
- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Articles
- Page Path
- HOME > Diabetes Metab J > Volume 36(1); 2012 > Article
-
Original ArticleClinical Significance of the Presence of Autonomic and Vestibular Dysfunction in Diabetic Patients with Peripheral Neuropathy
- Soo Kyoung Kim1, Kyeong Ju Lee1, Jong Ryeal Hahm1,2, Sang Min Lee1, Tae Sik Jung1,2, Jung Hwa Jung1,2, Sungsu Kim1, Deok Ryong Kim2,3, Seong-Ki Ahn2,4, Won-Hee Choi5, Soon Il Chung1,2
-
Diabetes & Metabolism Journal 2012;36(1):64-69.
DOI: https://doi.org/10.4093/dmj.2012.36.1.64
Published online: February 17, 2012
- 3,811 Views
- 45 Download
- 18 Crossref
1Department of Internal Medicine, Gyeongsang National University School of Medicine, Jinju, Korea.
2Institue of Health Science, Gyeongsang National University School of Medicine, Jinju, Korea.
3Department of Biochemistry, Gyeongsang National University School of Medicine, Jinju, Korea.
4Department of Otolaryngology, Gyeongsang National University School of Medicine, Jinju, Korea.
5Department of Nursing, Koje College, Geoje, Korea.
- Corresponding author: Jong Ryeal Hahm. Department of Internal Medicine, Gyeongsang National University Hospital, Gyeongsang National University School of Medicine, 79 Gangnam-ro, Jinju 660-702, Korea. jrhahm@daum.net
Copyright © 2012 Korean Diabetes Association
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
-
Background
- We investigated the prevalence of diabetic autonomic neuropathy (DAN) and vestibular dysfunction (VD) in diabetic patients with peripheral neuropathy.
-
Methods
- Thirty-five diabetic patients with peripheral neuropathy were enrolled from August 2008 to July 2009. All subjects underwent autonomic function tests. Nineteen of the patients (54.3%) underwent videonystagmography.
-
Results
- Diabetic autonomic neuropathy was observed in 28 patients (80%). A mild degree of autonomic failure was observed in 18 patients (64.3%), and a moderate degree of autonomic failure was observed in ten patients (35.7%). Factors related to DAN included diabetic nephropathy (P=0.032), degree of chronic kidney disease (P=0.003), and duration of diabetes (P=0.044). Vestibular dysfunction was observed in 11 of 19 patients (57.9%). There was no significant association between DAN and VD.
-
Conclusion
- Diabetic autonomic neuropathy was observed in 28 diabetic patients (80%) with peripheral neuropathy. Vestibular dysfunction was observed in nearly 60% of diabetic patients with peripheral neuropathy who complained of dizziness but showed no significant association with DAN. Diabetic patients who complained of dizziness need to examine both autonomic function and vestibular function.
- Diabetic autonomic neuropathy (DAN) is a type of diabetic polyneuropathy, usually accompanied by diabetic peripheral neuropathy [1,2]. Diabetic autonomic neuropathy is associated with the duration of the disease as well as poor glycemic control and has a negative effect on quality of life and life expectancy in affected patients [3,4].
- Previous reports indicated that the vestibular system is involved in autonomic neural control for blood pressure and respiration according to positional change [5,6]. Vestibular dysfunction (VD) is a common comorbidity in patients with diabetes. Klagenberg et al. [7] reported a VD prevalence rate of 60% in a survey of 30 subjects with type I diabetes. Gawron et al. [8] reported that children and young adults with type I diabetes were vulnerable to VD, and VD prevalence was significantly higher in diabetic individuals than non-diabetic individuals [7-9]. Vestibular function plays an important role in postural stability with upright posture, along with the somatic and visual systems. Thus, VD increases the risk of falls due to postural instability when walking, especially when it is accompanied by diabetic peripheral neuropathy [10,11].
- It is likely that diabetic patients with peripheral neuropathy will experience worsened symptoms or signs of the disorder when accompanied by DAN or VD. We investigated the prevalence and clinical significance of concomitant DAN and VD in patients with diabetic peripheral neuropathy.
INTRODUCTION
- Subjects
- From October 2008 to July 2009, we retrospectively investigated 35 patients who, based on their medical records, had been diagnosed with diabetic peripheral neuropathy. All patients had received autonomic and vestibular function tests in Gyeongsang National University Hospital. We also investigated age, sex, height, weight, body mass index (BMI), cigarette smoking, alcohol consumption, diabetes type, duration of diabetes, presence of microvascular complications and hypertension in all subjects.
- We excluded patients with diseases that could possibly cause peripheral neuropathy as well as disorders influencing autonomic function such as: 1) severe neuropathy involved with paresis, muscle atrophy, or vibratory/heat sensory dysfunction on at least five areas including bilateral first toes, first metatarsal bones, or dorsa of the feet; 2) peripheral arterial obstructive disease; 3) alcoholic neuropathy or chronic alcohol abuse; 4) drug history potentially related to neuropathy (cisplatin, taxol) or exerting substantial influence on the result of the survey (antidepressants, anticonvulsants, opiates, neuroleptics); 5) neuropathy due to a malignant tumor, Parkinson's disease, epilepsy, multiple sclerosis, spinal stenosis, vitamin B12 deficiency, or leprosy; 6) panhypopituitarism or hypothyroidism; 7) pheochromocytoma; 8) congestive heart failure or amyloidosis; or 9) other severe diseases (cancer, liver cirrhosis, chronic hepatitis, GOT/GPT ≥80 IU/L, serum creatinine ≥3.0 mg/dL, hemodialysis).
- Diagnosis of diabetic peripheral neuropath
- We included patients with abnormal results on a total symptom score questionnaire and neurologic tests (128 Hz vibration sense test, pin prick test, 10 g monofilament test) [12]. The following were defined as abnormal:
-
≥2 points in total symptom score based on questions targeting pain, burning sensation, dysesthesia, and anesthesia.
≥10-second discrepancy between subject and tester in a 128-Hz vibration test
≤7 incidences of pain perception during a pin-prick test on the foot
≤7 correct responses during a 10 g monofilament test
- Diagnosis of diabetic autonomic neuropathy
- Diabetic autonomic neuropathy was evaluated using tilt and Valsalva tests as well as by monitoring heart rate response to deep breathing (HRDB) using a finometer model-1 (Finapres Medical Systems BV Co., Ltd., Amsterdam, The Netherlands). A quantitative sudomotor axon reflex test (QSART) was conducted using QSWEAT (WR Medical Electronics Co., Stillwater, OK, USA). The severity of autonomic neuropathy was assessed using a composite autonomic scoring scale (CASS) from 0 to 10, calculated as the cumulative score of sudomotor (0 to 3), cardiovagal (0 to 3) and adrenergic subscores (0 to 4). In determining the severity of autonomic neuropathy, scores of 0 to 3, 4 to 6, and 7 to 10 were defined to be mild, moderate and severe, respectively [13]. A CASS score ≥1 confirmed the presence of DAN.
- The quantitative sudomotor axon reflex test assesses only postganglionic sudomotor function. This is a more sensitive test than a sympathetic skin response (SSR), which is associated with polysynaptic reactions.
- Evaluation of vestibular function
- Patients were given a questionnaire which elicited vertigo symptoms in the outpatient otolaryngology department. Patients then underwent examinations including the spontaneous nystagmus test, gaze nystagmus test, positional nystagmus test, head shaking test, Dix-Hallpike test, and the Roll test. A videonystagmography with caloric test was performed to reflect lateral semicircular canal and superior vestibular nerve functions.
- Statistical analysis
- Data are presented as the mean±standard deviation (SD) or median (25th to 75th percentile). A chi-square with Fisher's exact test or Mann-Whitney U test was used to detect differences between the groups. Spearman's correlation analysis was used to assess correlations. Statistical analysis was performed using PASW version 18.0 software (SPSS Inc., Chicago, IL, USA). For all statistical analyses, a P value of less than 0.05 (two-sided) was considered statistically significant.
METHODS
- Clinical characteristics of the subjects
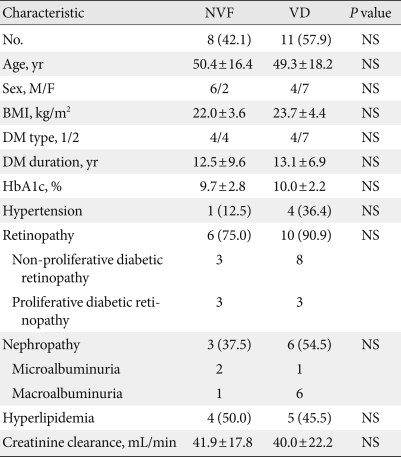
- The male to female ratio of the enrolled subjects was 18:17 with a mean age of 51.1±15.5 years. Twenty-five patients had type 2 diabetes, which was significantly higher than the number of patients with type 1 diabetes. The median duration of diabetes was 11 years (range, 1 to 28 years). The mean BMI was 23.5±4.2 kg/m2 and mean HbA1c was 9.8±2.9%. The numbers of the patients with dyslipidemia, diabetic retinopathy, and diabetic nephropathy were 18 (51.4%), 26 (74.3%), and 19 (54.3%), respectively (Table 1).
- Comparison of clinical characteristics based on the presence of diabetic autonomic neuropathy in patients with diabetic peripheral neuropathy
- Twenty-eight patients were diagnosed with diabetic autonomic neuropathy (80%). Of these, 18 exhibited mild severity and 10 exhibited moderate severity. The numbers of patients who obtained abnormal results for the tilt test, Valsalva test, heart rate response to deep breathing, and QSART were 20 (71.4%), 16 (57.1), 7 (25%), and 23 (82.1%), respectively. The prevalence of QSART was highest among these tests.
- The longer duration of diabetes and the presence of diabetic nephropathy were associated with a statistically significant increase in the prevalence of DAN (Table 2). However, diabetic retinopathy and the state of glucose control as determined by HbA1c were not statistically related to the presence of DAN (Table 2).
- The stage of chronic kidney disease and diabetic duration had a statistically significant relationship to the severity of DAN according to CASS (Table 3). Diabetes duration was significantly correlated with CASS (r=0.45, P<0.01). None of age, sex, height, or BMI were linked to DAN (data not shown).
- Relationship between vestibular function and autonomic neuropathy
- The vestibular function test was performed for patients with dizziness. Of 19 patients who underwent the vestibular function test, 11 were diagnosed with VD (57.9%). The symptoms of these patients included dizziness (79%), tinnitus (37%), headache and hearing problems (21%), ear fullness (11%) and vertigo (5%).
- No significant differences in age, sex, BMI, diabetic duration, state of glucose control, diabetic retinopathy, diabetic nephropathy, stage of chronic kidney disease, hypertension, and dyslipidemia were observed between the normal vestibular function group and the abnormal group (Table 4).
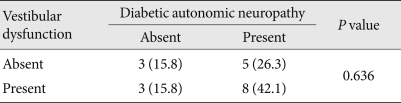
- Eight patients were identified as having both DAN and VD (42.1%). No statistical significance was observed between DAN and VD (Table 5).
RESULTS
- Diabetic peripheral neuropathy and autonomic neuropathy are common types of diabetic neuropathy. Diabetic peripheral neuropathy is frequently, although not always, accompanied by DAN [14].
- Diabetic peripheral neuropathy is associated with foot ulcers, postural instability while walking, and falls [15]. Similarly, DAN and VD are associated with postural instability while walking and abnormal distribution of foot pressure [11,16]. If DAN and VD coexist with diabetic peripheral neuropathy, the symptoms and signs may be aggravated.
- The present study demonstrated that 28 (80%) of 35 patients with diabetic peripheral neuropathy also had DAN. This high rate of occurrence can be explained by the fact that enrolled patients had already been diagnosed with diabetic peripheral neuropathy, and, unlike previous studies, our study protocol included cardiac autonomic neuropathy, sudomotor, and adrenergic dysfunction based on CASS. Twenty-three patients (82.1%) were abnormal according to the QSART, which was the test with highest frequency of abnormal tests in the current study. To our knowledge, QSART is more sensitive than SSR in the diagnosis of early diabetic neuropathy. A QSART conducted on a type 2 diabetic foot can predict postural hypotension, confirming its usefulness. Although the lack of equipment precludes broad utilization, QSART can be helpful in early diagnosis of DAN [17,18].
- The risk factors for DAN are old age, female sex, high BMI, height, type of diabetes, longer morbidity and poor glycemic control [9]. Age, sex, height, BMI, diabetes type and glucose control state were not significantly related to DAN (P>0.05). However, the duration of diabetic morbidity was significantly related to DAN (P=0.044). In this study, the longer duration of morbidity was the more severe DAN (P=0.009). The duration of diabetes was significantly correlated with CASS (r=0.45, P<0.01). Diabetic nephropathy (P=0.032) and stage of chronic kidney disease (P=0.003) were also significantly associated with DAN.
- Klagenberg et al. [7] reported that VD prevalence was 60% in a study of 30 patients with type 1 diabetes. Li et al. [9] reported that VD prevalence in 76 diabetic patients and 60 non-diabetic patients was 68.4% and 8.3%, respectively, demonstrating a significantly higher percentage in diabetics than in non-diabetics. Gawron et al. [8] reported that many children and young adults with type 1 diabetes had VD, which was linked to hypoglycemia frequency, duration of morbidity, and level of glucose control. Thus, we conducted the vestibular function test on 19 patients with otolaryngoloical problems of the 35 total patients who underwent the autonomic function test. Of the 19 patients, 11 (57.9%) had VD. This result was similar to previously described studies [7]. However, there is a limitation since the vestibular function test was conducted only on patients with dizziness. Vestibular dysfunction was not associated with age, sex, BMI, duration of diabetes, HbA1c, diabetic retinopathy, diabetic nephropathy, stage of chronic kidney disease, hypertension or hyperlipidemia. Furthermore, VD was not significantly associated with DAN (P=0.636).
- As previously described, patients with diabetic peripheral neuropathy have a high risk of postural instability and falls [15], which can be worsened by coexisting VD. However, VD can be ameliorated through vestibular rehabilitation treatment. Therefore, it is clinically important to detect VD in diabetic patients with peripheral neuropathy [19,20].
- The present study demonstrated that the most common symptom in the patients with VD was dizziness (79%), which can be caused by several conditions including cerebrovascular disease or adverse effects from drugs necessary for diabetic peripheral and autonomic neuropathy, hypoglycemia, or cardiac autonomic dysfunction. Thus, dizziness in diabetic patients can be caused by not only diabetic autonomic neuropathy like orthostatic hypotension, but also VD.
- Limitation in this study was the small sample size of patients with diabetic peripheral neuropathy. However, this study revealed that patients with diabetic peripheral neuropathy had a high prevalence of DAN, and both diabetic nephropathy and the duration of diabetic morbidity were associated with DAN. Furthermore many of the diabetic patients with dizziness also had VD. Thus, if a diabetic patient of long morbidity develops dizziness, it is so helpful in making a differential diagnosis, treating, and educating patients that we should evaluate DAN and VD via autonomic function test and vestibular function test, respectively.
DISCUSSION
-
Acknowledgements
- This survey was conducted with financial support from the Korean Institute of Medicine.
ACKNOWLEDGMENTS
- 1. Ewing DJ, Burt AA, Williams IR, Campbell IW, Clarke BF. Peripheral motor nerve function in diabetic autonomic neuropathy. J Neurol Neurosurg Psychiatry 1976;39:453-460. ArticlePubMedPMC
- 2. Valensi P, Huard JP, Giroux C, Attali JR. Factors involved in cardiac autonomic neuropathy in diabetic patients. J Diabetes Complications 1997;11:180-187. ArticlePubMed
- 3. The Diabetes Control and Complications Trial Research Group. The effect of intensive diabetes therapy on measures of autonomic nervous system function in the Diabetes Control and Complications Trial (DCCT). Diabetologia 1998;41:416-423. ArticlePubMedPMCPDF
- 4. Vinik AI, Erbas T. Recognizing and treating diabetic autonomic neuropathy. Cleve Clin J Med 2001;68:928-930. 932934-944. ArticlePubMed
- 5. Furman JM, Jacob RG, Redfern MS. Clinical evidence that the vestibular system participates in autonomic control. J Vestib Res 1998;8:27-34. ArticlePubMed
- 6. Yates BJ, Bronstein AM. The effects of vestibular system lesions on autonomic regulation: observations, mechanisms, and clinical implications. J Vestib Res 2005;15:119-129. ArticlePubMed
- 7. Klagenberg KF, Zeigelboim BS, Jurkiewicz AL, Martins-Bassetto J. Vestibulocochlear manifestations in patients with type I diabetes mellitus. Braz J Otorhinolaryngol 2007;73:353-358. ArticlePubMed
- 8. Gawron W, Pospiech L, Orendorz-Fraczkowska K, Noczynska A. Are there any disturbances in vestibular organ of children and young adults with type I diabetes? Diabetologia 2002;45:728-734. ArticlePubMedPDF
- 9. Li J, Zhang T, Shen J, Gong J, Wang H, Zhang J, Pang Y. The changes in vestibular function in patients with diabetes mellitus and its clinical significance. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 2008;22:10-13.
- 10. Cavanagh PR, Derr JA, Ulbrecht JS, Maser RE, Orchard TJ. Problems with gait and posture in neuropathic patients with insulin-dependent diabetes mellitus. Diabet Med 1992;9:469-474. ArticlePubMed
- 11. Agrawal Y, Carey JP, Della Santina CC, Schubert MC, Minor LB. Diabetes, vestibular dysfunction, and falls: analyses from the National Health and Nutrition Examination Survey. Otol Neurotol 2010;31:1445-1450. PubMed
- 12. Tesfaye S, Boulton AJ, Dyck PJ, Freeman R, Horowitz M, Kempler P, Lauria G, Malik RA, Spallone V, Vinik A, Bernardi L, Valensi P. Toronto Diabetic Neuropathy Expert Group. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care 2010;33:2285-2293. ArticlePubMedPMCPDF
- 13. Low PA. Composite autonomic scoring scale for laboratory quantification of generalized autonomic failure. Mayo Clin Proc 1993;68:748-752. ArticlePubMed
- 14. Toyry JP, Partanen JV, Niskanen LK, Lansimies EA, Uusitupa MI. Divergent development of autonomic and peripheral somatic neuropathies in NIDDM. Diabetologia 1997;40:953-958. ArticlePubMedPDF
- 15. Simoneau GG, Ulbrecht JS, Derr JA, Becker MB, Cavanagh PR. Postural instability in patients with diabetic sensory neuropathy. Diabetes Care 1994;17:1411-1421. ArticlePubMedPDF
- 16. Petrofsky J, Lee S, Macnider M, Navarro E. Autonomic, endothelial function and the analysis of gait in patients with type 1 and type 2 diabetes. Acta Diabetol 2005;42:7-15. ArticlePubMedPDF
- 17. Shimada H, Kihara M, Kosaka S, Ikeda H, Kawabata K, Tsutada T, Miki T. Comparison of SSR and QSART in early diabetic neuropathy: the value of length-dependent pattern in QSART. Auton Neurosci 2001;92:72-75. ArticlePubMed
- 18. Itoh H, Uebori S, Asai M, Kashiwaya T, Atoh K, Makino I. Early detection of orthostatic hypotension by quantitative sudomotor axon reflex test (QSART) in type 2 diabetic patients. Intern Med 2003;42:560-564. ArticlePubMed
- 19. Nashner LM, Shupert CL, Horak FB, Black FO. Organization of posture controls: an analysis of sensory and mechanical constraints. Prog Brain Res 1989;80:411-418. PubMed
- 20. Aranda C, Meza A, Rodriguez R, Mantilla MT, Jauregui-Renaud K. Diabetic polyneuropathy may increase the handicap related to vestibular disease. Arch Med Res 2009;40:180-185. ArticlePubMed
REFERENCES

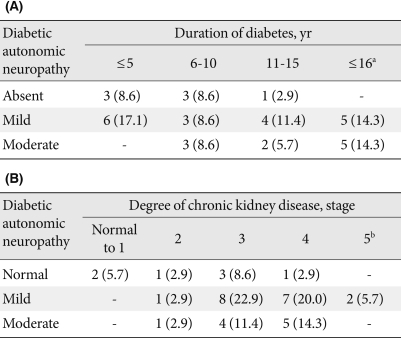
Figure & Data
References
Citations

- β-Glucans obtained from fungus for wound healing: A review
Chunhua Xu, Fengxia Wang, Shibing Guan, Lizhen Wang
Carbohydrate Polymers.2024; 327: 121662. CrossRef - Dynamic and static balance functions in hemodialysis patients and non‐dialysis dependent CKD patients
Nobuyuki Shirai, Suguru Yamamoto, Yutaka Osawa, Atsuhiro Tsubaki, Shinichiro Morishita, Ichiei Narita
Therapeutic Apheresis and Dialysis.2023; 27(3): 412. CrossRef - Micro (mi) RNA and Diabetic Retinopathy
Sadashiv, Praveen Sharma, Shailendra Dwivedi, Sunita Tiwari, Pankaj Kumar Singh, Amit Pal, Sandeep Kumar
Indian Journal of Clinical Biochemistry.2022; 37(3): 267. CrossRef - Physiotherapists’ Perspectives on Type 2 Diabetes Management and as a Primary Condition for Referral to Physiotherapy Services: A Qualitative Descriptive Study
Sarah M. Janssen, Denise M. Connelly, Heather Gillis
Physiotherapy Canada.2022;[Epub] CrossRef - Subclinical vestibular dysfunction in type 1 diabetes mellitus
Abdollah Moossavi, Moslem Shaabani, Ensieh Nasli Esfahani, Mohsen Vahedi, Zakaria Enayati
Hearing, Balance and Communication.2021; 19(2): 86. CrossRef Potential Applications of Nanomaterials and Technology for Diabetic Wound Healing
Que Bai, Kai Han, Kai Dong, Caiyun Zheng, Yanni Zhang, Qianfa Long, Tingli Lu
International Journal of Nanomedicine.2020; Volume 15: 9717. CrossRef- Recent advancements in biopolymer and metal nanoparticle-based materials in diabetic wound healing management
Veena Vijayakumar, Sushanta K. Samal, Smita Mohanty, Sanjay K. Nayak
International Journal of Biological Macromolecules.2019; 122: 137. CrossRef - Auditory function and motor proficiency in type 1 diabetic children: A case-control study
Jalali Mir Mohammad, Soleimani Robabeh, Koohmanai Shahin, Tizno Saeed, Akbari Maryam
International Journal of Pediatric Otorhinolaryngology.2018; 109: 7. CrossRef - Vestibular profile of type 1 versus type 2 chronic diabetes mellitus
Ola Abdallah Ibraheem, Mohammad Ramadan Hassaan, Mayada Mohamed Mousa
Hearing, Balance and Communication.2017; 15(3): 133. CrossRef - Glycemic variability is an important risk factor for cardiovascular autonomic neuropathy in newly diagnosed type 2 diabetic patients
Wen Xu, Yanhua Zhu, Xubin Yang, Hongrong Deng, Jinhua Yan, Shaoda Lin, Huazhang Yang, Hong Chen, Jianping Weng
International Journal of Cardiology.2016; 215: 263. CrossRef - Impact of Diabetic Complications on Balance and Falls: Contribution of the Vestibular System
Linda J. D'Silva, James Lin, Hinrich Staecker, Susan L. Whitney, Patricia M. Kluding
Physical Therapy.2016; 96(3): 400. CrossRef - Shedding light on miR-26a: Another key regulator of angiogenesis in diabetic wound healing
Carlos Zgheib, Kenneth W. Liechty
Journal of Molecular and Cellular Cardiology.2016; 92: 203. CrossRef - Augmented asymmetrical visual field dependence in asymptomatic diabetics: Evidence of subclinical asymmetrical bilateral vestibular dysfunction
Rima Abdul Razzak, Jeffery Bagust, Sharon Docherty, Wiam Hussein, Abdullah Al-Otaibi
Journal of Diabetes and its Complications.2015; 29(1): 68. CrossRef - Associations between autonomic dysfunction and pain in chemotherapy‐induced polyneuropathy
H. Nahman‐Averbuch, Y. Granovsky, E. Sprecher, M. Steiner, T. Tzuk‐Shina, D. Pud, D. Yarnitsky
European Journal of Pain.2014; 18(1): 47. CrossRef - Balance training in the intervention of fall risk in elderly with diabetic peripheral neuropathy: A review
Xi Pan, Jiao-jiao Bai
International Journal of Nursing Sciences.2014; 1(4): 441. CrossRef - Synkope aus der Sicht des Neurologen
A. Bickel, J. Röther
Herz.2014; 39(4): 443. CrossRef - The Role of MicroRNAs in Diabetic Complications—Special Emphasis on Wound Healing
João Moura, Elisabet Børsheim, Eugenia Carvalho
Genes.2014; 5(4): 926. CrossRef - Recent advances on the development of wound dressings for diabetic foot ulcer treatment—A review
Liane I.F. Moura, Ana M.A. Dias, Eugénia Carvalho, Hermínio C. de Sousa
Acta Biomaterialia.2013; 9(7): 7093. CrossRef

 KDA
KDA
 PubReader
PubReader Cite
Cite



