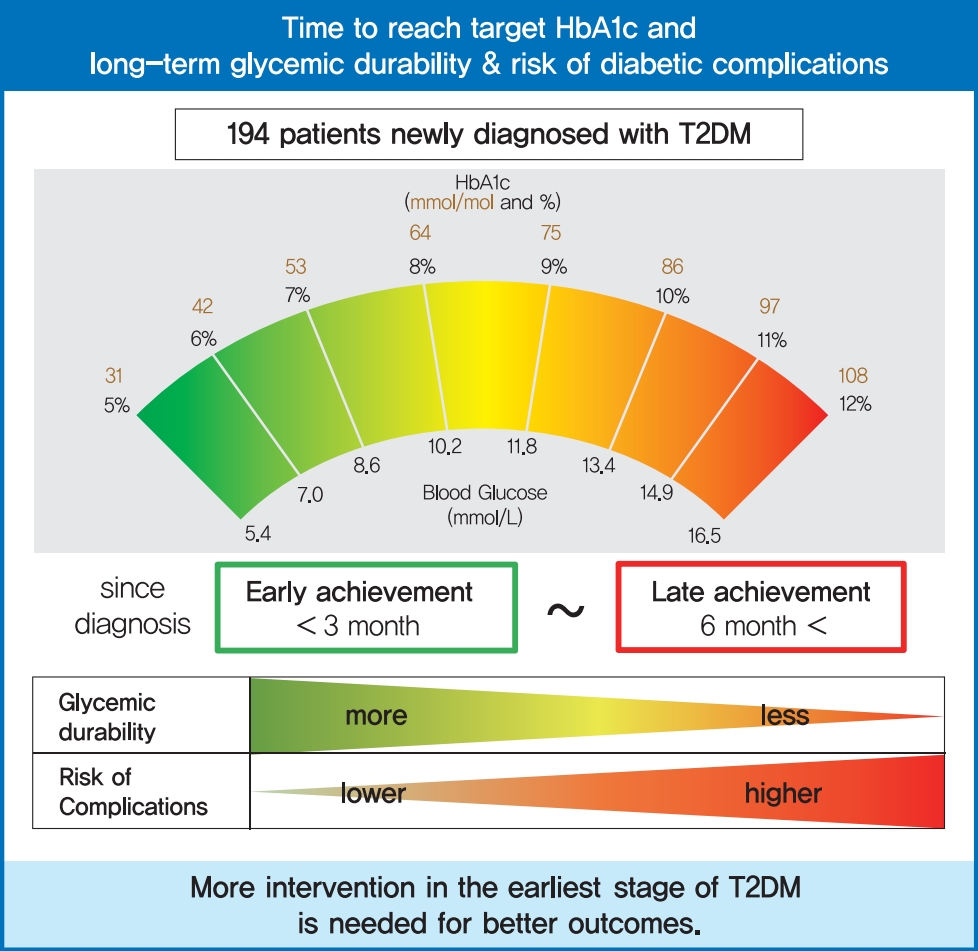
Time to Reach Target Glycosylated Hemoglobin Is Associated with Long-Term Durable Glycemic Control and Risk of Diabetic Complications in Patients with Newly Diagnosed Type 2 Diabetes Mellitus: A 6-Year Observational Study
Article information
Abstract
Background
To evaluate the association of time to reach the target glycosylated hemoglobin (HbA1c) level with long-term durable glycemic control and risk of diabetic complications in patients with newly diagnosed type 2 diabetes mellitus (T2DM).
Methods
In a longitudinal observational cohort, 194 patients with T2DM newly diagnosed between January 2011 and March 2013 were followed up over 6 years. Patients were classified according to the time needed to reach the target HbA1c (<7.0%): <3, 3 to 6 (early achievement group), and ≥6 months (late achievement group). Risks of microvascular complications including diabetic retinopathy, nephropathy, and neuropathy as well as macrovascular events including ischemic heart disease, ischemic stroke, and peripheral arterial disease were assessed by multivariable Cox proportional hazards analysis.
Results
During a median follow-up of 6.53 years, 66 microvascular and 14 macrovascular events occurred. Maintenance of durable glycemic control over 6 years was more likely in the early achievement groups than in the late achievement group (34.5%, 30.0%, and 16.1% in <3, 3 to 6, and ≥6 months, respectively, P=0.039). Early target HbA1c achievement was associated with lower risk of composite diabetic complications (adjusted hazard ratio [HR, 0.47; 95% confidence interval [CI], 0.26 to 0.86 in <3 months group) (adjusted HR, 0.50; 95% CI, 0.23 to 1.10 in 3 to 6 months group, in reference to ≥6 months group). Similar trends were maintained for risks of microvascular and macrovascular complications, although statistical significance was not reached for macrovascular complications.
Conclusion
Early target HbA1c achievement was associated with long-term durable glycemic control and reduced risk of diabetic complications in newly diagnosed T2DM.
INTRODUCTION
Hyperglycemia leads to development of vascular complications in patients with diabetes mellitus [1-4]. However, the impact of intensive glycemic control on diabetic complications differed according to patient characteristics [5]. The benefit of intensive glycemic control on diabetic complications has been relatively established early in the course of type 2 diabetes mellitus (T2DM) [6,7], whereas the effects of intensive glycemic control on macrovascular still remain inconsistent among patients with longstanding diabetes [8-12]. This so-called legacy effect, referred to as metabolic memory, has also been shown in several observational studies [13-15]. Such evidence has strongly influenced the changes in diabetes management guidelines that have introduced the concept of individual treatment strategies [16].
The United Kingdom Prospective Diabetes Study (UKPDS) has provided important evidence showing the beneficial effect of early intensive glycemic control on lowering diabetic vascular complications in patients with recent-onset T2DM [6,7]. However, the intensive treatment arm in the UKPDS did not reach the target glycosylated hemoglobin (HbA1c) level of below 7.0%, which is the generally recommended target in recent clinical guidelines [7]. These results are similar to those in the Diabetes Control and Complication Trial and the Steno-2 trial [2,17]. Currently, we do not have enough evidence regarding the effect of truly intensive glycemic control on diabetic complications in the early stage of diabetes. We also do not have enough information regarding how fast and how long we need to conduct the intensive glycemic control strategy in those patients.
In a previous retrospective cohort study, we evaluated the factors related to durable glycemic control in newly diagnosed T2DM [18]. From this study, one of the major determinants for durable glycemic control was how early patients achieved the target HbA1c level (<7.0%). Patients who reached the target HbA1c level within 3 months were more likely to maintain durable glycemic control up to 2 years compared with those who did not, suggesting that prompt engagement of an intensive glycemic control strategy right after a diagnosis of diabetes is needed. Based on those results, we hypothesized that early achievement of the target HbA1c level at the time of disease onset would lead to favorable outcomes in terms of diabetic complications as well as long-term durable glycemic control. The aim of this study was to evaluate the impact of early glycemic control on the prevention of future diabetic complications beyond glycemic durability in a real-world clinical setting.
METHODS
Study population
This study was conducted as part of a longitudinal observational T2DM patient cohort, the Anam Diabetes Observational Study (ADIOS). The cohort consisted of 194 men and women aged ≥18 years, who were newly diagnosed with T2DM between January 2011 and March 2013 at a single tertiary medical institution in Korea. The diagnosis of T2DM was based on the diagnostic criteria of the American Diabetes Association [19]. Patients were routinely followed up every 3 to 4 months during the observational period. Each individual’ medical history, demographic information, new or advanced symptoms related to diabetic complications, and prescribed medications were recorded at baseline and every visit. Anthropometric data such as systolic and diastolic blood pressure, body weight, and waist circumference were measured at each follow-up visit. The laboratory variables including HbA1c, fasting plasma glucose, serum creatinine, estimated glomerular filtration rate (eGFR), and lipid profiles were also evaluated. The urinary albumin to creatinine ratio was measured every 6 to 12 months. Development or progression of diabetic complications was assessed annually or more frequently based on the clinician’s judgement. From the original cohort, 145 patients (74.7%) completed follow-up of over 6 years until October 2018. There have been no regulations for clinicians’ judgement for choice of glucose-lowering agents; however, the target of HbA1c to be achieved was set to less than 7.0% at the onset of T2DM in all patients.
The Institutional Review Board of Korea University Anam Hospital approved this study protocol (2017AN0050). All participants provided informed written consent. The STROBE statement checklist is available as Appendix 1.
Outcomes
Long-term durable glycemic control was defined as the maintenance of optimal glycemic control (HbA1c <7.0%) from 6 months after diagnosis up to 6 years without adding other glucose-lowering agents.
Composite diabetic complications including microvascular and macrovascular events were assessed. The prespecified microvascular outcomes were onset or progression of diabetic retinopathy, nephropathy, and neuropathy. The incidence or progression of diabetic retinopathy was assessed by routine eye examination with funduscopy or by ophthalmologists. The incident nonproliferative diabetic retinopathy and progression from nonproliferative to proliferative diabetic retinopathy assessed by ophthalmologists as well as procedures involving vitrectomy or photocoagulation were counted as diabetic retinopathy outcomes. The outcomes of new or worsening nephropathy were defined as having any of the following: progression of albuminuria based on urinary albumin to creatinine ratio (incident microalbuminuria or progression from microalbuminuria to macroalbuminuria), doubling of serum creatinine, ≥30% decline in eGFR, or development of end-stage kidney disease [20]. Diabetic peripheral neuropathy was assessed by questionnaire, electromyography, or a nerve conduction study. The macrovascular outcomes included atherosclerotic cardiovascular events defined according to diagnostic or therapeutic procedures for ischemic heart disease, ischemic stroke, and peripheral artery disease.
Statistical analyses
Patients were classified into three groups according to the time needed to reach the target HbA1c level (<7.0%) after diagnosis: within 3, 3 to 6 (early achievement groups), and 6 months or over (late achievement group). Patient characteristics are described as number (percent) for categorical variables and mean (standard deviation) for continuous variables. Groups were compared using paired t-test, Mann-Whitney U test, and analysis of variance. A repeated-measures logistic model for the longitudinal analysis of HbA1c over time was performed to compare mean HbA1c trajectories between groups. Cox proportional hazards regression analysis was used to estimate the hazard ratio (HR) and 95% confidence interval (CI) for long-term durable glycemic control and microvascular and macrovascular complications. Possible confounders including age, sex, smoking, alcohol, education level, physical activity, body mass index (BMI), mean low-density lipoprotein cholesterol, mean systolic blood pressure, eGFR, glucose-lowering agents, antithrombotic agents, statins, antihypertensive drugs, and baseline HbA1c levels were adjusted for analyses. Kaplan-Meier curves were presented to describe the time until the first event of composite complications and to compare differences among groups using a log-rank test. A P<0.05 was considered statistically significant. All statistical analyses were performed using SPSS version 24.0 (IBM Co., Armonk, NY, USA).
RESULTS
The baseline characteristics of patients are summarized in Table 1. The mean age was 54.7 years, and mean BMI was 25.6 kg/m2. Patient characteristics according to time to reach the target HbA1c level (<3, 3 to 6, and ≥6 months) were generally comparable except for a few variables. Subjects in the early target HbA1c achievement groups had higher education levels, higher fasting C-peptide levels, and lower total cholesterol levels than those in the late achievement group. The mean HbA1c level at diagnosis was tended to be higher in the later achievement group than that in the early achievement groups (8.9%, 8.9%, and 9.7% in the <3, 3 to 6, and ≥6 months groups, respectively), but the difference was not significant. Accordingly, sulfonylurea (SU) and insulins were more frequently prescribed in those patients (Supplementary Table 1).
Time to target HbA1c achievement and long-term durable glycemic control
Of 194 patients in the original cohort, 145 patients completed the follow-up. We excluded patients who had positive autoantibodies, died from other underlying diseases and lost to follow-up (Supplementary Fig. 1). The mean follow-up duration was 6.53 years (range, 5 to 9). The HbA1c trajectory in each group is presented in Supplementary Fig. 2. Mean HbA1c levels during 6-year were 6.6%±0.6%, 6.7%±0.6%, and 7.3%±0.8% in the <3, 3 to 6, and ≥6 months, respectively.
We examined the association between time to reach the target HbA1c level (<7.0%) and long-term durable glycemic control (Fig. 1). Compared with patients who reached the target HbA1c over 6 months after diagnosis, those who reached the target value within 6 months were more likely to maintain durable glycemic control (34.5 %, 30.0%, and 16.1% in the <3, 3 to 6, and ≥6 months groups, respectively, P=0.039), suggesting that earlier glycemic target achievement is responsible for long-term durable glycemic control in new-onset T2DM.
Time to target HbA1c achievement and microvascular and macrovascular events
During follow-up, 66 microvascular and 14 macrovascular events occurred. Diabetic complication events occurred more frequently in the late achievement group than in the early achievement groups: 31.9%, 30.8%, and 48.7% in the <3, 3 to 6, and ≥6 months groups, respectively (Table 2). The risk of composite diabetic complications was significantly lower (adjusted HR, 0.47; 95% CI, 0.26 to 0.86) in the earliest target HbA1c achievement group (<3 months) than in the late target HbA1c achievement group (≥6 months) after adjusting for confounding variables. Similar results were found for microvascular complications. Macrovascular events also occurred less frequently in the early achievement groups than in the late achievement group; however, the HR was not significant probably because of the low number of events in each group. Further adjustment for mean HbA1c during 6 years in the same analyses did not largely change the direction of the results (Supplementary Table 2).
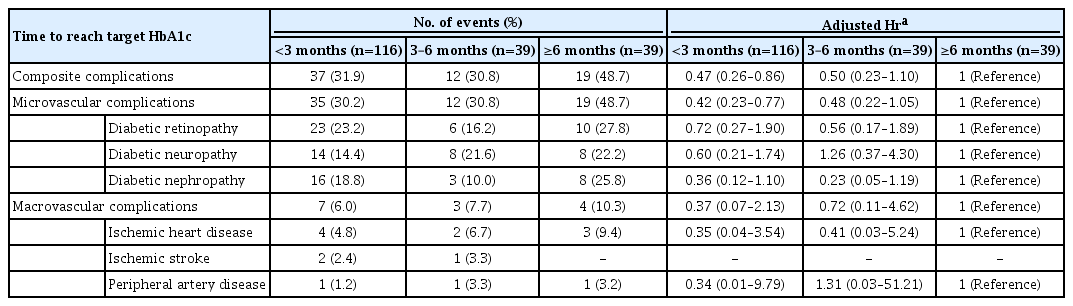
HRs for the risks of diabetic complications according to time to reach target HbA1c according to the Cox proportional hazards model
Kaplan-Meier curves also demonstrated a lower disease-free probability of composite diabetic complications in the early achievement groups than in the late achievement group although it showed borderline statistical significance (log-rank test, P=0.060) (Fig. 2).
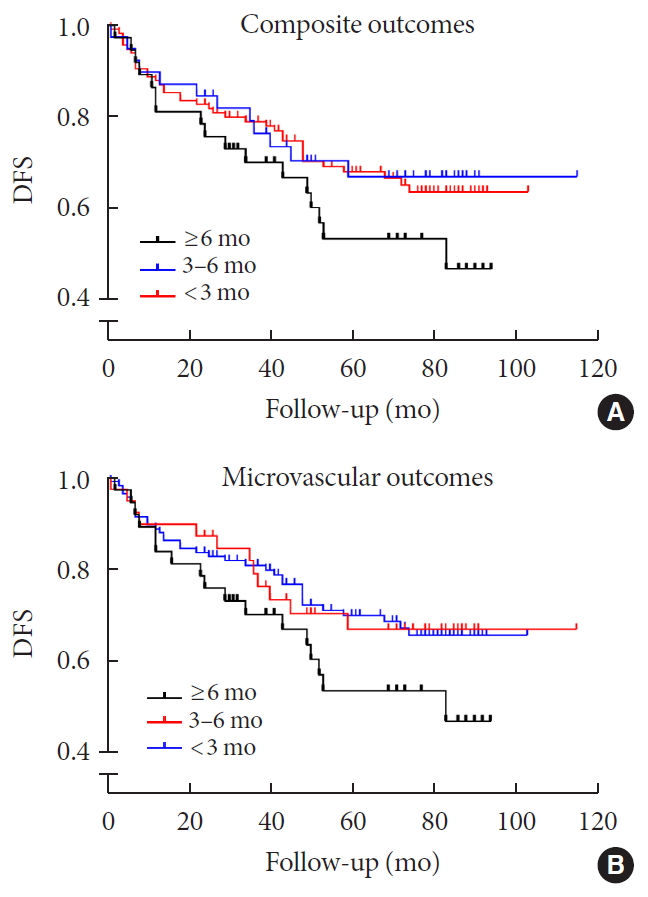
Kaplan-Meier curves for cumulative survival incidence of diabetic complications according to time to reach target glycosylated hemoglobin. (A) Composite complications (log rank test, early achievement groups vs. late achievement group; P=0.060). (B) Microvascular outcomes (log rank test, early achievement groups vs. late achievement group; P=0.034). DFS, disease free survival.
DISCUSSION
In this observational cohort study, we found that time to reach target HbA1c achievement was closely associated with long-term glycemic control and risk of diabetic complications in new-onset T2DM. These results have important clinical implications in the management of T2DM. First, the outcome of patients with T2DM, especially regarding development of diabetic complications, is determined very early in the course of disease progression. Second, early intervention in the management of hyperglycemia is important to reduce the risk of diabetic complications as well as to maintain long-term durable glycemic control.
The association between early glycemic control intervention and long-term favorable glycemic control was shown in several clinical trials. For example, short-term early intensive insulin therapy with multiple daily injections or the use of an insulin pump in drug-naive patients with T2DM resulted in a high rate of diabetes remission during 1 year [21]. Initial triple combination therapy with metformin, pioglitazone, and exenatide was superior in terms of 2-year glycemic control to conventional sequential add-on therapy in new-onset T2DM [22]. Both studies suggested that β-cell preservation from glucotoxicity in the early stage of diabetes could have beneficial effects on long-term glycemic control [23,24]. Our study provided a different level of evidence regarding this issue because of its observational, rather than interventional, nature. On the assumption that all patients received standard diabetes care, the results in this study clearly indicated that reaching the target HbA1c level within 6 months after diagnosis would lead to long-term glycemic control in routine clinical practice. The glycemic control in the early stage can be explained by many patient or clinician factors including the recovery rate from glucotoxicity, the response to drug therapy, the intensity of drug therapy, or the patient’s attitude to the diabetes management. Although currently we do not definitively know what the major factor is that determines early favorable glycemic control, such early control during the first 6 months after diagnosis could be a critical determinant for 6-year glycemic control in patients with new-onset T2DM.
The benefit of early intensive glycemic control for reducing diabetic complications has been shown in previous randomized controlled trials. The UKPDS reported that intensive glycemic control reduced the risk of microvascular complications by 25% compared with conventional treatment in recent-onset T2DM [6,25]. The 10-year follow-up of the UKPDS confirmed that intensive glycemic control was associated with a low incidence of macrovascular complications and similar results were reproduced in a few cohort studies [26]. The Diabetes and Aging Study reported that patients with HbA1c levels ≥6.5% for the first year after diagnosis had an increased risk of both microvascular and macrovascular events compared with patients with HbA1c levels <6.5% among patients with newly diagnosed T2DM [13]. A recent large cohort study also supported that the degree of glycemic control significantly affected the development of cardiac event in even asymptomatic diabetic individuals [27]. In line with those studies, our study added information about the importance of the earliest stage in the course of diabetes: the earlier the achievement of the target HbA1c, the lower the complication risk. We speculated that early target achievement is responsible for the low risk of diabetic complications via long-term durable glycemic control. In addition, we also found that visit-to-visit HbA1c variability was lower in the early achievement groups than that in the later achievement group (SD, 0.96, 0.99, and 1.27 in the <3, 3 to 6, and ≥6 months groups, respectively, P=0.042), which may have affected the development of diabetic complications. Glycemic variability has recently drawn much interest as a strong prognostic factor for diabetes complications and mortality [28-31]. It has been demonstrated that oscillating glucose could result in damage to endothelial cells and increase oxidative stress more than continuous hyperglycemia in both healthy and T2DM subjects [32].
We also noted that patients in the late target achievement group had low level of baseline C-peptide with frequent use of SU and insulins, which means they had dysfunctional pancreatic β-cell. Previous studies have already identified the relationship between β-cell dysfunction and the development of diabetic complications [33]. Glucotoxicity followed by prolonged hyperglycemia induces β-cell dysfunction possibly through mitochondrial dysfunction with production of reactive oxygen species, endoplasmic reticulum stress, and increased levels of intracellular calcium, which are also relevant to the development of complications such as neuropathy, nephropathy and cardiovascular disease [33,34]. Therefore, β-cell dysfunction in the late achievement group was likely to directly or indirectly associated with to the development of diabetic complication in our study.
The current study has several limitations. First, the number of subjects and the outcomes, especially of macrovascular events, was small. There were 61 microvascular and 13 macrovascular outcomes in total, which might limit the statistical analyses. Second, some clinicians’ and patients’ factors, specifically the engagement of glucose-lowering agents which might influenced the outcomes were not controlled during the observational period. Therefore, glucose lowering agents including insulins were adjusted for the subsequent analyses. Third, there might be a possibility that the low mean HbA1c levels at diagnosis, although it was not significant, in the early target achievement group may have affected the low risk of complications.
In conclusion, among patients with newly diagnosed T2DM, early achievement of the target HbA1c level was associated with long-term durable glycemic control and a lower rate of diabetic complications, especially microvascular complications, compared with late target HbA1c achievement. More intervention in the earliest stages of the disease is needed for better diabetes care.
SUPPLEMENTARY MATERIALS
Supplementary materials related to this article can be found online at https://doi.org/10.4093/dmj.2020.0046.
Proportion of glucose-lowering agents prescribed
HRs for the risks of diabetic complications according to time to reach target HbA1c according to the Cox proportional hazards model
Disposition of study subjects.
Mean glycosylated hemoglobin (HbA1c) trajectory according to time to reach the target HbA1c level. aDifference between groups, P<0.01.
Notes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTIONS
Conception or design: N.H.K.
Acquisition, analysis, or interpretation of data: K.J.K., J.C., J.H.B., K.J.K., H.J.Y., J.A.S., N.H.K., K.M.C., S.H.B., S.G.K., N.H.K.
Drafting the work or revising: K.J.K.
Final approval of the manuscript: K.J.K., J.C., J.H.B., K.J.K., H.J.Y., J.A.S., N.H.K., K.M.C., S.H.B., S.G.K., N.H.K.
FUNDING
This study was supported by a Korea University Grant K1625581 (author Nam Hoon Kim).
Acknowledgements
None