Effects of a DPP-4 Inhibitor and RAS Blockade on Clinical Outcomes of Patients with Diabetes and COVID-19
Article information
Abstract
Background
Dipeptidyl peptidase-4 inhibitor (DPP-4i) and renin-angiotensin system (RAS) blockade are reported to affect the clinical course of coronavirus disease 2019 (COVID-19) in patients with diabetes mellitus (DM).
Methods
As of May 2020, analysis was conducted on all subjects who could confirm their history of claims related to COVID-19 in the National Health Insurance Review and Assessment Service (HIRA) database in Korea. Using this dataset, we compared the short-term prognosis of COVID-19 infection according to the use of DPP-4i and RAS blockade. Additionally, we validated the results using the National Health Insurance Service (NHIS) of Korea dataset.
Results
Totally, data of 67,850 subjects were accessible in the HIRA dataset. Of these, 5,080 were confirmed COVID-19. Among these, 832 subjects with DM were selected for analysis in this study. Among the subjects, 263 (31.6%) and 327 (39.3%) were DPP-4i and RAS blockade users, respectively. Thirty-four subjects (4.09%) received intensive care or died. The adjusted odds ratio for severe treatment among DPP-4i users was 0.362 (95% confidence interval [CI], 0.135 to 0.971), and that for RAS blockade users was 0.599 (95% CI, 0.251 to 1.431). These findings were consistent with the analysis based on the NHIS data using 704 final subjects. The adjusted odds ratio for severe treatment among DPP-4i users was 0.303 (95% CI, 0.135 to 0.682), and that for RAS blockade users was 0.811 (95% CI, 0.391 to 1.682).
Conclusion
This study suggests that DPP-4i is significantly associated with a better clinical outcome of patients with COVID-19.
INTRODUCTION
People with risk factors for cardiovascular diseases such as diabetes mellitus (DM) and hypertension have a higher risk of coronavirus disease 2019 (COVID-19) infection than the general population and generally exhibit poor prognosis [1-7]. There is an urgent need to develop evidence-based treatment methods to effectively prevent and manage COVID-19 in people with chronic diseases. However, it is not easy to provide reliable evidence within a short period in a worldwide pandemic situation.
Recently, it has been suggested that dipeptidyl peptidase-4 (DPP-4) and angiotensin-converting enzyme 2 (ACE2) may be parts of the receptor proteins of the novel coronavirus and may have a significant impact on the clinical course of COVID-19 [8,9]. Their molecular pathways are well-known to play important roles in glucose homeostasis, cardiovascular hemodynamics, and physiologic responses associated with inflammatory cascades [10-14]. Therefore, DPP-4 inhibitor (DPP-4i) and renin-angiotensin system (RAS) blockade, which are widely used in general practice, are likely to have a significant impact on the clinical course of COVID-19 in patients with chronic diseases [15-17].
The evidence that DPP-4i and RAS blockade significantly affect the clinical course of COVID-19 infection is unclear. Therefore, it is generally recommended that COVID-19 patients with chronic diseases either not use or discontinue these drugs [18]. However, the clinical significance of DPP-4i and RAS blockade for COVID-19 in the absence of effective treatment or preventive measures other than symptomatic treatment can be very important.
Hence, we utilized the entire claims data related to COVID-19 from Korea’s national medical insurance database, and investigated the effects of DPP-4i and RAS blockade, which are commonly administered to patients with DM, on the shortterm clinical outcomes of COVID-19.
METHODS
Health Insurance Review and Assessment Service claims database
The Korean National Health Insurance is a single healthcare insurance system that covers approximately 97% of the total Korean population; the remaining 3% are “medical protection” beneficiaries. Information on individuals’ utilization of medical facilities, prescription records, and diagnostic codes configured in the format of International Classification of Diseases, 10th revision (ICD-10) is recorded in the Health Insurance Review and Assessment Service (HIRA) database [19]. This database is considered representative of the Korean population and is used in research through anonymization and de-identification.
National Health Insurance Service database
The National Health Insurance Service (NHIS) database is also national representative data based on the claim data of national health insurance, such as the HIRA database. In particular, the NHIS provides a biennial health check-up program for all beneficiaries aged ≥40 years, which consists of anthropometry, a self-administered questionnaire on past medical history, health-related behavior, and laboratory tests [20]. Because of these characteristics, this dataset differs from the HIRA database and the composition of the population, and further analysis is possible considering various clinical characteristics.
Study subjects of HIRA database
Currently, the HIRA is managing a separate research database using drug usage data for the past 5 years for subjects who have performed confirmatory tests for COVID-19. To protect personal information, the export of raw data is prohibited by law, and research is being performed in a way that provides deidentified results when researchers submit program codes for analysis [21]. As of May 17, 2020, the total number of Korean COVID-19 related claims released to researchers was 67,850 (Fig. 1). Of these, 5,080 and 832 were confirmed cases of COVID-19 and COVID-19 with DM, respectively.
Study subjects of NHIS database
The official name of the Korean NHIS database for COVID-19 research is National Health Information Database (NHID)-COVID. This consisted of data from COVID-19 patients in Korea that occurred up to June 4, 2020, and 15 times of age and sex matched controls. This dataset includes subject claim data from 2015 to 2020 along with their national health check-up data. In this database, only de-identified results of analysis codes are provided to researchers to protect the subject’s privacy. In addition, instead of the exact age of the subject, it is grouped in units of 10 years and provided to the researcher. The total number of subjects released to researchers was 129,120 (Fig. 2). Of these, 3,494 and 704 were confirmed cases of COVID-19 and COVID-19 with DM, respectively.
Definition of COVID-19 infection, clinical status, and other clinical variables
The clinical variables that can be identified in Korea’s COVID-19 claim database are the subject’s age, sex, diagnostic code, medication prescription, outpatient care, hospitalization, critical care, and death. COVID-19 diagnosis was defined as ICD10 diagnosis codes B34.2, B97.2, U18, U18.1, or U07.x. Intensive care was defined as a procedure code for endotracheal intubation or mechanical ventilation, or the charge of an intensive care unit management fee. We analyzed subjects according to a mild case of self-isolation or simple hospitalization, or a severe/lethal case of death or intensive care.
The subject’s comorbidity was defined as a diagnostic code. DM was defined as E10.x, E11.x, E12.x, E13.x, or E14.x, hypertension as I10.x, I11.x, I12.x, I13.x, or I15.x, dyslipidemia as E78.x, cardiovascular disease as I20.x, I21.x, I22.x, I23.x, I24.x, or I25.x, and cerebrovascular disease as I61.x, I62.x, I63.x, or I64.x. Chronic kidney disease was defined as N18.x, asthma as J45.x or J46.x, and chronic obstructive pulmonary disease as J44.x. Cancer was defined as C00.x, C01.x, C02.x, C03.x, C04. x, C05.x, C06.x, C07.x, C08.x, C09.x, C10.x, C11.x, C12.x, C13. x, C14.x, C15.x, C16.x, C17.x, C18.x, C19.x, C20.x, C21.x, C22. x, C23.x, C24.x, C25.x, C26.x, C30.x, C31.x, C32.x, C33.x, C34. x, C37.x, C38.x, C39.x, C40.x, C41.x, C43.x, C44.x, C45.x, C46. x, C47.x, C48.x, C49.x, C50.x, C51.x, C52.x, C53.x, C54.x, C55. x, C56.x, C57.x, C58.x, C60.x, C61.x, C62.x, C63.x, C64.x, C65. x, C66.x, C67.x, C68.x, C69.x, C70.x, C71.x, C72.x, C73.x, C74. x, C75.x, C76.x, C77.x, C78.x, C79.x, C80.x, C81.x, C82.x, C83. x, C84.x, C85.x, C86.x, C88.x, C90.x, C91.x, C92.x, C93.x, C94. x, C95.x, C96.x, and C97.x.
The subject’s medication use was assessed based on the initial date of COVID-19 diagnosis. If the prescription was confirmed within 180 days of diagnosis and was for at least 90 days, the subject was defined as a user of a specific drug. And the use of medication was considered both inpatient and outpatient, and both oral and injectable agents such as insulin were defined in the same method.
Anthropometric assessments of subjects were performed by health care professionals in the NHID-COVID dataset. Height, weight, waist circumference, and blood pressure were assessed. Information on the lifestyle was obtained through standardized self-reported questionnaires. Subjects were classified according to smoking status as nonsmoker, former smoker, or current smoker. Individuals who consumed 30 g of alcohol per day were classified as being heavy alcohol consumers [22,23]. Regular physical activity was defined as performance of strenuous exercise for at least once per week [23].
Thereafter, differences in characteristics and clinical status were compared according to whether the subject used DPP-4i and/or RAS blockade. We also investigated the possibility of synergy during the combined use of these two drugs.
Statistical analysis
Basic characteristics of subjects were expressed as mean±standard deviation for continuous variables in each subgroup and as number and percentage for categorical variables. The chi-square test, Fisher’s exact test, independent Student’s t-test, and one-way analysis of variance were used to compare the clinical characteristics of the subjects. Logistic regression analysis was used to adjust various clinical variables. Statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA), and P<0.05 was considered statistically significant.
Ethics statement
This study was approved by the Institutional Review Board of Kyung Hee University Hospital (no. KHUH 2020-04-067). The requirement for informed consent was waived by the Institutional Review Board because de-identified information was used for the analyses.
RESULTS
Clinical characteristics with or without use of DPP-4i
Clinical characteristics of subjects were compared according to whether DPP-4i was used. In the HIRA dataset, mean age of DPP-4i users was significantly higher than that of non-users (Supplementary Table 1). And males were 56.65%, which was not significantly different from the percentage among non-users. Hypertension and dyslipidemia were shown by 74.90% and 93.92% of the subjects, respectively, and were statistically significant. However, there were no significant differences in the prevalence of other comorbidities. In terms of medication, DPP-4i users had significantly higher rates of metformin, sulfonylurea, thiazolidinedione, RAS blockade, diuretics, statin, and fibrate usage. However, the usage rate of sodium glucose cotransporter-2 inhibitor (SGLT2i) was significantly higher in non-users.
In the NHID-COVID dataset, the age group of DPP-4i users showed no significant difference compared to non-users (Supplementary Table 2). The proportion of males in DPP-4i users was 47.4%, showing a significant difference compared to 38.0% in the non-users group. In addition, body mass index (BMI), waist circumference, systolic blood pressure, fasting glucose, and total cholesterol levels were significantly higher in DPP-4i users. Comorbidity and medication-related characteristics were similar to those of the HIRA dataset.
Clinical characteristics with or without the use of RAS blockade
Clinical characteristics of subjects were compared according to whether RAS blockade was used (Supplementary Table 3). RAS blockade users had a mean age of 64.85±13.23 years, which was significantly higher than that of non-users, and 56.57% of them were male. Most RAS blockade users had dyslipidemia, and the prevalence of cardiovascular and chronic kidney diseases among them was significantly higher than that among non-users. With regard to medication, the usage frequency of metformin, sulfonylurea, thiazolidinedione, DPP-4i, diuretics, calcium-channel blocker, and statin was significantly higher among RAS blockade users than among non-users.
In the NHID-COVID dataset, the age group of RAS blockade users also showed significant difference compared to non-users (Supplementary Table 4). The proportion of males in RAS blockade users was 39.6%, showing no significant difference compared to 40.7% in the non-users group. BMI, waist circumference, blood pressure, fasting glucose were significantly higher in RAS blockade users. And height, total cholesterol, and the proportion of current smoker were significantly lower RAS blockade users. The prevalence of dyslipidemia was significantly higher in RAS blockade users, but the differences between the two groups for other comorbidities were not significant. With regard to medication, the usage frequency of metformin, sulfonylurea, DPP-4i, beta blocker, diuretics, calcium-channel blocker, and statin was significantly higher among RAS blockade users than among non-users.
Differences in severity of COVID-19 infection upon use of DPP-4i and RAS blockade
In the HIRA dataset, the fractions of those who received intensive care or died were 3.42% and 4.39% among DPP-4i users and non-users, respectively (Table 1). The unadjusted odds ratio (OR) of these severe/lethal cases among DPP-4i users was 0.771 (95% confidence interval [CI], 0.355 to 1.676). However, the adjusted OR (aOR), considering age, sex, comorbidity, and medication, was 0.362 (95% CI, 0.135 to 0.971). The fractions of those who received intensive care or died were 3.98% and 4.16% among RAS blockade users and non-users, respectively (Table 1). The OR of severe/lethal cases among RAS blockade users was 0.954 (95% CI, 0.471 to 1.933). The aOR, considering the effects of various clinical variables, was also not significant, at 0.599 (95% CI, 0.251 to 1.431).

Differences in COVID-19 related clinical status on the use of DPP-4i and/or RAS blockade based on the data from the National Health Insurance Review and Assessment Service of Korea
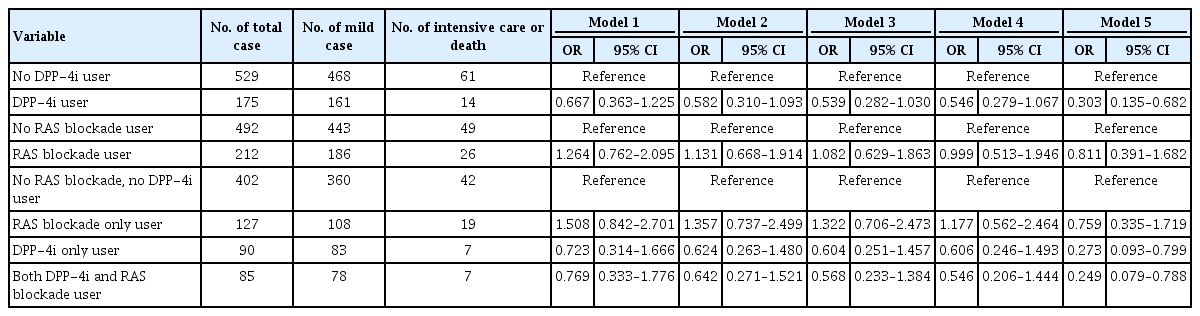
In the NHID-COVID dataset, the fractions of those who received intensive care or died were 8.00% and 11.53% among DPP-4i users and non-users, respectively (Table 2). The unadjusted OR of these severe/lethal cases among DPP-4i users was 0.667 (95% CI, 0.363 to 1.225). However, the aOR considering age, sex, health check-up variables, comorbidity, and medication, was 0.303 (95% CI, 0.135 to 0.682). The fractions of those who received intensive care or died were 12.26% and 9.95% among RAS blockade users and non-users, respectively (Table 2). The OR of severe/lethal cases among RAS blockade users was 1.264 (95% CI, 0.762 to 2.095). The aOR, considering the effects of various clinical variables, was also not significant, at 0.811 (95% CI, 0.391 to 1.682).
Combination effect of DPP-4i and RAS blockade
To evaluate the synergistic effect of the combination of these two drugs, subjects were divided into four groups according to whether DPP-4i and RAS blockade were used, and further analyzed. Their clinical characteristics are summarized for each dataset in Supplementary Tables 5 and 6.
In the HIRA dataset, compared to that of subjects who used neither DPP-4i nor RAS blockade, the aOR was 0.456 (95% CI, 0.158 to 1.314) for severe/lethal cases of RAS blockade only users, 0.232 (95% CI, 0.057 to 0.954) for DPP-4i-only users, and 0.251 (95% CI, 0.074 to 0.850) for subjects using both DPP-4i and RAS blockade (Table 1). And in the NHID-COVID dataset, compared to that of subjects who used neither DPP-4i nor RAS blockade, the aOR was 0.759 (95% CI, 0.335 to 1.719) for severe/lethal cases of RAS blockade only users, 0.273 (95% CI, 0.093 to 0.799) for DPP-4i-only users, and 0.249 (95% CI, 0.079 to 0.788) for subjects using both DPP-4i and RAS blockade (Table 2).
DISCUSSION
In this study, we compared the characteristics and clinical outcomes of COVID-19 infection upon administration of DPP-4i and RAS blockade, using two independent large database representatives of the Korean population. In the HIRA dataset, the aOR for severe/lethal cases of DPP-4i users was 0.362 (95% CI, 0.135 to 0.971), and that for RAS blockade users was 0.599 (95% CI, 0.251 to 1.431). The aOR for the subgroup of those who used both drugs was 0.251 (95% CI, 0.074 to 0.850), which was not significantly different from that of DPP-4i-only users. And results were consistent in the analysis results using the NHID-COVID dataset. These suggest that the use of DPP-4i for people with DM is associated with a better clinical outcome of COVID-19 infection, suggesting that the synergistic effect of the combination of these two classes of drug is not likely to be noticeable.
Our findings are consistent with the existing experimental research hypothesis that DPP-4i may reduce the severity of COVID-19 because DPP-4 acts as a receptor for coronavirus [8,24]. This hypothesis has not been confirmed in large population groups, and our findings may be among the important evidence that supports it. In our study, DPP-4i users were more likely to exhibit comorbidities than non-users, and thus were more likely to be taking medications of different classes. Despite these differences in characteristics, it is interesting to note that the use of DPP-4i is associated with better clinical outcomes of COVID-19 patients with DM. In particular, since DPP-4i rarely causes hypoglycemia when used alone, it is necessary to examine the possibility of this drug being used as an immunomodulator for systemic infections, and not solely as a diabetes drug [25,26].
It should also be noted that the number of users of SGLT2i was significantly higher among DPP-4i non-users. A large-scale clinical trial of SGLT2i has demonstrated that this drug has a significant effect on the prevention of cardiovascular disease [27,28]. Based on the results of this study, a randomized trial was recently performed to confirm the effect of SGLT2i on COVID-19 patients [29]. However, SGLT2i is likely to have a negative effect on patients with acute phase infections, for which it is important to maintain stable hemodynamics [30, 31]. Currently, we are conducting a study on the clinical impact of SGLT2i on COVID-19 patients; using the results, we plan to provide partial answers to important clinical questions as soon as possible.
Since ACE2 is also a known as a physiologic regulator of human coronavirus infection, the possibility that RAS blockade may negatively affect the susceptibility and severity of COVID-19 infection has been hypothesized [32,33]. However, it is known that RAS blockade has anti-inflammatory and antioxidant pleiotropic effects, and research results on preventing pulmonary complication in vulnerable patients have been reported [34,35]. A systemic review states that there is currently no clear evidence as to whether angiotensin-converting enzyme inhibitor or angiotensin receptor blocker needs to be started or stopped for viral infections, including COVID-19 [18].
In our study, RAS blockade tended to decrease the aOR for severe/lethal cases; however, this decrease was statistically insignificant. This implies that RAS blockade users are also more likely to exhibit comorbidities than non-users, and hence, it is possible that this is a vulnerable clinical condition that requires more medication. It is also possible that the number of subjects studied was not sufficient to confirm statistical significance. This may have been because the effects on the long-term clinical course and prognosis were not considered; owing to the limitation of our study design, only the short-term clinical outcomes of the subjects were analyzed. The synergistic effect of the combination of DPP-4i and RAS blockade could not be explained for the same reason.
This study has other limitations. Firstly, since this study was based on claim data, it was difficult to obtain detailed clinical information about the patients. In particular, it was difficult to evaluate the patient’s laboratory tests, images, and detailed clinical course information. Secondly, the study results demonstrated that DPP-4i users exhibited better clinical outcomes; however, these results did not imply a clear causal relationship. Thirdly, it was difficult to predict the long-term clinical course and prognosis of the patients because our analysis was only for short-term clinical outcomes. Fourthly, this study may be difficult to generalize with respect to other countries as it was conducted in Korea, where the proportion of severely ill patients and fatalities related to COVID-19 is lower than that in other countries. Lastly, further research is needed because a clear mechanism for explaining the facts obtained in this study has not been established. These limitations necessitate careful interpretation and generalization of the results.
However, this study was based on two large dataset representatives of the entire population of Korea, and such large-scale studies are rare. Moreover, with regard to situations where effective prevention or treatment of COVID-19 infection has not been established, our research results contain meaningful data on drugs that are commercially available and applicable to many people. In particular, using Korea’s national medical insurance claim and health check-up data, it was possible to consider the impact of comorbidities and various medications in the analysis. Currently, efforts are being made in Korea to increase the size and quality of the COVID-19 database used in research for public interest. In the future, detailed observations of the clinical courses of subjects will enable a more detailed understanding of the disease, and it is expected that a more effective methodology for prevention and treatment will be established soon.
In conclusion, this population-based study suggests that DPP-4i is significantly associated with a better clinical outcome of COVID-19 infection. However, the effect of RAS blockade is not significant.
Supplementary Materials
Supplementary materials related to this article can be found online at https://doi.org/10.4093/dmj.2020.0206.
Clinical characteristics according to the use of DPP-4i in Korean COVID-19 patients with diabetes based on the data from the National Health Insurance Review and Assessment Service of Korea
Clinical characteristics according to the use of DPP-4i in Korean COVID-19 patients with diabetes based on the NHID-COVID database from the National Health Insurance Service of Korea
Clinical characteristics according to the use of RAS blockade in Korean COVID-19 patients with diabetes based on the data from the National Health Insurance Review and Assessment Service of Korea
Clinical characteristics according to the use of RAS blockade in Korean COVID-19 patients with diabetes based on the NHID-COVID database from the National Health Insurance Service of Korea
Clinical characteristics according to whether DPP-4i and RAS blockade were used in Korean COVID-19 patients with diabetes based on the data from the National Health Insurance Review and Assessment Service of Korea
Clinical characteristics according to the use of DPP-4i and/or RAS blockade in Korean COVID-19 patients with diabetes based on the NHID-COVID database from the National Health Insurance Service of Korea
Notes
CONFLICTS OF INTEREST
Researchers from a pharmaceutical company participated in this study (Jeongwoo Lee, Hyewon Nam, and Dae-Sung Kyoung). They are experts on claims data, and their institutions have not intentionally influenced the basic hypothesis of the study, analysis plan, result arrangement, result interpretation, and manuscript preparation. The researchers from other institutions have no conflict of interest to declare.
AUTHOR CONTRIBUTIONS
Conception or design: S.Y.R., J.L.
Acquisition, analysis, or interpretation of data: J.L., H.N., D.
S.K., D.W.S., D.J.K.
Drafting the work or revising: S.Y.R., J.L.
Final approval of the manuscript: S.Y.R.
FUNDING
This work was supported by the Korean Endocrine Society of EnM Research Award 2020.
Acknowledgements
Since the COVID-19 database of the HIRA and the NHI are provided for public interest, it is a principle that all research results and publications be released to open access.
The authors thank emeritus professor Young Seol Kim of Kyung Hee University for his exceptional teaching and inspiration, which encouraged the authors to conduct the current study.