Association of Vaspin with Metabolic Syndrome: The Pivotal Role of Insulin Resistance
Article information
Abstract
Background
Previous studies evaluating the relationship between serum vaspin concentrations and metabolic syndrome (MetS) have yielded contrasting results. Additionally, contribution of general and abdominal obesity, chronic inflammation, and insulin resistance to this relationship remains unknown.
Methods
In a cross-sectional setting, we investigated the association between vaspin and MetS in 145 subjects ranging from normoglycemia to type 2 diabetes. Vaspin concentrations were measured using enzyme-linked immunosorbent assay.
Results
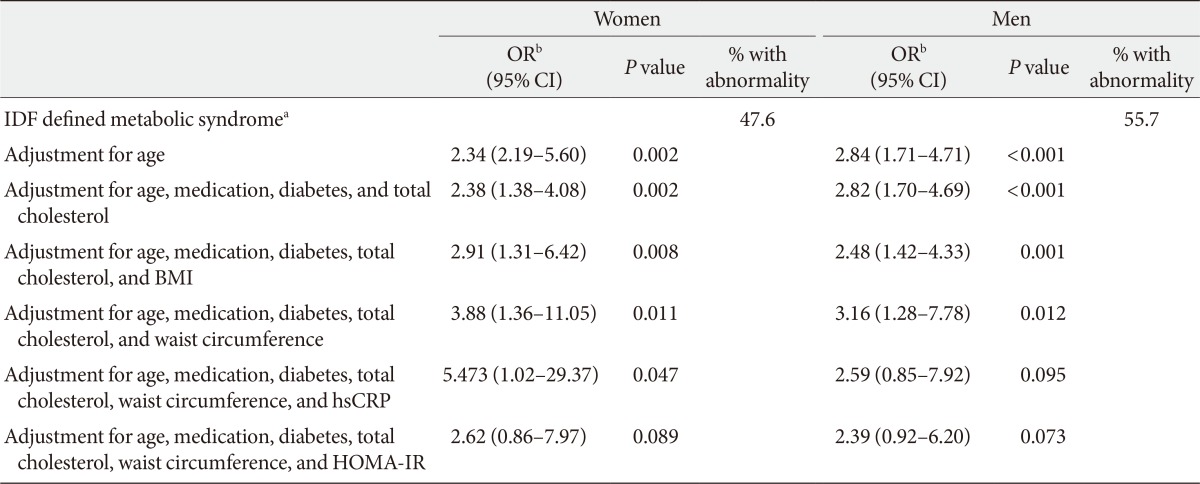
Women had 29% higher vaspin concentrations compared with men. Subjects with MetS (51% of all participants) had higher vaspin concentrations (P=0.019 in women and P<0.001 in men). In logistic regression, vaspin significantly predicted raised fasting plasma glucose (P<0.001), and raised triglycerides (P<0.001) after controlling for age in both sexes. Moreover, vaspin was the significant predictor for reduced high-density lipoprotein cholesterol and raised waist circumference in women and men, respectively. Considering MetS as a whole, vaspin predicted MetS even after adjustment for age, medications, diabetes, total cholesterol, and waist circumference in both sexes (odds ratio [OR], 3.88; 95% confidence interval [CI], 1.36 to 11.05; P=0.011 for women; OR, 3.16; 95% CI, 1.28 to 7.78; P=0.012 for men). However, this relationship rendered nonsignificant after introducing homeostasis model assessment of insulin resistance (HOMA-IR) in women (P=0.089) and high-sensitivity C-reactive protein (P=0.073) or HOMA-IR in men (P=0.095).
Conclusion
Vaspin is associated with some but not all components of MetS. Vaspin is a predictor of MetS as a single entity, independent of obesity. This relationship is largely ascribed to the effects of insulin resistance and chronic inflammation.
INTRODUCTION
Obesity, hyperglycemia, hyperlipidemia, and hypertension occur together more commonly than what is expected by chance and confer risk of atherosclerosis beyond a level that can be explained by sum of these risk factors [1]. This constellation of metabolic disturbances pertains to a single entity dubbed metabolic syndrome (MetS). Despite the immense literature published in the past decade investigating various aspects of MetS, understanding the underlying mechanism(s) that unite all these metabolic perturbances is a work in progress. Insulin resistance and chronic low grade inflammation have both been proposed as the core pathology in MetS and subsequent development of diabetes, and cardiovascular disease [2,3]. Adipose tissue dysfunction is involved in the emergence of both events via a cluster of adipocytokines including but not limited to leptin, adiponectin, interleukin-6, resistin, visfatin, omentin, and vaspin [4]. Vaspin, a novel member of the adipokine family, is a 395 amino-acid product with structure similar to serine protease inhibitors [5]. Evidence linking vaspin to MetS has been clouded with contrasting results [6,7,8]. Therefore, the purpose of this study was twofold: first, to assess the relationship between vaspin and MetS in a group of Iranian adults; and second, to investigate whether this association is mediated through abdominal obesity, insulin resistance, or chronic inflammation.
METHODS
From October 2010 to July 2011, 100 patients who visited diabetes clinic of Vali-Asr Hospital (Tehran, Iran) were enrolled in the current study. Healthy individuals accompanying patient (i.e., family members, friends, etc.) were also recruited; a group of 50 subjects was enrolled in this way. Finally, five cases were dropped due to having missing values on key variables and 145 subjects were included in the final analysis. Tehran University of Medical Sciences Ethics Committee approved the study protocol. Written informed consent was obtained from each subject before enrollment.
Participants' demographics along with medical history were recorded using a predesigned standard questionnaire. Blood pressure was measured employing a manual sphygmomanometer (Big Ben Adults; Rudolf Riester, Jungingen, Germany) with subjects in a sitting position. The average of two readings with 10-minute interval was recorded. Measurement of height was done using a standard stadiometer and the nearest 0.1 cm was recorded. Weight was measured with a digital scale (GS49; Beurer, Ulm, Germany) and recorded with 0.1 kg accuracy. Waist circumference was measured mid-line between inferior costal margin and anterior iliac crest. Hip circumference was measured at the point where hip is widest. Both readings were recorded with 0.1 cm precision. Body mass index (BMI) was calculated as weight in kilograms divided by height squared in meters.
After an overnight fasting of 12 hours, 10 mL of venous blood was drawn from each subject. Fasting plasma glucose (FPG) was measured by enzymatic calorimetric method of glucose oxidase test. Fasting insulin was determined by radioimmunoassay techniques (Immunotech, Prague, Czech Republic). Hemoglobin A1c (HbA1c) concentrations were assessed via high performance liquid chromatography. Serum concentrations of total cholesterol, high density lipoprotein cholesterol (HDL-C), low density lipoprotein cholesterol (LDL-C), and triglycerides were determined by enzymatic methods (Pars Azmun commercial kits; Pars Azmun, Karaj, Iran). Quantitative determination of serum high sensitivity C-reactive protein (hsCRP) was performed with commercial kits (Diagnotics Biochem Canada Inc., London, Canada) enzyme-linked immunosorbent assay (ELISA) technique. Homeostasis model assessment of insulin resistance (HOMA-IR) was defined according to formula developed by Matthews et al. [9]: fasting insulin concentrations (IU) multiplied by FPG (mg/dL), divided by 405. Serum concentrations of vaspin were measured with ELISA technique employing commercial kits (Kit AG-45A-0017EK-KI01; AdipoGen, San Diego, IL, USA) with inter- and intra-assay coefficients of variation of 3.27% to 9.06% and 1.31% to 3.85%, respectively.
Definitions
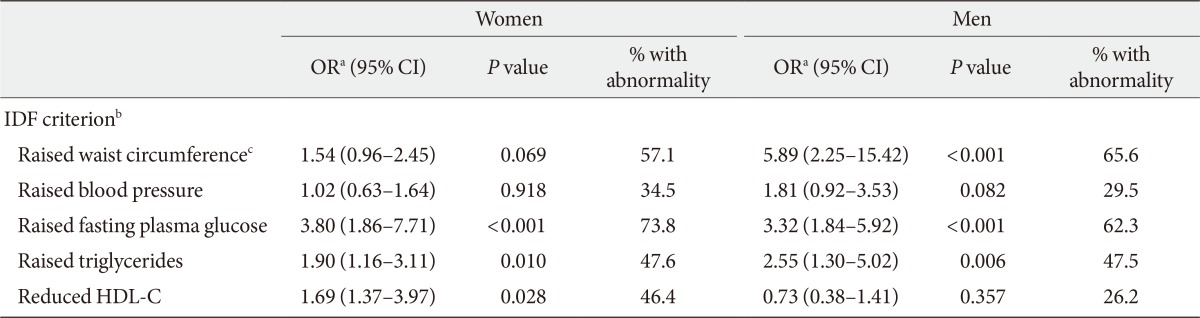
Diabetes mellitus was defined according to recent American Diabetes Association criteria of FPG ≥126 mg/dL or equivalently HbA1c ≥6.5%. However, no discrimination between type 1 and type 2 diabetes was made based on standard methods, i.e., glycemic clamp and C-peptid measurement. Nevertheless, since all subjects responded to lifestyle modification and oral medication and insulin therapy was not initiated in any of patients, all subjects are likely to be type 2 diabetes. Also, subject were defined as impaired fasting glucose (IFG) with FPG levels 100 to 125 mg/dL [10]. Diagnosis of MetS was established using International Diabetes Federation (IDF) criteria: MetS is diagnosed in the presence of raised waist circumference (≥90 cm for both sexes [11]) plus any two combination of the other four metabolic abnormalities as outlined below [1]: 1) raised waist circumference, 2) raised systolic blood pressure (SBP) ≥130 mm Hg or diastolic blood pressure (DBP) ≥85 mm Hg, 3) raised fasting plasma glucose ≥100 mg/dL or previous diagnosis of diabetes, 4) raised triglycerides ≥150 mg/dL or use of specific medications for this lipid abnormality, and 5) reduced HDL-C <40 mg/dL in men and <50 mg/dL in women, or use of specific medications for this lipid abnormality.
Statistical analysis
Statistical analyses were performed using SPSS version 19 (IBM Corp., New York, NY, USA). Because of well-described differences between women and men regarding adipokine concentrations and metabolic status, separate analyses were done for each sex.
Categorical variables are presented in percent and continuous variables with normal distribution in mean±standard deviation (SD). In all analyses, vaspin concentrations and HOMA-IR values were transformed to natural logarithm to correct for their right-sided skewness. For these variables, crude values are reported using median (interquartile range). Difference in vaspin levels between two genders was evaluated with Student t-test.
Pearson correlation was employed to determine the correlation between vaspin and metabolic variables. Logistic regression models were incorporated to assess the association between vaspin and each MetS criterion, controlling for age. Association between vaspin and MetS as a single entity was also assessed via binary logistic regression models (enter and stepwise).
In the enter model, possible confounding variables were placed into the model one-by-one according to a predefined order to assess the independency of vaspin-MetS relationship. A stepwise model was also constructed to investigate which set of variables best predict MetS.
In all regression models, odds ratios (OR) with 95% confidence intervals (95% CI) were calculated for one SD increment in log-vaspin. Given the logarithmic nature of vaspin, use of SD increments essentially means that what is the odds of vaspin for the presence of MetS in a patient with average vaspin concentration (relative to population distribution of the value) as opposed to a patient with more extreme values.
Additionally, linear association of vaspin with glycemic status (normoglycemic, to IFG, to type 2 diabetes), as well as number of metabolic abnormalities (from 0 to 1-3, and to 4-5) was investigated using analysis of variance (ANOVA); P for linear trend was calculated. In all analyses, P<0.05 was considered statistically significant.
RESULTS
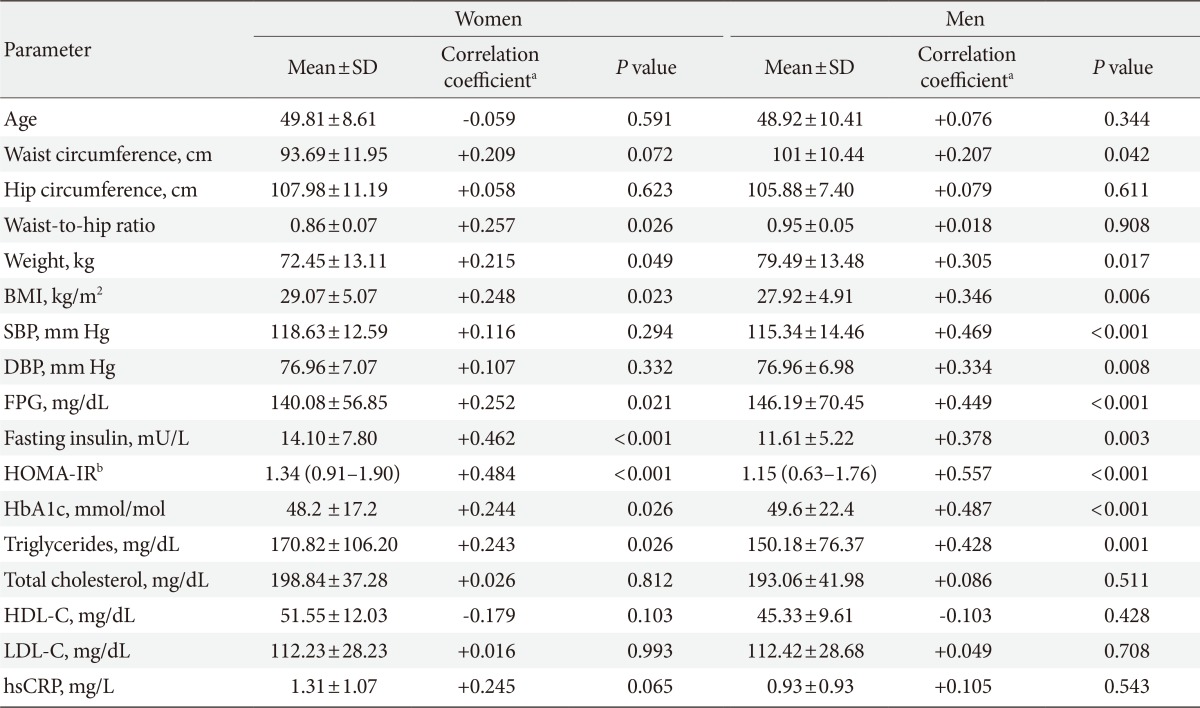
A total of 145 subjects participated in this study with age ranging from 26 to 72 years. Women comprised majority of cases (57.2%, n=84). The median of vaspin concentrations was 0.88 ng/mL (interquartile range, 0.4 to 1.42) and 0.69 ng/mL (interquartile range, 0.30 to 1.37) in women and men, respectively. On average, women had 29% higher vaspin values compared with men, although the difference was not statistically significant (P=0.086). Regarding glucose metabolism, 45 subjects (31%) were normoglycemic, 14 (9.7%) had IFG, and the remainder 86 (59.3%) were diagnosed with type 2 diabetes mellitus (data not shown). 13.1% and 20.0% of patients were taking medications for treatment of hypertension and hyperlipidemia, respectively. There were no significant differences in vaspin concentrations between medication-naive, and on-medication patients (P=0.217 for antihypertensive and P=0.241 for antihyperlipidemic). Correlation between vaspin concentrations and baseline anthropometric and metabolic characteristics of study participants are depicted in Table 1. There was a significant positive correlation between vaspin and BMI (r=0.248, P=0.023), FPG (r=0.252, P=0.021), fasting insulin (r=0.462, P<0.001), waist-to-hip ratio (r=0.257, P=0.026), and HOMA-IR (r=0.484, P<0.001) in women. In a similar fashion, significant correlations were observed among men regarding BMI (r=0.346, P=0.006), FPG (r=0.449, P<0.001), fasting insulin (r=0.378, P=0.003), HOMA-IR (r=0.557, P<0.001), and triglycerides (r=0.428, P=0.001). Although significant correlations were not observed between vaspin and SBP and DBP in women, vaspin was significantly associated with SBP (r=0.469, P<0.001) and DBP (r=0.334, P=0.008) in men. Based on IDF criteria, 74 subjects (51%) had MetS (47.6% of women and 55.7% of men). As depicted in Fig. 1, an increasing trend in vaspin concentrations was observed form normal glucose tolerance to type 2 diabetes (ANOVA F=13.67, P for trend <0.001 in women; F=14.39, P for trend <0.001 in men). Additionally, there was a linear trend between increase in number of metabolic abnormalities (from 0 to 1-3, and to 4-5), and increment in vaspin concentrations (ANOVA F=7.37, P for trend=0.008 in women; F=13.78, P for trend <0.001 in men).
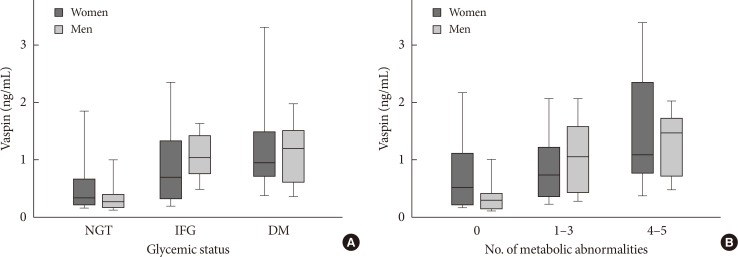
Vaspin concentrations stratified according to sex/glycemic status (A), or sex/number of metabolic abnormalities (B). Given the nonnormal distribution of vaspin, values are presented as median (25% to 75% percentiles) (boxes), along with 5% to 95% percentiles (whiskers). NGT, normal glucose tolerance; IFG, impaired fasting glucose; DM, diabetes mellitus.
Logistic regression models investigating the association between vaspin and each component of MetS are presented in Table 2. Vaspin was a significant predictor of all, but not raised blood pressure and raised waist circumference in women. In men, vaspin significantly predicted raised waist circumference, raised FPG, and raised triglyceride. Vaspin predicted MetS as a whole after accounting for effects of age (Table 3). This association persisted even after adjustment for use of antihypertensive, antihyperlipidemic, antihyperglycemic medications, diabetes, total cholesterol, and waist circumference in an additive multivariate logistic regression model in women (OR, 3.8; 95% CI, 1.36 to 11.05; P=0.008) and men (OR, 3.16; 95% CI, 1.28 to 7.78; P=0.012). However, when HOMA-IR was introduced in the model for women, the association between vaspin and MetS, rendered nonsignificant (P=0.089). For men, HOMA-IR and hsCRP proved to be major confounding variables rendering vaspin-MetS relationship nonsignificant (Table 3). When variables were entered into the model in a stepwise manner, only HOMA-IR contributed to predicting MetS in both women (OR, 5.59; 95% CI, 2.11 to 14.81; P=0.001) and men (OR, 5.88; 95% CI, 1.61 to 21.55; P=0.008), further confirming the role of HOMA-IR as the main confounding variable.
DISCUSSION
Vaspin (visceral adipose tissue-derived serine protease inhibitor) is a member of the serine protease inhibitor family, originally identified as an adipokine secreted from visceral adipocytes in Otsuka Long-Evans Tokushima fatty (OLETF), a rat model of obesity and type 2 diabetes [5]. In humans, vaspin has been found to be associated with insulin resistance, MetS, and type 2 diabetes [5,12]. Although the mechanisms by which vaspin relates to insulin insensitivity remains poorly understood, preliminary findings suggest that an upsurge in serum vaspin levels, as well as increment in vaspin mRNA expression in adipose tissue, might be a compensatory counter-effect against unknown upregulated proteases that are increased in obesity and insulin resistance states [5].
Here, we found a strong association between vaspin and some, but not all of the MetS components. After controlling for age, vaspin was an independent predictor of MetS. In line with our findings, Choi et al. [7] reported a significant association between vaspin and MetS; however, within MetS components, only raised triglycerides correlated with vaspin. Similarly, in a group of obese children and adolescents, vaspin significantly correlated with BMI, insulin, HOMA-IR, and triglycerides [8]. Moreover, El-Mesallamy et al. [13] reported a positive association between vaspin and BMI, waist-to-hip-ratio, along with glycemic indices including HbA1c, HOMA-IR, and FPG. Nonalcoholic fatty liver disease, a related entity found in as many as 90% of subjects with MetS, have also been associated with an elevated level of serum vaspin [14]. On the other hand, Auguet et al. [6] in a sample of women with BMI ≥40 kg/m2, found no correlation between vaspin and MetS; surprisingly, vaspin negatively correlated with waist circumference, leptin, and interleukin-6.
Herein, vaspin concentrations were not meaningfully different between sexes, albeit it was about one third higher in women. In agreement with this observation, Youn et al. [12] reported higher vaspin concentrations in normoglycemic female subjects; whereas this sex difference abrogated in subjects with type 2 diabetes. On the other hand, Choi et al. [7] in a sample of subjects with MetS demonstrated that circulating vaspin is in fact higher in men suggesting sex predilection might be reversed in patients with multiple metabolic disturbances.
We further investigated the vaspin-MetS association by controlling for BMI (a general index of obesity), waist circumference (a marker of abdominal obesity), hsCRP (hepatocyte derived marker of inflammation), and HOMA-IR (representative of peripheral insulin resistance). After entering HOMA-IR in the multivariate model, vaspin no longer predicted MetS in women, indicating that vaspin-MetS relationship is mediated to a large part via insulin resistance. This finding is further supported by the observation that raised FPG produced the highest OR amongst MetS components. The same role of insulin resistance was found where vaspin levels was correlated significantly with visceral adipose tissue area in subjects with higher HOMA-IR but not in the lower HOMA-IR subjects [15]. In the same line, Chang et al. [16] observed that the association of vaspin decreasing with improvement in anthropometric indices was mainly modified by insulin resistance.
However, we observed that vaspin-MetS relationship in men is modulated by chronic inflammation indicated by hsCRP, as well as insulin resistance. These findings are in the same line with accumulating evidence suggesting that contribution of obesity and adipose tissue dysfunction in metabolic abnormalities and obesity-related complications owes to chronic low-grade inflammation [17]. In other words, release of adipokines from adipocytes and adipose tissue leads to chronic inflammatory state that may play a central role in development of insulin resistance and type 2 diabetes [18].
Vaspin was initially introduced as an insulin-sensitizing adipokine based on observations made in OLETF rats [5]. Experiments with human subjects, nonetheless, indicated that vaspin concentrations are often raised in diabetes and insulin resistance [19,20]. In a group of 30 type 2 diabetes patients, 2-week therapy with subcutaneous insulin infusion resulted in a significant decrease in vaspin concentrations [14]. It is suggested that elevated vaspin levels in fact provide a compensatory mechanism to abrogate the chain of events leading to insulin resistance [19]. Unwrapping this conundrum is a focus of future research. The findings presented here should be interpreted considering limitations corollary to cross-sectional setting. Future prospective studies with larger sample are needed to explore the direction of causality between adipokines and metabolic abnormalities. Another limitation of this study is that we discriminated type 1 and type 2 diabetes based on subjects' clinical presentation and their response to lifestyle modification along with oral treatment instead of standard laboratory assessment (i.e., measurement of autoantibodies).
In conclusion, vaspin is associated with MetS independent age, gender, general and abdominal obesity. Vaspin-MetS association is dependent upon insulin resistance and chronic inflammation, to the most part.
Notes
No potential conflict of interest relevant to this article was reported.