Metformin and Cancer in Type 2 Diabetes
Article information
Many epidemiologic studies have demonstrated a link between type 2 diabetes and an increased risk of certain cancers, and cancer-related mortality [1,2]. A number of meta-analysis studies have shown an increase in the risk of breast, endometrial, colorectal, hepatocellular, pancreatic, bladder cancer, and non-Hodgkin's lymphoma in diabetes patients, compared to nondiabetic subjects [1,3]. Some observational studies have shown that diabetes is positively associated with the mortality of endometrial, breast, and colorectal cancer patients [2,4-6]. Since the prevalence of type 2 diabetes is increasing globally, this association may impart a great burden to public health, and, therefore, has garnered much research attention. There are potential risk factors such as aging, obesity, and physical inactivity, which are shared by both diabetes and cancer. In addition, multiple biologic factors seem to play a role in the development of cancer in type 2 diabetes. These include hyperinsulinemia, hyperglycemia, sex hormones, oxidative stress, and inflammatory cytokines [7].
Insulin resistance and hyperinsulinemia are important characteristics of type 2 diabetes. Chronic hyperinsulinemia may increase the risk of cancer because insulin has a mitogenic and antiapoptotic effect. Insulin can bind and activate insulin receptor as well as insulin-like growth factor 1 receptor (IGF-1R), which has a potent mitogenic and transforming activity. Insulin can also increase free IGF-1 levels by decreasing IGF-1-binding proteins, which can enhance its mitogenic potential [1]. Several epidemiologic findings support this link, demonstrating that hyperinsulinemia is associated with an increased risk of developing endometrial and breast cancers in postmenopausal women without diabetes [8,9]. It is quite difficult to differentiate the specific effect of hyperglycemia on the cancer risk from that of hyperinsulinemia, because the two are usually present in most diabetes patients. A meta-analysis has recently shown that patients having lower hemoglobin A1c levels with intensive glucose control had no difference in cancer incidence, compared to those having higher A1c levels with standard control. However, cancer risk was not the primary outcome of the trials, suggesting that hyperglycemia may not be directly linked to increased cancer risk [10]. Hyperinsulinemia can reduce sex-hormone binding globulin (SHBG), leading to increased bioavailable estrogen, and may affect hormone-sensitive cancers in women with type 2 diabetes. A recent study has shown that higher estrogen, IGF-1, and C-peptide levels increased the risk of postmenopausal breast cancer, suggesting that the interaction between insulin, SHBG, and estrogen may influence the development of breast cancer [11]. Metabolic derangements in type 2 diabetes cause proinflammatory conditions and increased oxidative stress, increasing inflammatory cytokines such as tumor necrosis factor-α (TNF-α) and interleukin 6. It is known that TNF-α can induce development and progression of some tumors [12].
Given the relationship between hyperinsulinemia and the increased risk of cancer in diabetic patients, medications such as insulin or secretagogues, which increase circulating insulin levels, may increase the cancer risk. It is reasonable to expect that insulin sensitizers such as metformin can reduce the cancer risk by decreasing hyperinsulinemia. Recently there have been a number of reports suggesting that diabetes medications are related to an increased risk of cancer. Although there are difficulties complicated by multiple medications and different exposure durations, epidemiologic studies have demonstrated that insulin secretagogues and insulin use may increase the cancer risk, while metformin is associated with a lower risk of cancer [13-18]. Metformin treatment can inhibit hepatic glucose output and improve insulin sensitivity, thereby lowering circulating insulin levels, which may decrease the risk of developing cancer in diabetes patients. Evans et al. [13] first reported that metformin use was associated with a reduced risk of cancer in type 2 diabetic patients. Furthermore, they found a dose-response relationship between metformin and cancer incidence. Thereafter, many, if not all, observational studies have shown a reduced risk of certain cancers, such as breast, pancreas, prostate, and colorectal cancers in diabetic patients treated with metformin [19-22]. In addition to the reduced cancer risk, metformin use has also been associated with reduced cancer-related mortality [23-26]. Recently, there was a report that metformin use in breast cancer led to a higher pathologic complete response rate, compared to no use in diabetic patients [27]. Taken together, these findings are in line with in vitro and in vivo preclinical results showing both antiproliferative and antineoplastic action of metformin on several cancer cell lines and various cancers in animal models [28].
In this issue, Chung et al. [29] presented an article showing a reduced risk of cancer in type 2 diabetes patients treated with metformin, compared to patients not treated with metformin. They enrolled a total of 1,217 patients who had at least 5 years' follow-up, and investigated the influences of metformin and other factors on the risk of cancer. They found that only metformin was associated with a reduced risk of cancer, and that, interestingly, patients taking less than 1,000 mg of metformin per day had a lower cancer occurrence, compared to those taking higher than 1,000 mg per day. In contrast to this finding, several studies have demonstrated a dose-response relationship between the amount of exposure to metformin and cancer risk [13,15]. As the authors suggest, the discrepancy may be due to survival bias or analysis of the daily dose of metformin, rather than the total amount of metformin exposure. Despite of some limitations, this study provides valuable information showing that metformin is associated with a reduced risk of cancer in Korean diabetes patients.
Metformin use in diabetes patients can lower circulating insulin levels by inhibiting hepatic glucose production and improving insulin sensitivity. This insulin-lowering action of metformin may have an anticancer effect because insulin has mitogenic properties. This indirect action of metformin may, in turn, attenuate the activation of phosphatidylinositol-3-kinase (PI3K), the mammalian target of rapamycin (mTOR) signaling in cancer cells [30-32]. As previously noted, given that hyperinsulinemia is related to an increased cancer risk, metformin action on lowering circulating insulin may, in part, explain the association between metformin use and a reduced cancer risk in diabetes patients. In addition, metformin appears to inhibit tumor growth directly because metformin can activate AMP-activated protein kinase (AMPK), and thereby, lead to inhibition of mTOR signaling and subsequent pathways controlling cellular proliferation in various cancer cells [33,34]. Metformin can also inhibit mTOR signaling independently (Fig. 1) [35]. Interestingly, recent studies have shown that metformin may target breast cancer stem cells, or be involved in regulating stem cell ontogeny [36,37]. Although the underlying mechanisms are not yet fully understood, the inhibition of mTOR signaling pathways may be a major mechanism of antitumor action of metformin.
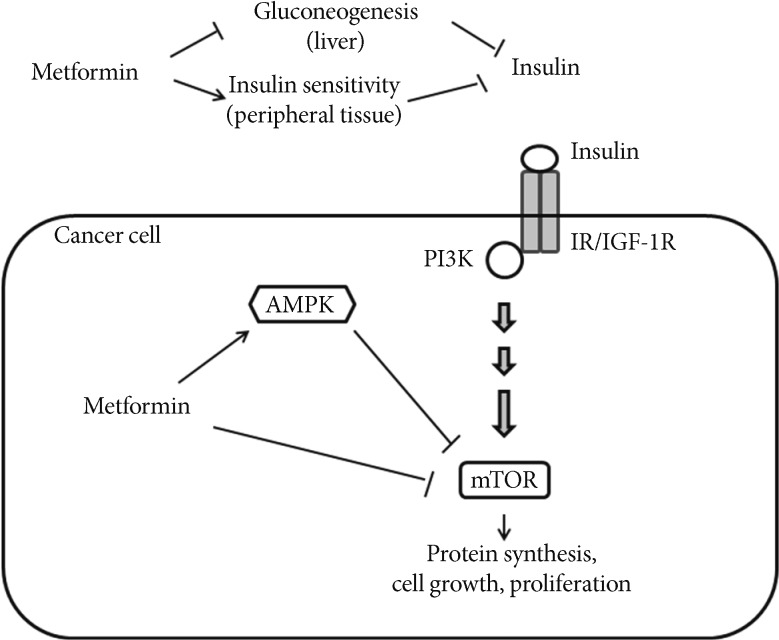
Effects of metformin on cancer. Metformin inhibits hepatic gluconeogenesis and stimulates glucose uptake in peripheral tissue, thereby lowering circulating insulin levels, and indirectly reducing phosphatidylinositol-3-kinase (PI3K)/mammalian target of rapamycin (mTOR) signaling. Metformin also activates AMP-activated protein kinase (AMPK) leading to inhibition of mTOR signaling and protein synthesis in cancer cells. Metformin may directly inhibit mTOR signaling [30-32]. IR/IGF-1R, insulin receptor/insulin-like growth factor-1 receptor.
In summary, epidemiologic evidence suggests that type 2 diabetes is associated with an increased risk of various cancers and mortality. Insulin resistance and resulting hyperinsulinemia may play a major role in cell proliferation and tumor growth. A number of studies have shown that metformin is associated with a reduced risk of developing cancer and also reduced cancer-related mortality. It should be of note that most of these studies are mainly based upon observational, not randomized controlled trials and, therefore, should be interpreted with caution. Nevertheless, they provide important information that deserves further research. Metformin has also been shown to have antiproliferative action in in vitro and in vivo animal models. Metformin may exert an antitumor action through several mechanisms. For example, metformin may decrease PI3K/mTOR signaling by alleviating hyperinsulinemia, and also, directly inhibit mTOR by activating AMPK. Ongoing prospective studies of metformin use in nondiabetic cancer patients will provide additional clues to help determine mechanisms and clinical efficacy of metformin in various cancers.
Notes
No potential conflict of interest relevant to this article was reported.