Efficacy and Safety of Biphasic Insulin Aspart 30/70 in Type 2 Diabetes Suboptimally Controlled on Oral Antidiabetic Therapy in Korea: A Multicenter, Open-Label, Single-Arm Study
Article information
Abstract
Background
The purpose of this study was to evaluate change in glycosylated hemoglobin (HbA1c), side effects, and quality of life (QOL) after a 16-week treatment period with Biphasic insulin aspart 30/70 (BIasp30) in patients with type 2 diabetes mellitus (T2DM) who had been suboptimally controlled with oral antidiabetic drugs (OADs).
Methods
The study consisted of a 4-week titration period when concurrent OAD(s) were replaced with BIasp30 and followed by a 12-week maintenance period. All patients completed the Diabetes Treatment Satisfaction Questionnaire at the beginning and the end of the trial. Hypoglycemic episodes were recorded by the patient throughout the trial.
Results
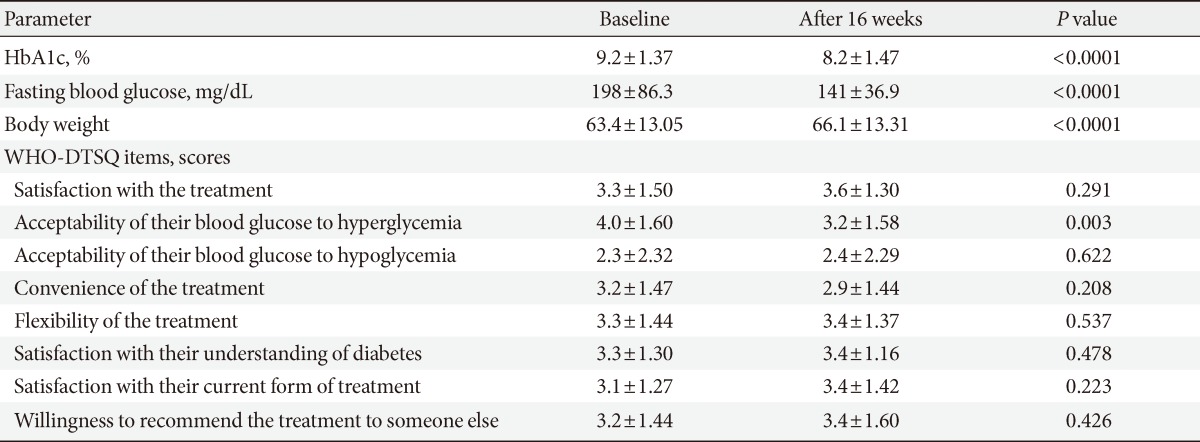
Sixty patients were included, of whom 55 patients (92%) completed the full 16-week treatment period. Seven-point blood glucose was significantly improved as compared with the baseline, except for the postlunch blood glucose level. HbA1c at the end of period was significantly improved from 9.2% to 8.2% (P<0.001). Eleven percent (n=6) of patients achieved HbA1c values ≤6.5% and 22% (n=12) of patients achieved <7.0%. There were 3.4 episodes/patients-year of minor hypoglycemia and 0.05 episodes/patients-year of major hypoglycemia. QOL showed significant changes only in the acceptability of high blood glucose category (P=0.003).
Conclusion
Treatment with once or twice daily BIasp30 may be an option for the patients with T2DM suboptimally controlled with OADs in Korea. However, considering the low number of patients achieving the HbA1c target and the high postlunch blood glucose levels, additional management with another modality may be required for optimal control.
INTRODUCTION
Many patients with type 2 diabetes mellitus (T2DM) in Korea are still not receiving appropriate pharmacologic treatment to control and manage their glucose levels, as shown in reports of large number of patients with glycosylated hemoglobin (HbA1c) levels above the International Diabetes Federation target of 6.5% or the American Diabetes Association (ADA) target of 7.0% [1,2]. Poor glucose control can contribute to the development of microvascular complications. T2DM is a progressive metabolic disease in which increased insulin resistance and deterioration of β-cell secretory function result in a decline of glucose homeostasis [3]. Progressive failure of β-cells despite gradual intensification of glucose-lowering therapy leads to hyperglycemia in most patients with T2DM. To compensate for inadequate insulin secretion, insulin treatment is initiated in patients who do not achieve desired glucose control on oral antidiabetic agents (OADs) [4,5].
Patients with T2DM commonly start a once or twice-daily insulin treatment of basal or biphasic insulin formulation [4,5]. Biphasic insulin formulation has become increasingly popular because of its simplicity, the substitution of both basal and mealtime insulin with a single injection, and the possibility of administration immediately before or after a meal [6]. Biphasic insulin analogs improve HbA1c to a greater extent than basal insulin analogs [7,8]. Biphasic insulin aspart 30/70 (BIasp30) consists of 30% soluble insulin aspart and 70% protamine-crystallized aspart. The soluble fraction is absorbed quickly, which effectively controls prandial glucose, and the protamine-crystallized fraction, which has a delayed absorption, controls basal glucose levels [9]. Biphasic insulin analogues provide glycemic control similar to that of biphasic human insulin and better glycemic control than long acting insulin analogues and OADs [10].
Many trials with biphasic insulin analogues have included obese Western patients, and studies of patients with T2DM treated by BIasp30 in Asia are scarce. Unlike Western diabetic patients, Asians with diabetes are primarily young to middle-aged adults [11] and have a lower body mass index (BMI) [12]. On average, Asian patients also have a greater amount of visceral adipose tissue for a given BMI [12], which is significantly associated with subclinical atherosclerosis [13]. Asians also have higher levels of postprandial glycemia and lower insulin sensitivity than Western population in response to a 75-g carbohydrate load [14].
The purpose of this study was to evaluate change in HbA1c after 16 weeks of treatment with BIasp30, to document side effects, and to assess the quality of life (QOL) in patients with T2DM in Korea who failed to achieve optimal glucose control with OADs.
METHODS
Patients
This study was conducted at six centers in Korea from July 2007 to October 2008. Patients who fulfilled the following criteria were included in the study: 1) a diagnosis of T2DM according to the World Health Organization (WHO) classification; 2) older than 18 years; 3) insulin naïve; 4) current treatment with OAD(s) for at least 3 months; and 5) willingness and ability to start insulin therapy and to perform self-blood glucose monitoring. Patients were excluded if they had a history of drug or alcohol abuse, were currently being treated with any other drug known to affect blood glucose (i.e., monoamine oxidase-inhibitors, β-adrenergic agents, anabolic steroids, and systemic glucocorticoids), had impaired renal function with serum creatinine ≥1.7 mg/dL, had significant cardiac disease defined as: decompensated heart failure (New York Heart Association classification III and IV), unstable angina pectoris, myocardial infarction within the last 12 months, or severe uncontrolled hypertension (systolic blood pressure >180 mm Hg and/or diastolic blood pressure >110 mm Hg when seated), or had proliferative retinopathy and/or advanced neuropathy as judged by the investigator. Patients who were pregnant or breast-feeding, or had the intention to become pregnant were not enrolled. The study was approved by the Institutional Review Boards of each individual participating institution. All patients provided written informed consent before the start of the study. This trial was registered in Clinical Research Information Service in Korea (http://cris.cdc.go.kr ; identifier, KCT0000388).
Study design
After the screening period of 3 weeks during which each patient continued his/her usual OAD(s) treatment, a 4-week titration period and a 12-week maintenance period were followed. Patients visited the outpatient clinic twice during the titration period and once each month during the maintenance period to evaluate vital signs, anthropometric measurements, adverse events, and compliance. During the titration period, concurrent OAD(s) therapy was replaced with BIasp30 Flex-Pen (Novo Nordisk A/S, Bagsvaerd, Denmark) and the patients were asked to complete the WHO Diabetes Treatment Satisfaction Questionnaire (WHO-DTSQ) [15]. Insulin therapy was initiated at a total daily dose of 0.3 U/kg body weight, with insulin given at breakfast. Insulin was injected immediately before the meal. If the total daily-calculated dose exceeded 30 U, it was recommended to give BIasp30 twice daily at breakfast and at main evening meal at a 2:1 ratio, respectively. Total daily doses were individually titrated in steps of 2 U per injection (when necessary, adjustments in steps of 4 U were considered) based on plasma glucose values from the 3 preceding days (measured with an OneTouch Ultra Glucometer; LifeScan, Milpitas, CA, USA). If two or more of the three readings for a specific time period were not in the target range, the insulin dosage was adjusted. The targets for self-monitored blood glucose (SMBG) values were as follows: 1) fasting, preprandial, and night time 6.0 to 8.0 mmol/L (108 to 143 mg/dL); 2) 120 minutes postprandial 6.0 to 10.0 mmol/L (108 to 179 mg/dL). Healthcare providers were available by telephone to discuss dose adjustment and/or changes in the dosing regimen between the scheduled follow-up visits as needed throughout the study. At the end of the trial, the WHO-DTSQ was again administered to evaluate the QOL following the BIasp30 treatment.
Efficacy assessments
HbA1c was measured at the initial screening and at the end of trial at a local laboratory using high performance liquid chromatography. Fasting plasma glucose was also obtained at the same time as the HbA1c measurement. Daily 7-point (before and after each meal and at bed time) SMBG was checked at the beginning of trial, at the maintenance phase and at the end of trial using a glucometer. QOL was assessed using the WHO-DTSQ at the beginning and at the end of the study. This questionnaire addresses eight areas: satisfaction with the treatment, perceived acceptability of their blood glucose levels (i.e., frequency of hyperglycemia and hypoglycemia), convenience of the treatment, flexibility of the treatment, patient satisfaction with diabetes knowledge, satisfaction with the current form of treatment, and willingness to recommend the treatment to someone else.
Safety assessments
Hypoglycemic episodes were recorded by the patient in a diary throughout the trial (i.e., date, time of hypoglycemic episode, time and dose of last trial medication, time of last main meal prior to the hypoglycemic episode, or a blood glucose value whenever possible). In addition to this basic information, patients responded to the following three questions for each episode: 1) Was it noted with a home glucose monitor? 2) Was the episode symptomatic? 3) Was intravenous glucose infusion required? A major hypoglycemic episode was defined as an episode with severe central nervous system symptoms consistent with hypoglycemia in which the patient was unable to self-treat and which had at least one of the following characteristics: blood glucose <2.8 mmol/L (50 mg/dL) and/or reversal of symptoms after intravenous glucose infusion or intramuscular glucagon injection. A minor hypoglycemia episode was defined as an episode with symptoms consistent with hypoglycemia, confirmed by SMBG measurement <3.8 mmol/L (70 mg/dL) and which was handled by the patient or any asymptomatic blood glucose measurement <3.8 mmol/L (70 mg/dL).
Statistical analysis
Analyses were performed on an intention-to-treat population defined as all patients who were exposed to the experiment and had any efficacy data recorded. Categorical variables are presented here as numbers and percentages. Continuous variables are expressed as means±standard deviation (SD) or as medians with a range. Statistical analysis was performed using a two-tailed paired t-test to compare pretreatment and post-treatment differences in blood glucose profiles, body weight, and WHO-DTSQ scores. We performed repeated-measures analysis of variance (ANOVA) for the comparison of glycemic efficacy across four subgroups, by quartiles of age, BMI, duration of diabetes, and initial HbA1c, and in three subgroups by previous treatment regimen, according to the number of oral hypoglycemic agents used previously. All P values were two sided and a P value of less than 0.05 was considered statistically significant. All statistical analyses were performed using SAS version 9.2 for Windows (SAS Institute Inc., Cary, NC, USA).
Calculation of the sample size was based on the following parameters: 80% power for detecting a difference of 0.4% for HbA1c with a two-sided t-test (α=0.05), an assumed SD of 1.2% for HbA1c, and a 15% drop out rate.
RESULTS
Baseline characteristics
A total of 60 patients enrolled and 59 patients completed this 16-week trial. One patient withdrew due to personal reasons. Baseline demographic data and the presence of various diabetic complications are described in Table 1. We analyzed data from 55 patients in the assessment of efficacy of the insulin regimen to bring about changes in HbA1c, excluding four patients who did not undergo the final HbA1c.
Efficacy
After the 16-week treatment period, HbA1c was significantly improved as compared with the baseline. The mean decrease was 0.99% (from 9.19% to 8.19%; P<0.001) (Table 2). Of a total of 55 patients, 11% (n=6) of patients achieved HbA1c values ≤6.5% and 22% (n=12) achieved HbA1c values <7.0% (data not shown). The mean 7-point SMBG profile for subjects at the end of trial showed a significant decrease in glucose values at all time points except the postlunch time point, when compared with the start of the study (Fig. 1). Prebreakfast values decreased by a mean of 50.1 mg/dL from 198±86.3 mg/dL at baseline to 141±36.9 mg/dL at the final visit (P<0.001). Postbreakfast (P<0.001), prelunch (P=0.001), predinner (P=0.001), postdinner (P=0.001), and bedtime values all decreased as well (P<0.001). Postlunch values also decreased by 9.9 mg/dL without statistical significance (P=0.442). The mean total daily dose of insulin was 0.52±0.19 U/kg at the end of trial. The results of the QOL assessment and the treatment satisfaction questionnaire showed a significant change in acceptability of blood glucose (hyperglycemia) (P=0.003), but no significant change was observed for the other items (Table 2).

Mean 7-point blood glucose profiles at baseline (▪) and after 16-week of treatment with biphasic insulin aspart 30/70 (○). Error bars represent standard error of the mean. P values were calculated from two-tailed paired t-tests. BB, before breakfast; PB, 2 hours postbreakfast; BL, before lunch; PL, 2 hours postlunch; BS, before supper; PS, 2 hours postsupper; HS, bedtime. aP<0.001, bP=0.001 for baseline vs. after 16 weeks.
Efficacy according to subgroups
Results from repeated-measures ANOVA by age, BMI, duration of diabetes, initial HbA1c, and previous treatment regimen are shown in Table 3. Each factor was divided into four groups on the basis of quartiles, with the exception of previous treatment regimen, which was divided into three subgroups according to the number of oral hypoglycemic agents. Across all four HbA1c groups, there was a significant difference in the degree of improvement of HbA1c (P<0.001). Patients with a higher initial HbA1c had significantly greater degrees of improvement. There were no other significant differences identified in the subgroup analyses; differences in age, BMI, duration of diabetes, and previous treatment regimen did not influence the extent of HbA1c improvement.
Safety
There were 62 episodes of minor hypoglycemia reported by 28 of the 55 patients, and only one severe hypoglycemia, which resolved with intravenous dextrose infusion.
DISCUSSION
In this study, we evaluated change in HbA1c, the occurrence of side effects, and alterations in QOL after 16-weeks of treatment with BIasp30 once or twice daily in patients with T2DM in Korea who had failed to reach optimal glucose control with OAD(s). Based on our results, treatment with once or twice daily BIasp30 is an alternative treatment regimen for patients with T2DM suboptimally controlled with OADs in Korea, although it does not necessarily lead to optimal control.
Insulin is generally considered to be the standard of care if glycemic level control with OADs alone is not satisfactory. Although conversion from OADs to insulin treatment can be done in a variety ways, it must be individualized to fit the patient's condition, and each treatment method has its own advantages and disadvantages. Up to 60% of patients will require ultimately insulin within 6 to 10 years of their initial diagnosis; even sooner if they have had long-standing undetected disease [16].
Intermediate-acting insulin or long-acting insulin is recommended as the initial insulin choice according to the recent consensus statement of the ADA and the European Association for the Study of Diabetes [17]. However, in the Treating to Target in Type 2 Diabetes (4-T) study [7], the glycemic efficacy of the biphasic insulin group was better than that of the basal insulin group. The proportions of patients with an HbA1c level of 6.5% or less in the biphasic group versus the basal group were 17.0% and 8.1%, respectively [7]. After 3 years, however, the efficacy of the biphasic insulin, the prandial insulin, and the basal insulin were similar [18]. These results might be due to the fact that sulfonylureas were replaced by additional insulin therapy if the HbA1c level exceeded 6.5%. It is thus difficult to conclude that postprandial glucose level is lowered by using intermediate-acting insulin or long-acting insulin alone.
Importantly, several epidemiological studies have shown the associations between postprandial hyperglycemia and increased risk of cardiovascular disease and death, making this an important measure of diabetes control [19,20]. In the 1-2-3 study [21] and the Sapporo 1-2-3 study [22], biphasic insulin resulted in hypoglycemic effects that were comparable to those of the four-times-daily basal-bolus regimen, an improvement over the basal insulin-only regimen. This suggests that biphasic insulin may be used as an initial insulin treatment in the conversion from OAD(s) to insulin treatment. In the twice daily injections with BIasp30 group in the 1-2-3 study, HbA1c goals ≤6.5% and <7.0% were achieved by 52% and 70%, respectively [21].
We could select biphasic insulin as the initial insulin therapy, however, in our study the twice-daily injection of BIasp30 did not show a satisfactory hypoglycemic effect. Only 11% of patients achieved HbA1c values ≤6.5% and a total of 22% achieved HbA1c <7.0%. There are several possible explanations for the low efficacy of glycemic control of this regimen. First, postlunch glucose control failed. As shown by the mean daily 7-point SMBG, the blood glucose level decreased at all the time points, except postlunch. At an HbA1c level of <7.5%, postprandial glucose contributes approximately 70% to the overall glycemic burden [17]. Some studies have suggested that, in particular, the postlunch blood glucose level and HbA1c are most closely correlated [22]. As such, additional measures should likely be taken to control the postlunch glycemic level.
Second, in this study, all of the patients previous OAD(s) were stopped in the transition to insulin. In other studies using biphasic insulin, metformin and/or other OAD(s) therapies were continued [7,8,18,21,23]. Lund et al. [24] reported that metformin treatment plus insulin prevented weight gain, improved glycemic control, and reduced insulin requirements. Ebato et al. [25] showed that continuing glimepiride with BIasp30 allowed for better glycemic control with less daily insulin doses than did discontinuation of the therapy. It has been suggested that if metformin treatment is combined with insulin treatment, that the HbA1c level is lowered by an additional 0.4%, which would bring the total decrease achieved in our study closer to 1.6%, the average level reported in previous studies. In addition, the percentage of patients achieving the target HbA1c would be much higher. We interpret our results to indicate that OAD treatment should be combined with insulin, even after conversion to insulin treatment, as OAD(s) plays an important role in blood glucose control.
Third, relatively insufficient insulinization may have limited the effect of the therapy. In nine randomized trials investigating insulin initiation with basal insulin, there were dose-responsive relationships between the reduction in HbA1c levels and both the end point insulin dose and the frequency of patient contact. This suggests that substantial decreases in HbA1c can be achieved, provided that the daily insulin dose and the contact frequency are adequate [26]. This same principle could likely be applied to biphasic insulin. In this study, the mean total dose of insulin was 34 U, that is, 0.52 U/kg. In several studies using biphasic insulin, the mean total dose of insulin (0.5 to 1.0 U/kg) was higher, and was related to an achievement ratio of HbA1c ≤6.5% [7,8,18,21,23].
Fourth, the follow-up period may have been too short to confirm the glycemic efficacy of biphasic insulin. However, other studies with a similar follow-up period as this study have shown higher glycemic efficacy than ours [21,23]. In the biphasic insulin group of the Sapporo 1-2-3 study [23], HbA1c goals ≤6.5% were achieved by 21.2% of all patients over a 16-week period. Garber et al. [21] reported that HbA1c goals ≤6.5% were achieved by 52% of all patients over a 16-week period. However, in the 4-T trial, the ratio in achievement of HbA1c ≤6.5% was only 17% at 1 year, and only increased to 31.9% over 3 years [7,18]. Another study took 28 weeks to achieve 42% of patients with HbA1c values ≤6.5% [8].
Fifth, about it is not entirely clear whether all patients in our study were compliant with their insulin dose titration. We used a step-up titration of BIasp30 from once daily injection before breakfast and a patient-driven algorithm, with patients increasing their insulin dose by two units every 3 days. This constitutes a practical approach that has been shown to be equally or more effective than the physician-led titration [27,28]. Rapid titration of the dose is indispensable for successful insulin therapy [26]. Despite this, although patients were encouraged to titrate their doses every 3 days, many reported hesitating for fear of hypoglycemia and weight gain. Therefore, frequent visits to the clinic and patients' compliance would be needed in a patient-driven or doctor-driven algorithm.
In the QOL assessment, only "How often have you felt that your blood sugars have been unacceptably high recently?" showed a statistically significant change. Contrasting with our expectations, patients were less satisfied with their current blood glucose levels after changing to the insulin-based regimen. This suggests that patients expected a higher degree of change in their blood glucose control with the first use of the insulin than was realistic.
In this study, there were 62 episodes of minor hypoglycemia, or, 3.4 episodes per patient-year. Compared to other studies [7,21,29], this is a relatively favorable safety profile. For example, in the insulin glargine group of the APOLLO trial, the number of overall hypoglycemia episodes/patient-year was 5.2 [29]. In the 4-T trial, that of the basal group was 2.3 episodes/patient-year, and the biphasic group was 5.7 [7].
In addition to those mentioned above, this study is not without limitations. It was composed of a relatively small number of patients and was conducted as an open-label, single arm study. Further studies are needed to assess the long-term effect of BIasp30 on glycemic control in Korean patients.
In conclusion, treatment with BIasp30 may be an alternative for patients with T2DM that is only suboptimally controlled with OADs in Korea. For optimal glycemic control, however, an additional approach to the management of postlunch blood glucose, such as continuation of oral medications, may be necessary.
Notes
No potential conflict of interest relevant to this article was reported.