 130101705, and National Health and Medical Research Council of Australia grant No. 1063907.