- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Articles
- Page Path
- HOME > Diabetes Metab J > Volume 46(5); 2022 > Article
-
Original ArticleTechnology/Device Glucose Profiles Assessed by Intermittently Scanned Continuous Glucose Monitoring System during the Perioperative Period of Metabolic Surgery
-
Kyuho Kim1
 , Sung Hee Choi1,2, Hak Chul Jang1,2, Young Suk Park3
, Sung Hee Choi1,2, Hak Chul Jang1,2, Young Suk Park3 , Tae Jung Oh1,2
, Tae Jung Oh1,2
-
Diabetes & Metabolism Journal 2022;46(5):713-721.
DOI: https://doi.org/10.4093/dmj.2021.0164
Published online: January 24, 2022
1Department of Internal Medicine, Seoul National University Bundang Hospital, Seongnam, Korea
2Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea
3Department of Surgery, Seoul National University Bundang Hospital, Seongnam, Korea
-
Corresponding authors: Young Suk Park,
 , Department of Internal Medicine, Seoul National University Bundang Hospital, Seoul National University College of Medicine, 82 Gumi-ro 173beon-gil, Bundang-gu, Seongnam 13620, Korea E-mail: ohtjmd@gmail.com
, Department of Internal Medicine, Seoul National University Bundang Hospital, Seoul National University College of Medicine, 82 Gumi-ro 173beon-gil, Bundang-gu, Seongnam 13620, Korea E-mail: ohtjmd@gmail.com
Copyright © 2022 Korean Diabetes Association
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (https://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
-
Background
- Continuous glucose monitoring (CGM) has been widely used in the management of diabetes. However, the usefulness and detailed data during perioperative status were not well studied. In this study, we described the immediate changes of glucose profiles after metabolic surgery using intermittently scanned CGM (isCGM) in individuals with type 2 diabetes mellitus (T2DM).
-
Methods
- This was a prospective, single-center, single-arm study including 20 participants with T2DM. The isCGM (FreeStyle Libre CGM) implantation was performed within 2 weeks before surgery. We compared CGM metrics of 3 days before surgery and 3 days after surgery, and performed the correlation analyses with clinical variables.
-
Results
- The mean glucose significantly decreased after surgery (147.0±40.4 to 95.5±17.1 mg/dL, P<0.001). Time in range (TIR; 70 to 180 mg/dL) did not significantly change after surgery in total. However, it was significantly increased in a subgroup of individuals with glycosylated hemoglobin (HbA1c) ≥8.0%. Time above range (>250 or 180 mg/dL) was significantly decreased in total. In contrast, time below range (<70 or 54 mg/dL) was significantly increased in total and especially in a subgroup of individuals with HbA1c <8.0% after surgery. The coefficient of variation significantly decreased after surgery. Higher baseline HbA1c was correlated with greater improvement in TIR (rho=0.607, P=0.005).
-
Conclusion
- The isCGM identified improvement of mean glucose and glycemic variability, and increase of hypoglycemia after metabolic surgery, but TIR was not significantly changed after surgery. We detected an increase of TIR only in individuals with HbA1c ≥8.0%.
- Metabolic surgery is an effective treatment modality for individuals with obesity and diabetes in terms of their significant and sustained weight loss and diabetes remission [1]. The improvement of glucose homeostasis occurs within days after the surgery before weight loss occurs [2,3]. Therefore, the rapid tapering of antidiabetic drugs is mandatory [4]. There is a need to assess delicate glucose profiles to perform precise adjustment of medication and nutritional support. However, few studies have investigated immediate changes in glucose levels during the perioperative period.
- It has been well known that continuous glucose monitoring (CGM) systems are useful in both diabetes management and clinical research [5,6]. Two types of CGM systems are available: real-time CGM (rtCGM) and intermittently scanned CGM (isCGM). Compared with rtCGM, isCGM does not provide real-time results or alerts for current or impending glucose events and it must be used actively to obtain data. However, isCGM does not require capillary glucose calibration and it is easy to use periodically. It is also less expensive than rtCGM because it does not require a separate transmitter [7]. Therefore, isCGM is a good option for individuals who undertake CGM for the first time and during certain limited times, such as the perioperative period.
- Previous studies have reported CGM data immediately after metabolic surgery. Yip et al. [8] obtained 6-day CGM recordings from obese individuals with type 2 diabetes mellitus (T2DM) starting 3 days before Roux-en-Y gastric bypass (RYGB; n=11) or sleeve gastrectomy (SG; n=10) using CGMS Gold (Medtronic, Northridge, CA, USA). Wysocki et al. [9] obtained 10-day CGM recordings from obese individuals with T2DM starting 1 day before RYGB (n=10) or SG (n=6) using FreeStyle Libre CGM (Abbott Diabetes Care, Alameda, CA, USA). However, these studies did not report CGM data using standardized metrics. In addition, the CGM device used in Yip et al.’s study [8] was an outdated model with low accuracy (mean absolute relative difference [MARD], 14%) [10]. These limitations indicate the need for a study with sufficient sample size and standardized CGM metrics to determine the immediate improvement of hyperglycemia and detect hypoglycemia after metabolic surgery.
- In this study, we aimed to investigate the changes in standardized CGM metrics according to the international consensus [11,12] by using isCGM to determine the degree and rapidity of glycemic changes, including hypoglycemia before and after metabolic surgery.
INTRODUCTION
- Study participants
- This was a prospective, single-center, single-arm study at the Seoul National University Bundang Hospital (SNUBH). The inclusion criteria were: age ≥19 years and diagnosis of T2DM with body mass index (BMI) ≥30.0 kg/m2, or medically uncontrolled T2DM with BMI ≥27.5 kg/m2 according to the National Health Insurance reimbursement in South Korea. The exclusion criteria were: previous metabolic surgery, isCGM (FreeStyle Libre CGM) incompatible smartphone user, or concurrent use of antiobesity medications.
- Procedures
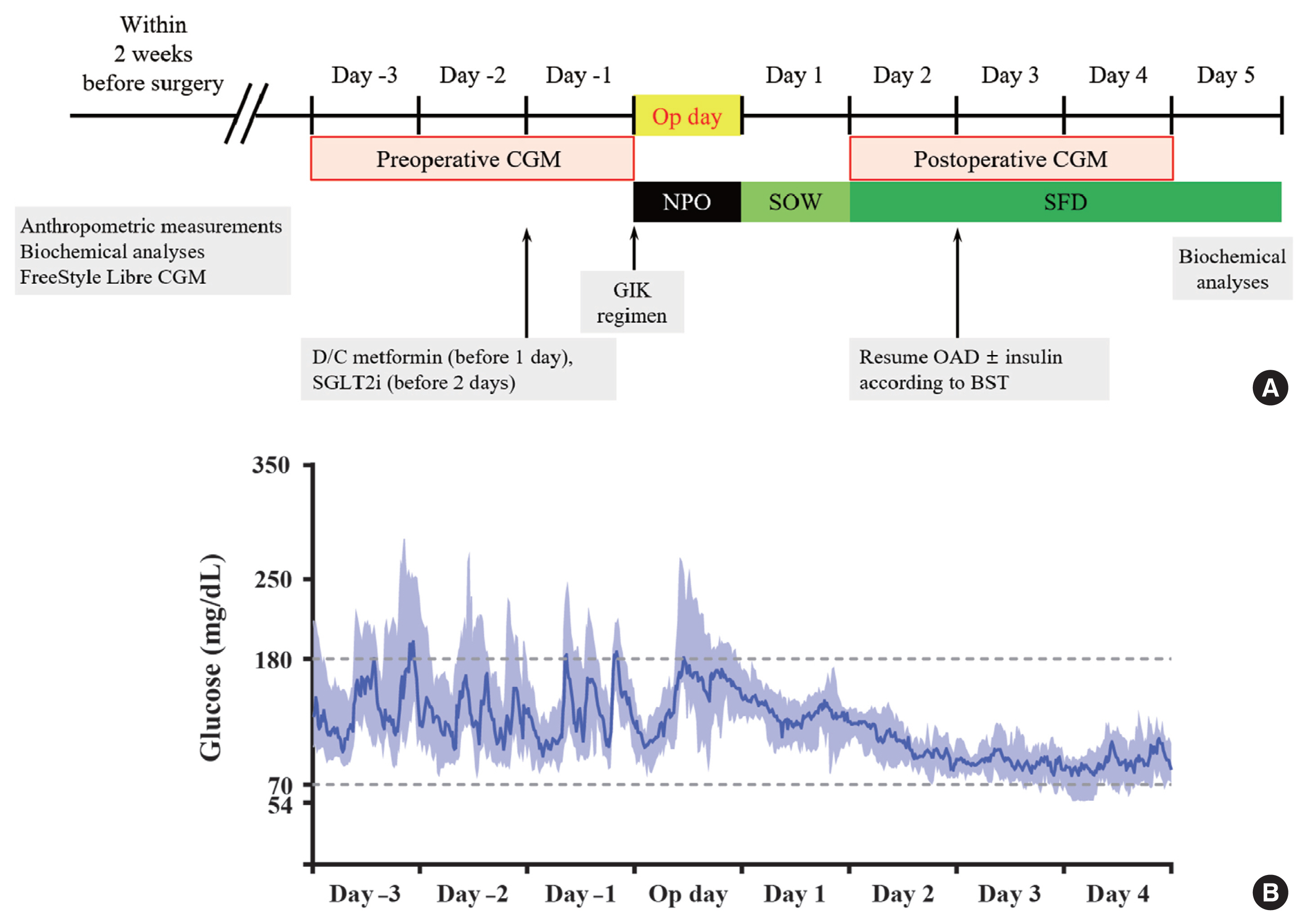
- The study design is presented in Fig. 1A. Anthropometric assessment and isCGM (FreeStyle Libre CGM) implantation were performed within 2 weeks before surgery. The isCGM continued for 14 consecutive days and was changed after 14 days. All CGM data were downloaded using LibreView software (Newyu Inc., Orlando, FL, USA), and transformed into Excel (Microsoft, Redmond, WA, USA) data files for analysis. To minimize the missing values, the CGM data collected during the 3 days before surgery (days −3, −2, and −1) and 3 days after surgery (days 2, 3, and 4) were compared. We excluded the data from the operation day and day 1 because of stress-induced hyperglycemia during the 24 hours immediately after metabolic surgery [8]. During the admission period, point-of-care capillary glucose testing (POCT) was performed by ward nurses using a glucometer (BAROZEN H expert Plus, i-SENS Inc., Seoul, Korea) four times a day before meals and at bedtime. Each POCT blood glucose was paired with the corresponding CGM value within 5 minutes and used for accuracy analysis.
- Blood samples were collected after an overnight fast within 2 weeks before surgery and at days 3 to 5 after surgery. Plasma glucose levels were measured using the hexokinase method and glycosylated hemoglobin (HbA1c) levels were measured by high-performance liquid chromatography (Bio-Rad, Hercules, CA, USA). Total cholesterol, triglycerides, high-density lipoprotein cholesterol, and low-density lipoprotein cholesterol were measured by enzymatic colorimetric assay. Serum creatinine was measured by the protocol of the central laboratory of SNUBH and estimated glomerular filtration rate was calculated by the Modification of Diet in Renal Disease equation. Serum insulin (DIAsource ImmunoAssays, Nivelles, Belgium) and C-peptide (Izotop, Budapest, Hungary) were measured by radioimmunoassay. Free fatty acid (FFA) was measured by AU5800 clinical chemistry analyzer (Beckman Coulter, Brea, CA, USA).
- Sodium-glucose cotransporter-2 inhibitor and metformin were discontinued 2 days and 1 day before the surgery, respectively. On admission day, a normocaloric diet was supplied. Nothing by mouth and a standardized glucose–insulin–potassium infusion [13] were started at midnight before surgery. Sips of water started on day 1 after surgery (day 1), followed by soft fluid diet on day 2 after surgery (day 2). From day 2, oral antidiabetic drugs (OADs) and insulin were resumed to achieve a target glucose range of 140 to 180 mg/dL.
- The study was approved by the Institutional Review Board of SNUBH (No. B-2007-624-305) and each participant provided written informed consent. The study was conducted in accordance with the Declaration of Helsinki and good clinical practice guidelines. This study was registered with the Clinical Research Information Service (CRiS, Korea, https://cris.nih.go.kr; registration number: KCT0005240).
- Outcomes
- The primary endpoint was the mean difference in time in range (TIR; 70 to 180 mg/dL) before and after surgery. The secondary endpoints were the mean difference in mean glucose (total, daytime, and nighttime), the mean difference in coefficient of variation (CV; total, daytime, and nighttime), the mean difference in time above range (TAR; >180 or >250 mg/dL) and the mean difference in time below range (TBR; <70 or <54 mg/dL; total, daytime, and nighttime) before and after surgery. In addition, we assessed these outcomes stratified by HbA1c level (HbA1c <8.0% and ≥8.0%) and surgery types (SG and bypass surgery), and correlations between preoperative clinical variables with the difference in CGM metrics and homeostatic model assessment for insulin resistance (HOMA-IR).
- We defined daytime from 6:00 AM to midnight, nighttime from midnight to 6:00 AM. The MARD was calculated using matched glucose pairs from POCT and isCGM, and expressed as a percentage. Glucose variability was calculated as CV= standard deviation (SD)/mean glucose×100%.
- Calculations
- HOMA-IR was calculated as follows: fasting insulin (μIU/mL)× fasting plasma glucose (FPG, mg/dL)/405. Homeostatic model assessment for beta cell function (HOMA-B) was calculated as follows: 360×fasting insulin (μIU/mL)/(FPG, mg/dL–63). Adipose tissue insulin resistance (Adipo-IR) was calculated as follows: fasting insulin (pmol/L)×fasting FFA (mmol/L) [14]. Improvement in TIR was calculated as follows: TIR after surgery–TIR before surgery. Decrease in HOMA-IR was calculated as follows: HOMA-IR before surgery–HOMA-IR after surgery.
- Statistical analysis
- We calculated that a sample size of 11 participants would provide 90% power with a type I error rate (two-sided) of 5% to reject the null hypothesis of no difference in the TIR before and after the surgery, under the assumption that the TIR after the surgery would be 30% higher than TIR before the surgery, with a SD of 30% [8]. Data were expressed as mean±SD or number (%). Comparisons of continuous variables were performed using Wilcoxon signed-rank test. Spearman’s correlation coefficient was used to evaluate the correlation between variables. In all cases, P<0.05 was considered statistically significant. Statistical analyses were performed using IBM SPSS software version 25.0 (IBM Corp., Armonk, NY, USA). Figures were drawn using GraphPad Prism software version 9.1.2 (GraphPad Software Inc., San Diego, CA, USA).
METHODS
- Between July 2020 and April 2021, 148 subjects underwent metabolic surgery in SNUBH, a tertiary academic hospital in South Korea. Among them, 45 subjects had T2DM and 22 participants were enrolled. After two participants dropped out due to poor compliance, the remaining 20 participants (five men, 15 women) were included in the final analysis (Supplementary Fig. 1). Table 1 shows the baseline characteristics of the study participants. The participants were 47.2±9.1 years old with a diabetes duration of 5.6±7.0 years. Preoperative BMI was 37.2±5.7 kg/m2 and HbA1c was 8.1%±1.8%. Nine participants used insulin therapy. Ten participants underwent laparoscopic sleeve gastrectomy (LSG) with duodenojejunal bypass, six underwent LSG, three underwent RYGB, and one underwent laparoscopic biliopancreatic diversion.
- Fig. 1B shows the CGM profiles before and after metabolic surgery. Both median and interquartile range of glucose reduced rapidly after surgery. Five out of nine participants discontinued insulin therapy after surgery. Sulfonylureas was discontinued after surgery in all participants.
- The CGM metrics were compared before and after metabolic surgery (Table 2). The percentage of time CGM is active was 90.4%±13.3% before surgery and 79.8%±19.7% after surgery. The MARD value was 19.6%. The mean total, daytime, or night-time glucose levels were significantly decreased after surgery compared with before surgery (147.0±40.4 mg/dL vs. 95.5± 17.1 mg/dL, P<0.001; 149.8±39.4 mg/dL vs. 95.9±17.4 mg/dL, P<0.001; 138.0±64.5 mg/dL vs. 91.1±20.0 mg/dL, P=0.002, respectively). CVs for total or daytime glucose were significantly decreased after surgery compared with those before surgery (29.2%±9.9% vs. 20.1%±9.0%, P=0.005; 28.3%±9.6% vs. 19.6%±8.8%, P=0.012, respectively). The TIR was not significantly changed after surgery. TAR (>250 or 180 mg/dL) was significantly decreased (6.8%±12.4% vs. 0.0%±0.0%, P=0.005; 23.9%±25.3% vs. 1.1%±5.0%, P<0.001), and in contrast, TBR (<70 or 54 mg/dL) was significantly increased after the surgery (3.0%±5.7% vs. 16.1%± 23.9%, P=0.019; 0.4%±0.9% vs. 5.4%± 10.7%, P=0.035). During nighttime, TBR (<54 mg/dL) was significantly increased after surgery (0.1%±0.5% vs. 10.2%± 21.0%, P=0.043).
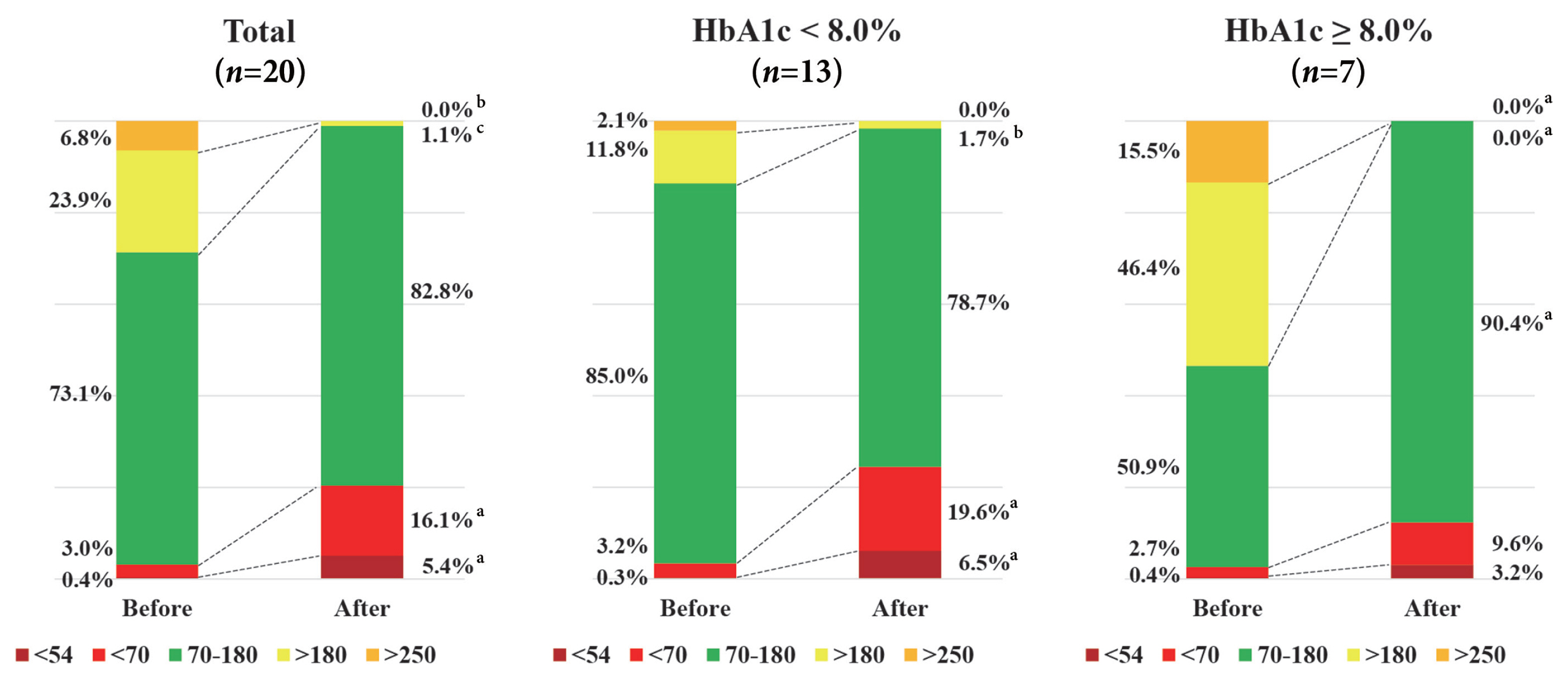
- We performed subgroup analysis stratified by preoperative HbA1c levels (Fig. 2). Among individuals with HbA1c <8.0% (n=13), TAR (>180 mg/dL) was significantly decreased (11.8%± 13.4% vs. 1.7%±6.2%, P=0.002), but TBR (<70 or 54 mg/dL) was significantly increased after surgery (3.2%±5.6% vs. 19.6%± 25.4%, P=0.013; 0.3%±0.7% vs. 6.5%±12.1%, P=0.046). Overall, this resulted in no significant change in TIR after surgery in this group. In contrast, among individuals with poor glycemic control (HbA1c ≥8.0%, n=7), TAR (>250 or 180 mg/dL) was significantly decreased (15.5%±16.7% vs. 0.0%±0.0%, P= 0.027; 46.4%±27.7% vs. 0.0%±0.0%, P=0.018) and as a result TIR was increased after surgery (50.9%±24.8% vs. 90.4%± 20.9%, P=0.018). In addition, we performed subgroup analysis stratified by surgery types (SG [n=6] and bypass surgery [n= 14]) (Supplementary Table 1). Individuals in SG group were younger (40.0±7.8 years old) and had a higher BMI (42.1±6.4 kg/m2) compared with those in bypass surgery group (age of 50.2±8.0 years old and BMI of 35.1±3.9 kg/m2). Mean glucose levels (total and daytime) and TAR (>180 mg/dL) were consistently decreased after surgery compared to before surgery regardless of surgical types. However, mean nighttime glucose levels, CV (total and daytime), and TAR (>250 mg/dL) were significantly decreased after bypass surgery, but they were not significantly decreased after SG.
- Insulin, FPG, C-peptide, and HOMA-IR were all significantly decreased immediately after surgery (within 3 to 5 days). However, there was no significant change in HOMA-B and Adipo-IR (Table 3). Interestingly, higher HbA1c was correlated with greater improvement in TIR (rho=0.607, P=0.005) and younger age was correlated with greater decrease in HOMA-IR (rho=−0.560, P=0.030) (Supplementary Fig. 2).
- Based on a total of 179 paired POCT-CGM measurements, the Clarke error grid analysis showed 99.4% of glucose values falling into clinically acceptable error zones A and B; 41.3% of values fell within zone A, 58.1% within zone B, and 0.5% within zone D (Supplementary Fig. 3).
RESULTS
- In this prospective study, we successfully detected the degree and rapidity of glycemic changes using isCGM in individuals who underwent metabolic surgery even though the compliance of isCGM was attenuated a little after surgery. In total, TBR (<70 or 54 mg/dL) was significantly increased and TAR (>250 or 180 mg/dL) was significantly decreased after surgery. Overall, this resulted in no significant change in TIR after surgery. A significant increase in TIR was only observed in individuals with poorly controlled diabetes (HbA1c ≥8.0%). However, the mean glucose of total, either daytime or nighttime, and CV for total or daytime glucose were decreased consistently after surgery compared with before surgery.
- Both TIR and CV have the benefit of assessing an individual’s glycemic profile more detailed than an assessment of HbA1c alone. Furthermore, these two metrics associate with diabetes complications [15]. In our study, we found that the change in TIR after metabolic surgery depended on baseline HbA1c. Improvement in TIR was significantly higher in individuals with HbA1 ≥8.0% compared with individuals with HbA1c <8.0% (P=0.004). In addition, a significant decrease in CV during nighttime was observed in individuals with HbA1c ≥8.0%, which means that dietary intake had little impact on the improvement of glycemic variability in this group. In this regard, metabolic surgery might be an appropriate option for rapid glucose control in individuals with poorly controlled diabetes.
- An earlier study of 26 individuals undergoing cardiac surgery using the FreeStyle Libre CGM showed reliable, but lower accurate results (Clarke error grid: 99.1% within zones A and B, 18.9% in zone A) compared with the Eirus intravascular microdialysis CGM (Maquet Critical Care, Solna, Sweden) conducted from the day before surgery to the day after surgery [16]. Another study of 15 individuals undergoing cardiopulmonary bypass surgery using the Dexcom G6 CGM (Dexcom, San Diego, CA, USA) showed that some sensors maintained precision, but lost accuracy after surgery [17]. In our study, the MARD value of 19.6% was higher than a previously reported value of 11.4% [18]. In addition, the MARD value of 15.3% before surgery increased significantly to 21.7% after surgery. A higher proportion of TBR after surgery might affect this phenomenon. Nevertheless, it is necessary to consider the possibility that the accuracy of sensors might be reduced during surgery.
- Previous studies showed that CGM could detect hypoglycemia effectively in individuals who underwent metabolic surgery at over 1 year after surgery [19,20]. In our study, we could detect rapid glycemic changes by applying isCGM during the perioperative period and discontinued insulin and OAD proactively. In addition, there was no difference in TBR after surgery between individuals stratified by surgical type (with or without bypass) or baseline antidiabetic drugs (data not shown). Although TBR (<70 or 54 mg/dL) was significantly increased after surgery, only one individual experienced symptomatic hypoglycemia. Considering that increased TBR was more frequently observed in clinical trials using isCGM compared with those using rtCGM [21], and that accuracy of FreeStyle Libre CGM was lower in the hypoglycemic range (<70 mg/dL) [22], we should be cautious in interpretation of the TBR results. Nevertheless, considering significant increase in TBR after surgery among individuals with HbA1c <8.0%, we should be cautious to resume antidiabetic drugs during the postoperative period of metabolic surgery to avoid hypoglycemia in this subgroup.
- In general, bypass surgery seems to be more effective than simple restrictive surgery in terms of diabetes remission during long-term follow-up [23]. In our study, we added early improvement of glycemic variability after bypass surgery, which finding was not statistically significant in SG. However, our study was not designed to evaluate the difference of bypass surgery and SG, and baseline characteristics of participants were not comparable between two surgical types. In addition, a previous study showed significant decreases of both mean glucose concentration and glycemic variability after SG in subjects with T2DM [24]. Further large scale study is necessary to see the early difference in glucose profiles between bypass surgery and SG.
- For preoperative prediction of T2DM remission after metabolic surgery, score systems such as ABCD score [25] and DiaRem score [26] have been proposed. These score systems include age as a factor, which is associated with T2DM remission rate. Interestingly, we observed greater decrease in HOMA-IR in individuals with younger age within 1 week after surgery in this study. This observation raises the possibility that early glycemic improvement after metabolic surgery could predict long-term T2DM remission. In this clinical perspective, we are planning a 1-year follow-up study with isCGM to evaluate whether this glycemic improvement after metabolic surgery will be maintained.
- Previous study of obese patients with T2DM showed that fasting plasma FFA levels increased by approximately 20% at 1 week, returned to preoperative values at 3 months, and was slightly decreased after 1 year after RYGB [27]. Another study showed that fasting plasma FFA levels were higher at 2 weeks after RYGB compared with those at baseline, and suggested that energy intake deficit led to decrease plasma insulin levels, thereby reducing inhibition of lipolysis [28]. Stress response of general anesthesia and surgery itself can increase catabolic hormones (epinephrine, norepinephrine, cortisol, glucagon, and growth hormone), and promote lipolysis and finally release FFA into the circulation [29]. Even though we did not measure any counter-regulatory hormones, we could assume that both a decrease of insulin levels and an increase of catabolic hormones were responsible for an increase of serum FFA levels within 1 week after metabolic surgery.
- Our study has several limitations. First, the actual time-periods of CGM application were diverse between participants. In the final analysis, we compared the CGM metrics using only 3-day values before and after surgery, respectively to minimize the missing value. As a result, the duration of isCGM application was short. Even though the data from 7 days before surgery and those from 3 days before surgery were not statistically different (data not shown), further longer-term comparison might be necessary. Second, we did not monitor individuals’ caloric intake during the perioperative period. Third, the MARD values after surgery were higher than those before surgery. Despite these limitations, this study has several strengths. This is the first study that provides a detailed picture of the degree and rapidity of glycemic improvement during the perioperative period using isCGM. In addition, this study suggests the feasibility of using isCGM during the perioperative period. We found no complications related to the isCGM device, no interference with surgical devices, such as an electronic coagulator, and reliable performance after surgery.
- In conclusion, the isCGM can provide the detailed information about immediate dynamic changes of glucose levels. We identified improvement of both mean glucose and glycemic variability, and increase of hypoglycemia after metabolic surgery. However, TIR was not different between pre- and post-operative periods. We identified an increase of TIR only in individuals with HbA1c ≥8.0%.
DISCUSSION
SUPPLEMENTARY MATERIALS
-
CONFLICTS OF INTEREST
Sung Hee Choi has been editorial board member of the Diabetes & Metabolism Journal since 2021. She was not involved in the review process of this article. Otherwise, there was no conflict of interest.
-
AUTHOR CONTRIBUTIONS
Conception or design: K.K., Y.S.P., T.J.O.
Acquisition, analysis, or interpretation of data: K.K., S.H.C., H.C.J., Y.S.P., T.J.O.
Drafting the work or revising: K.K., Y.S.P., T.J.O.
Final approval of the manuscript: K.K., S.H.C., H.C.J., Y.S.P., T.J.O.
-
FUNDING
None
NOTES
-
Acknowledgements
- The authors sincerely thank all the participants for their cooperation. In addition, we thank Ms. Gayoung Shin for her assistance.
ACKNOWLEDGMENTS

Values are presented as mean±standard deviation or number (%).
BMI, body mass index; BP, blood pressure; FPG, fasting plasma glucose; HbA1c, glycosylated hemoglobin; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; eGFR, estimated glomerular filtration rate; LSG/DJB, laparoscopic sleeve gastrectomy with duodenojejunal bypass; RYGB, Roux-en-Y gastric bypass; LBPD, laparoscopic biliopancreatic diversion.
| Before surgery | After surgery | Mean difference (95% CI) | P value | |
|---|---|---|---|---|
| Time CGM active, % | 90.4±13.3 | 79.8±19.7 | 10.59 (0.8 to 20.4) | 0.033 |
| Mean glucose, mg/dL | 147.0±40.4 | 95.5±17.1 | 51.6 (33.9 to 69.2) | <0.001 |
| Mean glucose during daytimea, mg/dL | 149.8±39.4 | 95.9±17.4 | 53.9 (38.0 to 69.8) | <0.001 |
| Mean glucose during nighttimeb, mg/dL | 138.0±64.5 | 91.1±20.0 | 46.9 (13.0 to 80.8) | 0.002 |
| CV, % | 29.2±9.9 | 20.1±9.0 | 9.1 (3.2 to 14.9) | 0.005 |
| CV during daytimea, % | 28.3±9.6 | 19.6±8.8 | 8.7 (2.7 to 14.7) | 0.012 |
| CV during nighttimeb, % | 21.2±11.3 | 17.1±11.3 | 4.2 (−4.9 to 13.2) | 0.184 |
| TAR >250 mg/dL, % | 6.8±12.4 | 0.0±0.0 | 6.8 (1.0 to 12.6) | 0.005 |
| TAR >180 mg/dL, % | 23.9±25.3 | 1.1±5.0 | 22.8 (10.8 to 34.7) | <0.001 |
| TIR 70−180 mg/dL, % | 73.1±24.2 | 82.8±24.5 | −9.7 (−26.7 to 7.3) | 0.247 |
| TBR <70 mg/dL, % | 3.0±5.7 | 16.1±23.9 | −13.1 (−23.5 to −2.7) | 0.019 |
| TBR <70 mg/dL during daytimea, % | 2.3±4.9 | 13.7±23.0 | −11.4 (−21.1 to −1.7) | 0.021 |
| TBR <70 mg/dL during nighttimeb, % | 4.1±8.4 | 17.3±27.5 | −13.2 (−26.8 to 0.4) | 0.068 |
| TBR <54 mg/dL, % | 0.4±0.9 | 5.4±10.7 | −5.0 (−9.8 to −0.2) | 0.035 |
| TBR <54 mg/dL during daytimea, % | 0.4±1.1 | 3.9±8.1 | −3.5 (−7.1 to 0.1) | 0.050 |
| TBR <54 mg/dL during nighttimeb, % | 0.1±0.5 | 10.2±21.0 | −10.1 (−20.2 to 0.0) | 0.043 |
| Before surgery | After surgery | Mean difference (95% CI) | P value | |
|---|---|---|---|---|
| FPG, mg/dL | 164.1±64.6 | 113.1±21.7 | 51.0 (19.0 to 82.9) | 0.001 |
| Insulin, μIU/mLa | 16.4±12.9 | 10.2±2.8 | 6.2 (−0.2 to 12.6) | 0.016 |
| C-peptide, ng/mLa | 3.9±2.3 | 2.3±1.3 | 1.6 (0.7 to 2.5) | 0.001 |
| FFA, μEq/Lb | 470.3±195.8 | 689.4±225.9 | −219.1 (−343.5 to −94.7) | 0.008 |
| HOMA-IRa | 6.6±4.9 | 2.8±1.2 | 3.8 (1.3 to 6.3) | 0.003 |
| HOMA-Ba | 81.8±74.3 | 111.2±96.1 | −29.4 (−103.4 to 44.6) | 0.173 |
| Adipo-IRc | 61.4±61.9 | 53.8±30.5 | 7.6 (−19.4 to 34.5) | 0.959 |
Values are presented as mean±standard deviation.
CI, confidence interval; FPG, fasting plasma glucose; FFA, free fatty acid; HOMA-IR, homeostatic model assessment for insulin resistance; HOMA-B, homeostatic model assessment for beta cell function; Adipo-IR, adipose tissue insulin resistance.
a n=15,
b n=11,
c n=10.
- 1. Nguyen NT, Varela JE. Bariatric surgery for obesity and metabolic disorders: state of the art. Nat Rev Gastroenterol Hepatol 2017;14:160-9.ArticlePubMedPDF
- 2. Wickremesekera K, Miller G, Naotunne TD, Knowles G, Stubbs RS. Loss of insulin resistance after Roux-en-Y gastric bypass surgery: a time course study. Obes Surg 2005;15:474-81.ArticlePubMedPDF
- 3. Pories WJ, Swanson MS, MacDonald KG, Long SB, Morris PG, Brown BM, et al. Who would have thought it?: an operation proves to be the most effective therapy for adult-onset diabetes mellitus. Ann Surg 1995;222:339-52.ArticlePubMedPMC
- 4. Busetto L, Dicker D, Azran C, Batterham RL, Farpour-Lambert N, Fried M, et al. Practical recommendations of the Obesity Management Task Force of the European Association for the Study of Obesity for the post-bariatric surgery medical management. Obes Facts 2017;10:597-632.ArticlePubMedPMCPDF
- 5. Vigersky R, Shrivastav M. Role of continuous glucose monitoring for type 2 in diabetes management and research. J Diabetes Complications 2017;31:280-7.ArticlePubMed
- 6. Cappon G, Vettoretti M, Sparacino G, Facchinetti A. Continuous glucose monitoring sensors for diabetes management: a review of technologies and applications. Diabetes Metab J 2019;43:383-97.ArticlePubMedPMCPDF
- 7. Edelman SV, Argento NB, Pettus J, Hirsch IB. Clinical implications of real-time and intermittently scanned continuous glucose monitoring. Diabetes Care 2018;41:2265-74.ArticlePubMedPDF
- 8. Yip S, Signal M, Smith G, Beban G, Booth M, Babor R, et al. Lower glycemic fluctuations early after bariatric surgery partially explained by caloric restriction. Obes Surg 2014;24:62-70.ArticlePubMedPDF
- 9. Wysocki M, Szopa M, Stefura T, Dudek A, Torbicz G, Gajewska N, et al. Continuous glucose monitoring in bariatric patients undergoing laparoscopic sleeve gastrectomy and laparoscopic Roux-En-Y gastric bypass. Obes Surg 2019;29:1317-26.ArticlePubMedPDF
- 10. Diabetes Research in Children Network (DirecNet) Study Group. The accuracy of the Guardian RT continuous glucose monitor in children with type 1 diabetes. Diabetes Technol Ther 2008;10:266-72.ArticlePubMedPMC
- 11. Battelino T, Danne T, Bergenstal RM, Amiel SA, Beck R, Biester T, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the International Consensus on Time in Range. Diabetes Care 2019;42:1593-603.PubMedPMC
- 12. Danne T, Nimri R, Battelino T, Bergenstal RM, Close KL, DeVries JH, et al. International consensus on use of continuous glucose monitoring. Diabetes Care 2017;40:1631-40.ArticlePubMedPMCPDF
- 13. Oh TJ, Kook JH, Jung SY, Kim DW, Choi SH, Kim HB, et al. A standardized glucose-insulin-potassium infusion protocol in surgical patients: use of real clinical data from a clinical data warehouse. Diabetes Res Clin Pract 2021;174:108756.ArticlePubMed
- 14. Sondergaard E, Espinosa De Ycaza AE, Morgan-Bathke M, Jensen MD. How to measure adipose tissue insulin sensitivity. J Clin Endocrinol Metab 2017;102:1193-9.ArticlePubMedPMCPDF
- 15. Yoo JH, Kim JH. Time in range from continuous glucose monitoring: a novel metric for glycemic control. Diabetes Metab J 2020;44:828-39.ArticlePubMedPMCPDF
- 16. Schierenbeck F, Franco-Cereceda A, Liska J. Accuracy of 2 different continuous glucose monitoring systems in patients undergoing cardiac surgery. J Diabetes Sci Technol 2017;11:108-16.ArticlePubMedPMCPDF
- 17. Perez-Guzman MC, Duggan E, Gibanica S, Cardona S, Corujo-Rodriguez A, Faloye A, et al. Continuous glucose monitoring in the operating room and cardiac intensive care unit. Diabetes Care 2021;44:e50-2.ArticlePubMedPMCPDF
- 18. Bailey T, Bode BW, Christiansen MP, Klaff LJ, Alva S. The performance and usability of a factory-calibrated flash glucose monitoring system. Diabetes Technol Ther 2015;17:787-94.ArticlePubMedPMC
- 19. Kefurt R, Langer FB, Schindler K, Shakeri-Leidenmuhler S, Ludvik B, Prager G. Hypoglycemia after Roux-En-Y gastric bypass: detection rates of continuous glucose monitoring (CGM) versus mixed meal test. Surg Obes Relat Dis 2015;11:564-9.ArticlePubMed
- 20. Hanaire H, Dubet A, Chauveau ME, Anduze Y, Fernandes M, Melki V, et al. Usefulness of continuous glucose monitoring for the diagnosis of hypoglycemia after a gastric bypass in a patient previously treated for type 2 diabetes. Obes Surg 2010;20:126-9.ArticlePubMedPDF
- 21. Pleus S, Heinemann L, Freckmann G. Blood glucose monitoring data should be reported in detail when studies about efficacy of continuous glucose monitoring systems are published. J Diabetes Sci Technol 2018;12:1061-3.ArticlePubMedPMCPDF
- 22. Galindo RJ, Migdal AL, Davis GM, Urrutia MA, Albury B, Zambrano C, et al. Comparison of the FreeStyle Libre Pro Flash continuous glucose monitoring (CGM) system and point-of-care capillary glucose testing in hospitalized patients with type 2 diabetes treated with basal-bolus insulin regimen. Diabetes Care 2020;43:2730-5.ArticlePubMedPMCPDF
- 23. Hofso D, Fatima F, Borgeraas H, Birkeland KI, Gulseth HL, Hertel JK, et al. Gastric bypass versus sleeve gastrectomy in patients with type 2 diabetes (Oseberg): a single-centre, triple-blind, randomised controlled trial. Lancet Diabetes Endocrinol 2019;7:912-24.ArticlePubMed
- 24. Wang L, Shi C, Yan H, Xia M, Zhu X, Sun X, et al. Acute effects of sleeve gastrectomy on glucose variability, glucose metabolism, and ghrelin response. Obes Surg 2021;31:4005-14.ArticlePubMedPDF
- 25. Lee WJ, Hur KY, Lakadawala M, Kasama K, Wong SK, Chen SC, et al. Predicting success of metabolic surgery: age, body mass index, C-peptide, and duration score. Surg Obes Relat Dis 2013;9:379-84.ArticlePubMed
- 26. Still CD, Wood GC, Benotti P, Petrick AT, Gabrielsen J, Strodel WE, et al. Preoperative prediction of type 2 diabetes remission after Roux-en-Y gastric bypass surgery: a retrospective cohort study. Lancet Diabetes Endocrinol 2014;2:38-45.ArticlePubMedPMC
- 27. Bojsen-Moller KN, Dirksen C, Jorgensen NB, Jacobsen SH, Serup AK, Albers PH, et al. Early enhancements of hepatic and later of peripheral insulin sensitivity combined with increased postprandial insulin secretion contribute to improved glycemic control after Roux-en-Y gastric bypass. Diabetes 2014;63:1725-37.ArticlePubMedPDF
- 28. Camastra S, Gastaldelli A, Mari A, Bonuccelli S, Scartabelli G, Frascerra S, et al. Early and longer term effects of gastric bypass surgery on tissue-specific insulin sensitivity and beta cell function in morbidly obese patients with and without type 2 diabetes. Diabetologia 2011;54:2093-102.ArticlePubMedPDF
- 29. Dagogo-Jack S, Alberti KG. Management of diabetes mellitus in surgical patients. Diabetes Spectr 2002;15:44-8.ArticlePDF
REFERENCES
Figure & Data
References
Citations

- Comparative Effect of Glucose-Lowering Drugs for Type 2 Diabetes Mellitus on Stroke Prevention: A Systematic Review and Network Meta-Analysis
Ji Soo Kim, Gyeongsil Lee, Kyung-Il Park, Seung-Won Oh
Diabetes & Metabolism Journal.2024; 48(2): 312. CrossRef - Use of Continuous Glucose Monitoring in Patients Following Bariatric Surgery: A Scoping Review
Yang Yu, Susan W. Groth
Obesity Surgery.2023; 33(8): 2573. CrossRef - Asymptomatic Hypoglycemia after Metabolic Surgery: New Insights from Perioperative Continuous Glucose Monitoring
Sang-Man Jin
Diabetes & Metabolism Journal.2022; 46(5): 675. CrossRef

 KDA
KDA PubReader
PubReader ePub Link
ePub Link Cite
Cite