Diabetogenic Effect of Statins: A Double-Edged Sword?
Article information
Abstract
Statins are widely prescribed cholesterol-lowering agents, which have been demonstrated to significantly reduce cardiovascular morbidity and mortality. However, recent trials have reported that statins cause worsening of hyperglycemia and increase the risk of new-onset diabetes. The association between the diabetogenic effect of statins with intensive dose and accompanying major risk factors for diabetes has been demonstrated. However, statins do not appear to have a class effect on insulin sensitivity in non-diabetic patients. Numerous mechanisms have been suggested to explain how statins cause β-cell insulin secretory dysfunction and peripheral insulin resistance leading to incident diabetes. According to findings from an aggregate of large clinical trials, the benefits of statin treatment appear to outweigh the risk of new-onset diabetes. Therefore, it would be inappropriate to discontinue the use of statins for prevention of cardiovascular events because of its potential risk for development of incident diabetes. This review addresses the currently available evidence related to statin use and new-onset diabetes from a clinical perspective.
INTRODUCTION
Statins are widely prescribed cholesterol-lowering agents and are considered to be relatively well-tolerated and effective drugs that significantly reduce cardiovascular morbidity and mortality. In addition, aggressive lowering of low density lipoprotein cholesterol (LDL-C) with higher doses of statins is increasingly encouraged for the reduction of cardiovascular events in primary and secondary prevention [1]. The widely known adverse effects of statins include liver enzyme elevation, lack of energy, muscle weakness, and myalgia [2]. Meanwhile, statins are known to modulate insulin secretion and sensitivity [3,4], and recent trial data have suggested that statins cause worsening of hyperglycemia and increase the risk of new-onset diabetes [5-8]. The first report on an association between statin use and incident diabetes was derived from post hoc analyses of West of Scotland Coronary Prevention Study (WOSCOP) published in 2001 [9], which reported a 30% risk reduction for incident diabetes with the use of 40 mg of pravastatin. However, the conflicting evidence on the diabetogenicity of statins became widely apparent after the release of 2008 report on Justification for the Use of Statins in Primary Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER) [10]. For this reason, the U.S. Food and Drug Administration placed a warning regarding the risk of diabetes on the labels of all statin agents in March 2012 [11].
Statins appear to cause diabetes. Therefore, it is necessary to address the messages of several clinical trials for study on diabetogenic effects of statins that should be weighed against the cardiovascular benefits that have been extensively established by clinical trials.
CLINICAL TRIAL FINDINGS ON STATINS AND DIABETES
As previously described, WOSCOP, while introducing the notion of direct association between statins and incident diabetes, showed that 5-year treatment of pravastatin 40 mg/day in 5,974 middle-aged men resulted in decreased new-onset diabetes by 30% (P=0.042) [9]. However, in this post hoc analysis, the definition of new-onset diabetes included at least one glucose level of ≥2.0 mmol/L (36 mg/dL) above baseline, which was a nonstandardized diagnostic criteria for diabetes. Sattar et al. [7], who conducted a reanalysis of this trial using standard criteria for diabetes, found that the risk of incident diabetes with pravastatin was no longer significantly increased (odds ratio [OR], 0.79; 95% confidence interval [CI], 0.58 to 1.10). In the aftermath, numerous randomized placebo-controlled trials of statins have reported inconsistent results on statin use and incident diabetes [10,12-15].
Given these inconsistencies, a meta-analysis of five available randomized placebo-controlled trials-Heart Protection Study (HPS; simvastatin 40 mg), Long-Term Intervention with Pravastatin in Ischemic Disease (LIPID; pravastatin 40 mg), Anglo-Scandinavian Cardiac Outcomes Trial (ASCOT; atorvastatin 10 mg), Controlled Rosuvastatin Multinational Study in Heart Failure (CORONA; rosuvastatin 10 mg), and JUPITER (rosuvastatin 20 mg)-was conducted in order to identify the possible effect of statin therapy in the development of type 2 diabetes [6]. The results of this hypothesis-testing trial including 51,619 participants, among whom 1,943 developed diabetes, showed a slight increase in the risk of diabetes (relative risk, 1.13; 95% CI, 1.03 to 1.23) with no evidence of heterogeneity across trials.
Recently, Sattar et al. [7] reported large meta-analyses of published and unpublished data providing a stronger concept that statins may have a diabetogenic effect. In their analysis of 13 trials including 91,140 participants, they demonstrated a 9% increased risk for incident diabetes (OR, 1.09; 95% CI, 1.02 to 1.17) during a mean of 4 years, with little heterogeneity between trials (Fig. 1) [7]. In addition, meta-regression showed that the risk for development of diabetes with statins was highest in trials with older subjects. Neither the change in LDL-C nor baseline body mass index (BMI) showed an association with the risk of new-onset diabetes. Collectively, the findings from the two independent meta-analyses described above suggest an association between statin use and the risk of incident diabetes; furthermore, it also implies that the increased incidence of diabetes is secondary to a class effect.
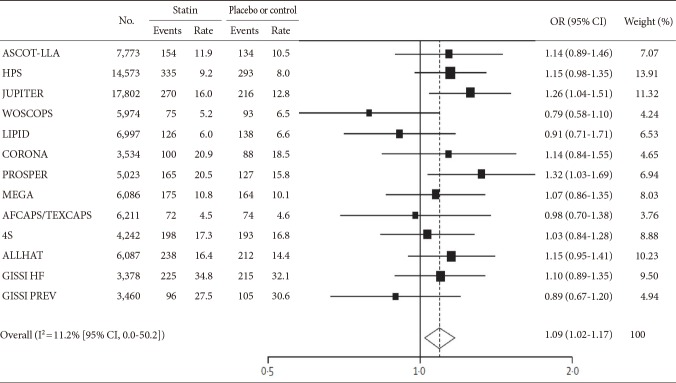
Association between statin therapy and incident diabetes in 13 major cardiovascular trials (weights are from random-effects analysis). OR, odds ratio; CI, confidence interval; ASCOT-LLA, Anglo-Scandinavian Cardiac Outcomes Trial-Lipid Lowering Arm; HPS, Heart Protection Study; JUPITER, Justification for the Use of Statins in Primary Prevention: an Intervention Trial Evaluating Rosuvastatin; WOSCOP, West of Scotland Coronary Prevention Study; LIPID, Long-Term Intervention with Pravastatin in Ischemic Disease; CORONA, Controlled Rosuvastatin Multinational Study in Heart Failure; PROSPER, Pravastatin in Elderly Individuals at Risk of Vascular Disease; MEGA, Management of Elevated Cholesterol in the Primary Prevention Group of Adult Japanese; AFCAPS/TEXCAPS, Air Force/Texas Coronary Atherosclerosis Prevention Study; 4S, Scandinavian Simvastatin Survival Study; ALLHAT, Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial; GISSI-HF, Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico-Heart Failure Trial; GISSI-PREV, GISSI-Prevenzione Trial. Adapted from Sattar et al. Lancet 2010;375:735-42, with permission from Elsevier [7].
The JUPITER study is generally considered to have triggered the current debate. It was a randomized placebo-controlled primary prevention trial, which included 17,802 middle aged men and women (mean age of 66 years) with serum LDL-C less than 130 mg/dL and high-sensitivity C-reactive protein levels of 2.0 mg/L. The primary outcome was the occurrence of myocardial infarction, stroke, unstable angina, arterial revascularization, or death from cardiovascular causes. Treatment with rosuvastatin 20 mg/day resulted in a 43% decrease of primary endpoint at the expense of a 25% increase in physician-diagnosed diabetes [10]. Therefore, they provided a post hoc analysis from the JUPITER trial in order to directly address the balance of cardiovascular benefits and diabetes risk of statin use [16]. In this analysis, an important prespecified secondary aim of the trial was to address the effects of rosuvastatin on incident type 2 diabetes. History of diabetes was an exclusion criterion for the trial; however, many participants in JUPITER trial had major risk factors for diabetes, such as metabolic syndrome, impaired fasting glucose, BMI ≥30 kg/m2, and hemoglobin A1c ≥6% at study entry. In individuals with one or more risk factors, statin treatment showed an association with a 39% reduction in the primary endpoint (P=0.0001) and a 28% increase in diabetes (P=0.01). In subjects with no risk factors, statin treatment showed an association with a 52% reduction in primary endpoint (P=0.0001), and no increase in diabetes (P=0.99) (Table 1) [16]. In analysis limited to the 486 participants who developed diabetes, reduction in cardiovascular risk associated with statin therapy (hazard ratio [HR], 0.63; 95% CI, 0.25 to 1.60) was consistent with that for the trial as a whole (HR, 0.56; 95% CI, 0.46 to 0.69). In either case, the absolute cardiovascular event lowering benefit by statin therapy was greater than the risk of developing new onset diabetes, both in subjects with and without risk factors of diabetes. The results of another post hoc analysis of JUPITER based on gender indicated that women in the rosuvastatin group showed a striking 50% increase in new onset diabetes (P=0.008), equal to the absolute decrease in cardiovascular events. By contrast, men in the rosuvastatin group exhibited a statistically nonsignificant 13% increase in new onset diabetes (P=0.29) [17].
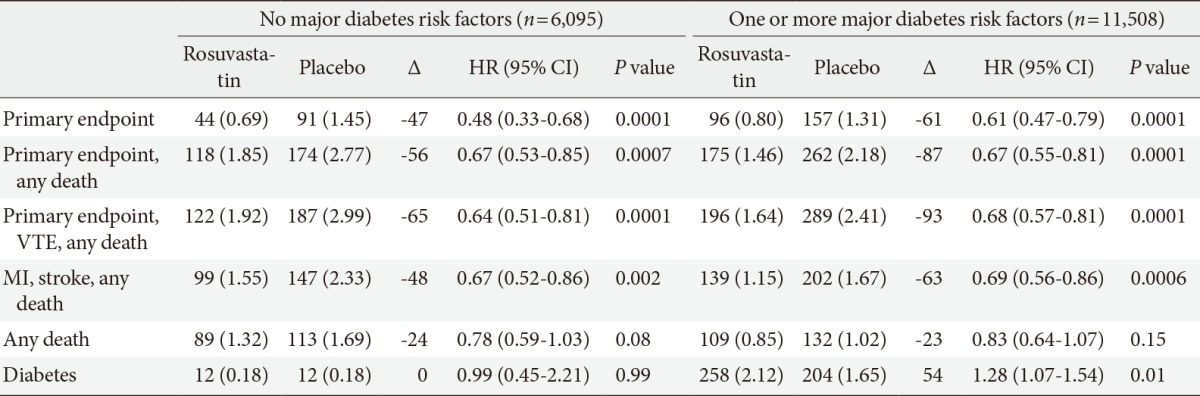
Absolute number of events, incidence rates, and hazard ratios for cardiovascular endpoints, death, and diabetes in the JUPITER trial in participants with or without major diabetes risk factors, according to random allocation to rosuvastatin or placebo
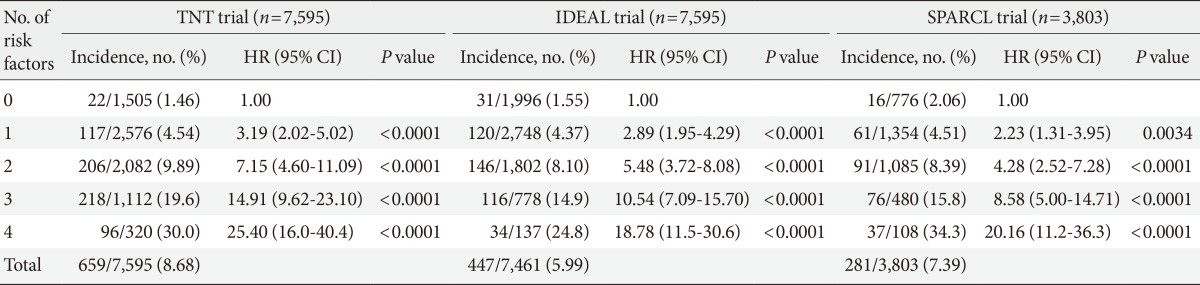
Intensive-dose statin therapy has been shown to further reduce cardiovascular events compared with moderate-dose statin, whereas side effects of statins appear to be dose related. Therefore, it is important to know whether an intensive dose of statin is associated with a greater risk of incident diabetes. Results of meta-analysis from five intensive-dose statin trials (n=32,752)-Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis In Myocardial Infarction 22 (atorvastatin 80 mg/pravastatin 40 mg), the Aggrastat to Zocor (A to Z trial; simvastatin 40 mg, simvastatin 80 mg/placebo, simvastatin 20 mg), Treating to New Target (TNT; atorvastatin 80 mg/atorvastatin 10 mg), Incremental Decrease in End Points through Aggressive Lipid Lowering (IDEAL; atorvastatin 80 mg/simvastatin 20 mg or 40 mg), and the Study of the Effectiveness of Additional Reductions in Cholesterol and Homocysteine (simvastatin 80 mg/simvastatin 20 mg)-revealed that the risk of new-onset diabetes over a weighted mean follow-up of 4.9 years increased by 12% (95% CI, 1.04 to 1.22) in subjects in intensive-dose statin therapy group compared with those in moderate-dose statin group. On the other hand, cardiovascular events decreased by 16% in intensive-dose group compared with the moderate-dose group (95% CI, 0.75 to 0.94) [8]. Waters et al. [18] also investigated the effect of high-dose statin on new-onset diabetes within three large randomized trials with atorvastatin. Among them, the TNT and IDEAL trials showed a trend toward an increase in new-onset diabetes for the atorvastatin 80 mg group compared with atorvastatin 10 mg (HR, 1.10; 95% CI, 0.94 to 1.29) or simvastatin 20 mg (HR, 1.19; 95% CI, 0.98 to 1.43). However, in the Stroke Prevention by Aggressive Reduction in Cholesterol Levels trial, an 80-mg dose of atorvastatin showed an association with an increased risk of incident diabetes compared with placebo (HR, 1.34; 95% CI, 1.05 to 1.72) [18]. This is slightly higher, but it still overlaps with the HR of 1.09 reported in the meta-analysis of 13 placebo-controlled statin trials [7]. According to multivariate analysis, fasting glucose >100 mg/dL, BMI >30 kg/m2, fasting triglycerides >150 mg/dL, and hypertension were strong predictors of new-onset diabetes in all three trials and in each of the three trials the risk of developing new-onset type 2 diabetes mellitus increased with an increasing number of risk factors (Table 2) [18].
Along with dose-related adverse effects of statins, there are also concerns regarding increased risk for patients who may have increased sensitivity to statins. Asians usually achieve heightened responses to therapeutic drugs compared to Westerners. The potential mechanisms are related to genetic differences between Asians and Caucasians in the metabolism of statins at the level of hepatic enzymes and drug transporters [19]. An analysis of 153,840 postmenopausal women without diabetes participating in the Women's Health Initiative reported an association of statin use with a 71% increase in new onset diabetes and a 48% increase after adjustment for other potential confounders (age, race, BMI, etc.) for all types of statins [20]. A subgroup analysis based on race/ethnicity showed a HR of 1.78 (95% CI, 1.32 to 2.40) for new-onset diabetes in Asian and Pacific Islander statin users; by contrast, Caucasian statin users had a HR of 1.49 (95% CI, 1.38 to 1.62). Thus, this finding indicates that genetic differences cause Asians to be more susceptible to the diabetogenic effects of statin therapy.
The question of whether distinct statins have differential effects on insulin sensitivity and metabolic homeostasis remains controversial. However, pravastatin has been reported to improve insulin sensitivity in patients with metabolic syndrome [21] and in patients with coronary artery disease and impaired glucose tolerance [22,23], and this effect was accompanied by a significant increase of plasma adiponectin levels [23,24]. On the other hand, other statins, including atorvastatin, rosuvastatin, and simvastatin, significantly contribute to an increase in insulin levels and a decrease in plasma adiponectin levels and insulin sensitivity [22]. These varying effects of statins on insulin sensitivity in patients without pre-existing diabetes were supported by systemic review and meta-analysis (Fig. 2) [25]. In addition, a recent population-based cohort study also showed that, compared with pravastatin, atorvastatin (adjusted HR, 1.22; 95% CI, 1.15 to 1.29), simvastatin (adjusted HR, 1.10; 95% CI, 1.04 to 1.17), and rosuvastatin (adjusted HR, 1.18; 95% CI, 1.10 to 1.26) increased the risk of incident diabetes, whereas no significantly increased risk was observed for fluvastatin (HR, 0.95) or lovastatin (HR, 0.99) [26]. Taken together, statins do not appear to have a class effect on insulin sensitivity in nondiabetic patients, and differences between individual statins are likely to exist.
Statins have been shown to lower LDL-C levels in patients with diabetes in addition to lowering the risk of major cardiovascular diseases (CVDs). The meta-analysis conducted by the Cholesterol Treatment Trialists reported a 21% reduction in the incidence of major vascular events per 1 mmol of LDL-C reduction (P<0.001) in 18,686 subjects with diabetes from 14 randomized controlled trials of primary and secondary CVD prevention [27]. Overall, in subjects with diabetes, statins have been shown to lower LDL-C by 22% to 40%, with a significant reduction in the 10-year risk of major CVD by 8% to 50% (P=0.007) [28]. Consequently, it seems necessary to weigh the risk of diabetes and the cardiovascular benefits of statins. Sattar et al. [7] determined that treatment of 255 patients with a statin for 4 years would result in one extra case of new-onset diabetes but would prevent 5.4 major coronary events (coronary heart disease death and nonfatal myocardial infarction) for a reduction of 1.0 mmol/L in LDL-C. This benefit would be greater if strokes and coronary revascularizations were included [7]. The rate of cardiovascular events in patients with new-onset diabetes was much lower than that of patients with diabetes at baseline and was not appreciably higher than that of patients without new-onset diabetes (adjusted HR, 1.02; 95% CI, 0.77 to 1.35) [18]. Post hoc analysis of JUPITER demonstrated a 28% increase in incident diabetes yet also exhibited a reduction of 39% in the primary endpoint among subjects assigned to rosuvastatin with at least one risk factor. That is, 134 vascular events or deaths were avoided for every 54 cases of new-onset diabetes. In addition, subjects with no risk factors showed no increased risk of diabetes (HR, 0.99; 95% CI, 0.45 to 2.21) and a 52% reduction in the primary endpoint (HR, 0.48; 95% CI, 0.33 to 0.68) [16]. These results demonstrate that the benefit for cardiovascular events and mortality of statin exceed the risk of diabetes.
HYPOTHETICAL MECHANISMS FOR STATIN-INDUCED DIABETES
Many mechanisms have been suggested in order to explain how statin might cause diabetes, and it seems that interaction of each mechanism is variably involved, such as combined pathophysiologic nature of diabetes characterized by a combination of β-cell dysfunction and insulin resistance of peripheral tissue [29]. A hypothetical paradigm for statin-induced impairment of glucose metabolism was proposed by Sampson et al. [30] with available experimental evidence (Fig. 3) [30, 31]. Statins (especially lipophilic statins) have been shown to inhibit glucose-induced calcium signaling and insulin secretion by blocking L-type Ca2+ channels in β-cells [32,33]. Glucokinase, the rate limiting enzyme for intracellular glucose metabolism, is inhibited by highly increased uptake of plasma LDL-C by statins, thus impairing glucose-induced calcium signaling for insulin secretion [34]. Moreover, statins reduce the synthesis of coenzyme Q 10 (CoQ10), an essential factor in the mitochondrial electron-transfer system, resulting in inhibition of insulin secretion by reduced production of ATP [35]. Reduction of CoQ10 levels in blood and muscle also leads to disruption of muscle mitochondrial function, which is related to the pathogenesis of insulin resistance and decreased exercise tolerance [36,37]. In in vitro and in animal studies, statins inhibit synthesis of isoprenoids leading to down-regulation of GLUT4 expression on adipocytes, resulting in impairment of glucose uptake [38,39].

Hypothetical paradigm for statin-induced impairment of glucose metabolism. 1) Statins inhibit cascade of closure of adenosine triphosphate (ATP)-dependent potassium channel, depolarization, and calcium influx leads to insulin secretion. 2) Glucokinase is inhibited by highly increased uptake of plasma low density lipoprotein cholesterol (LDL-C; plasma-derived) by statins. 3) Statins suppress synthesis of CoQ10, resulting in inhibition of insulin secretion due to reduced production of ATP. 4) Statin suppresses the synthesis of isoprenoids, thus causing downregulation of glucose transporter 4 (GLUT4) expression on adipocyte cells. 5) Statins cause unregulation of LDL receptors (LDL-Rs), leading to enhanced uptake of LDL-C. 6) Oxidation of LDL-C may incite an inflammatory cascade. 7) Over-production of nitric oxide (NO) has been shown to induce β-cell apoptosis via activation of calpain. CHOL, cholesterol (de novo synthesized); HMG-CoA, 3-hydroxy-methylglutaryl coenzyme A; OxLDL, oxidized low density lipoprotein. Adapted from Sampson et al. Curr Opin Cardiol 2011;26:342-7, with permission from Wolters Kluwer Health [30] and Sattar et al. Atheroscler Suppl 2012;13:1-10, with permission from Elsevier [31].
From the perspective of β-cell inflammation, statins enhance uptake of plasma LDL-C by up-regulation of LDL receptors and oxidation of LDL-C, triggering intracellular inflammatory response that compromises the functional and structural integrity of islet β-cells and ultimately leads to insulin secretory dysfunction [30]. In addition, cytokine-induced excessive production of nitric oxide has been shown to induce β-cell apoptosis via activation of calcium dependent protease, calpain [40]. Comprehensive interaction of these processes may contribute to development of diabetes by statin and may become more significant in elderly persons with age-dependent loss of β-cells. Also, statin-induced cholesterol lowering per se contributes to myocyte damage of skeletal muscle fiber in a majority of patients, despite their being asymptomatic, which may cause skeletal muscle insulin resistance [41]. Furthermore aging-related skeletal muscle wasting may accelerate statin-induced peripheral insulin resistance [29,42].
CONCLUSIONS
Although most of clinical trials of statins are conducted during relatively short duration, available data suggest that statin therapy increases the risk of new-onset diabetes, and the risk of diabetes increased with higher dose of statins and increased in susceptible groups, such as the the elderly, women, and Asians. Meanwhile, statins do not appear to have a class effect on insulin sensitivity in nondiabetic patients. However, it is important to note that statins are highly effective for prevention of cardiovascular events in individuals with or without diabetes. Therefore, the benefits of statin treatment appear to outweigh the risk of new-onset diabetes. In particular, the benefits of atorvastatin clearly outweigh the risks in patients with coronary or cerebrovascular disease. Therefore, it would not be appropriate to argue that potential risk of incident diabetes should result in discontinuation of statins for primary or secondary prevention in any patient with moderate to high cardiovascular risk.
It is more reasonable to investigate the question of whether individuals known to be susceptible to statins with a lower cardiovascular risk show the similar benefits of statin therapy outweighing the potential risk of incident diabetes. Avoidance of high-dose statin therapy, if possible, and more careful monitoring for development of diabetes are recommended. Moreover, proactive and intensive lifestyle intervention should be implemented in individuals with a high risk of incident diabetes when statins are used.
Notes
No potential conflict of interest relevant to this article was reported.