Relationship between Opium Abuse and Severity of Depression in Type 2 Diabetic Patients
Article information
Abstract
Background
Opium use in diabetic populations is associated with major depressive disorder (MDD). This study was designed to investigate the relationship between opium use and severity of depression in Iranian diabetic patients.
Methods
In this case-control study, 642 type 2 diabetic patients were recruited from those presenting at two outpatient clinics at the Akhavan Hospital in Kashan, Iran; of them, 600 diabetic patients were included in the study and divided into two groups: opium-abusers (150 patients) and non-opium-abusers (450 patients). Clinical and demographic information was obtained through a detailed questionnaire. Depression symptomalogy and severity were assessed with the Beck Depression Inventory (BDI), and a corresponding diagnosis was made based on the Diagnostic and Statistical Manual of Mental Disorders-IV, Text Revision, 2000 (DSM-IV TR) criteria.
Results
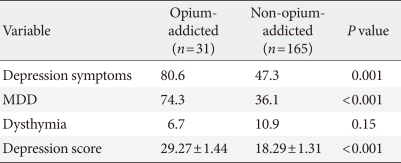
The mean depression score was higher in the opium abuse group than in the non-abuser group (29.27±1.44 vs. 18.29±1.31, P<0.001). In general, a significant association was found between opium abuse and depression among patients (odds ratio [OR], 4.54; 95% confidence interval [CI], 2.87 to 7.44; P=0.001). No significant relationship was found between dysthymia and opium abuse (OR, 0.68; 95% CI, 0.18 to 1.192; P=0.155), while MDD was significantly higher in the opium abuser group (OR, 7.32; 95% CI, 5.20 to 12.01; P<0.001).
Conclusion
Depression is more frequent in opium-dependent diabetic patients, and its severity is also greater. Given these findings, opium-dependent diabetic patients should be advised about the increased risks of depression and related comorbidities.
INTRODUCTION
Diabetes mellitus is a chronic disease that can have many complications, such as retinopathy, neuropathy, and nephropathy; it affects patients' quality of life, and it is often associated with depression [1-3]. Dietary restrictions, constant dependence on medicine, short- and long-term side effects, and the cost-burden of the disease may help to explain the high prevalence of depression among these patients [4].
Opium has traditionally been consumed in Iran as a treatment for pain, diarrhea, insomnia, and premature ejaculation. It is also used for enjoyment and elimination of tension [5,6]. Some Iranian patients consume opium for their diabetes, although the reasons and prevalence are not clear. The pain reduction effects of opium and a lack of adequate knowledge may explain their opium usage to some extent, but research is needed to gain a better understanding. However, it is known that opium abuse among diabetics is associated with an increased risk of severe depression [7]. Overall, about 90% of opioid-dependent patients are recognized to have a psychiatric disorder, which most typically include depression [8], antisocial character, anxiety [9], and memory and psychosomatic disorders [10,11].
Depression is one of the most common psychiatric disorders [12]. Initial presenting symptoms tend to include an increasingly reduced sense of self-efficacy, reduced self-care behaviors, mood swings, and in some cases increased suicidal ideation [9,13]. These symptoms, although significant in and of themselves, can also exacerbate problems for patients with diabetes [3], such as poor medical adherence, functional impairment, and high health care costs (up to 86% in primary care) [14].
Although opium abuse is known to have negative effects on diabetic patients [7], few studies have specifically assessed the impact of opium abuse on depression in this population. Therefore, the current study is designed to investigate the association between opium abuse and severity of depression in Iranian diabetic patients.
METHODS
In this prospective case-control study, we recruited 642 type 2 diabetic patients presenting at two outpatient clinics of the Akhavan Hospital in Kashan, Iran: 173 with a diagnosis of opium abuse (cases) and 469 without this diagnosis (controls). We obtained clinical and demographic information about all participants through a detailed questionnaire. Opium use status was ascertained by self report; however, because of variation in opium quality and difficulty in uniform measurement across all study patients, the quantity of opium intake was not estimated. However, all opium abusers reported having regularly used opium for at least 12 months prior to admission (as indicated by the question, "Has the client continued to use opium despite recurrent problems aggravated by the substance use within the last 12-month period?") [15]. Data on the frequency and duration of opium intake was missing or incomplete in some cases.
Depressive symptomalogy and severity were assessed through administration of the Beck Depression Inventory (BDI) [16], and patients scoring over 16 were considered to be depressed. A corresponding diagnosis was made based on the Diagnostic and Statistical Manual of Mental Disorders-IV, Text Revision, 2000 (DSM-IV TR) criteria [17]. To distinguish opium-induced major depressive disorder (MDD) from independent MDD, patients with a history of depression before beginning opium use, such as those who had used antidepressants or been hospitalized due to depression, were excluded. Patients were considered to have hypertension if they were currently using antihypertensive medications or if they had blood pressure readings above 140/90 mm Hg on two different occasions. Hypercholesterolemia was defined as having one or more serum cholesterol level readings above 6.2 mmol/L (240 mg/dL) [18]. Blood glucose control was assessed by measuring HbA1c levels, and values above 7% were considered uncontrolled [19]. The study protocol was approved by the ethics committee of Kashan University of Medical Sciences (Kashan, Iran). Informed consent was obtained from the patients before enrollment.
Statistical analysis
In a case-control study, cases and controls ideally match on all characteristics except the variable of interest. In order to consider the confounding effects of unmatched characteristics, we treated them as covariates in a multiple logistic regression analysis. Variables with P values less than 0.2 for differences between the two groups were considered to be covariates: these included age, marital status, literacy, hypertension, and hyperlipidemia. Results were reported as mean±standard deviation for quantitative variables and percentages for categorical variables. The groups were compared using the Student's t-test for continuous variables and the chi-square test (or Fisher's exact test as appropriate) for categorical variables. Odds ratios (OR) and 95% confidence intervals (CI) for OR were calculated. To decrease the effects of probable confounders, adjusted ORs were estimated when appropriate. Results for which P values were 0.05 or less were considered statistically significant. All the statistical analyses were performed using SPSS version 13.0 for Windows (SPSS Inc., Chicago, IL, USA).
RESULTS
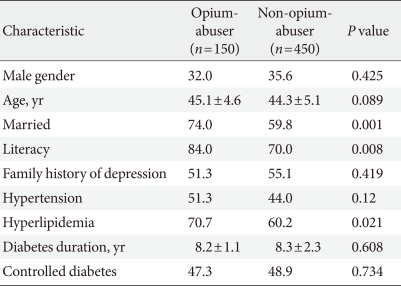
Of the 642 Iranian subjects with type 2 diabetes who satisfied the inclusion criteria, 600 (150 opium-abusers and 450 nonopium-abusers) patients were selected for analysis in this study. Their demographic and clinical characteristics are summarized in Table 1.
Depression and MDD were found to be more prevalent among opium-abusing diabetic patients than in non-abusers, and the mean depression score was higher in the opium abuse group. In contrast, dysthymia was diagnosed in 6.7% of abusers and 10.9% of non-abusers. Table 2 shows the prevalence of the different types of depression detected in the two groups.
In general, depression was found to be over four times more likely among opium abusers than non-abusers (OR, 4.54; 95% CI, 2.87 to 7.44; P=0.001). No significant relationship was found between dysthymia and opium abuse (OR, 0.68; 95% CI, 0.18 to 1.192; P=0.155). However, MDD was over seven times as likely in the opium abuse group (OR, 7.32; 95% CI, 5.20 to 12.01; P<0.001).
DISCUSSION
Depression is frequently comorbid in type 2 diabetic patients, with a prevalence of unipolar depression of about 10.9% and that of significant depressive symptoms of about 32.9% [20]. However, depression is under-recognized, and it is estimated to be untreated in approximately two-thirds of depressed diabetic patients. This is unfortunate, since the course of depression tends to be chronic and severe in patients with diabetes [21].
The association between diabetes and depression seems to be interactional, with some studies indicating that diabetes increases the incidence of depression [1-3], and others reporting that depressed adults have an increased risk of developing type 2 diabetes [22-24]. The latter might be explained by an increased likelihood for depressed adults to have a sedentary lifestyle, obesity, and smoking [21]. Conversely, Golden et al. [25] described a reduced risk of depression in patients with impaired fasting glucose levels and untreated type 2 diabetes. In addition, depression has been seen as a predictive factor for the severity and number of diabetic complications in some studies [26,27], while others have not found any such associations [28].
A surprising report states that up to 17.9% of Iranians have used opium at least once, and 8.8% are regular users [29]. Opium contains as much as 12% morphine and is the crudest and least potent form of opiate. Opium abuse has propagated among youth and in various nations for reasons such as ample accessibility and affordability of the drug, unemployment, and mental health problems.
From a physiopathological point of view, opiates act at the basomedial hypothalamus to inhibit the release of gonadotropin releasing factor and thus decrease the circulating concentrations of luteizing hormone and follicular-stimulating hormone. As a result of these decreased pituitary hormone concentrations, the testosterone plasma level declines [30]. McIntyre et al. [31] found that depressed men had lower mean bioavailable and total testosterone levels; likewise, several lines of evidence suggest that testosterone might be effective for the treatment of depression [32,33].
In several studies, it was established that opiate addicts were significantly more depressed than normal controls [8] and even slightly more depressed than psychiatric patients [34]. The reason why opium abuse appears to worsen depression severity in diabetic patients has not been clarified. Although opium use was once recommended as a treatment for diabetes mellitus [35], studies have demonstrated that opium addiction adversely affects some serum factors in diabetic patients by increasing serum glucose, decreasing high density lipoprotein cholesterol, and thus adding to metabolic derangements [36]. Further, serum glucose elevation in diabetics appears to be correlated with MDD [3].
Association between opium and depression or diabetes and depression has been discussed in several studies, and recent studies have found a significant association between opium abuse and depression in diabetic patients [7]. The current study also found this association to be significant, with 74.3% of opium abusing diabetic patients diagnosed with MDD, compared to 36.1% of non-abusing diabetic patients. In contrast, Shiri et al. [7] detected MDD in only 22.8% of diabetic men which was 1.7 times more than non-abuser diabetics. The reason for this considerable difference in results is not clear, but study design differences and confounders are likely contributors. In another study, Khamseh et al. [1] reported a 71.8% prevalence of MDD in all diabetic patients (abuser or non-abuser), just slightly less than the 74.3% in our study's abuser group. Their study included both type 1 and type 2 diabetic patients, while only type 2 diabetics were enrolled in our study. This appears to highlight the direct impact of opium consumption on diabetics' depressive symptoms compared to the effects of diabetes alone.
There is a fallacy among Iranian people that opium has favorable effects on diabetes mellitus. A previous study that addressed this possibility found that opium temporarily decreased blood glucose, but it had no distinct, long-lasting effects on blood glucose, as determined by HbA1c measurements [37]. Cerebral vascular involvement [38], vascular impairment following retinopathy, multiple hospital admissions, and sexual dysfunction seem to be worsened with comorbid depression among diabetic patients [39]. This may be related to the negative effects of psychiatric disorders on blood glucose control, which implies the importance of treatment for comorbid depression [40].
In the present study, opium use was ascertained by self report, and the quality of the opium intake was not quantified. It is possible that varying quality and frequency of opium use among abusing patients affected study outcomes. Additionally, while one of the exclusion criteria was a documented history of depression before beginning opium use, it is difficult to distinguish opium-induced major MDD from independent MDD, and there may have been pre-existing MDD that was not diagnosed. Group differences were adjusted for unmatched variables, but there are many possible confounders which were not measured or presented in this study. These include other aspects of physical health, mental health, and social support which might have modified the observed association between opium addiction and depression. Similarly, other possible confounders, such as thyroid function and folate levels, which may impact depression, were not matched between the groups. Findings could also have been enhanced by adding another control group consisting of non-diabetic opium abusers to assess the direct effects of opium abuse on depression.
We found that depression is both more frequent and more severe in diabetic patients who regularly use opium than in those who do not. Thus, our results suggest that opium-dependent diabetic patients should be advised about the associated increased risks of depression and related comorbidities. Further research is indicated to assess whether consultation about opium abuse in diabetic patients with depression can reduce the severity of depressive symptoms.
ACKNOWLEDGMENTS
The authors would like to thank Dr. Siamak Mehdizadeh Seraj for language revision of the manuscript.
Notes
No potential conflict of interest relevant to this article was reported.