
- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Articles
- Page Path
- HOME > Diabetes Metab J > Volume 36(2); 2012 > Article
-
Original ArticleRelationship between Opium Abuse and Severity of Depression in Type 2 Diabetic Patients
- Sepehrmanesh Zahra1, Sarmast Hossein2, Kord Valeshabad Ali3,4
-
Diabetes & Metabolism Journal 2012;36(2):157-162.
DOI: https://doi.org/10.4093/dmj.2012.36.2.157
Published online: April 17, 2012
1Department of Psychiatry, Kashan University of Medical Sciences, Kashan, Iran.
2Emergency Center, Kashan University of Medical Sciences, Kashan, Iran.
3Students' Scientific Research Center, Tehran University of Medical Sciences, Tehran, Iran.
4National Elites Foundation, Tehran, Iran.
- Corresponding author: Sarmast Hossein. Emergency Center, Kashan University of Medical Sciences, Kashan, Iran. beheena2002@yahoo.com
• Received: January 8, 2011 • Accepted: September 8, 2011
Copyright © 2012 Korean Diabetes Association
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
-
Background
- Opium use in diabetic populations is associated with major depressive disorder (MDD). This study was designed to investigate the relationship between opium use and severity of depression in Iranian diabetic patients.
-
Methods
- In this case-control study, 642 type 2 diabetic patients were recruited from those presenting at two outpatient clinics at the Akhavan Hospital in Kashan, Iran; of them, 600 diabetic patients were included in the study and divided into two groups: opium-abusers (150 patients) and non-opium-abusers (450 patients). Clinical and demographic information was obtained through a detailed questionnaire. Depression symptomalogy and severity were assessed with the Beck Depression Inventory (BDI), and a corresponding diagnosis was made based on the Diagnostic and Statistical Manual of Mental Disorders-IV, Text Revision, 2000 (DSM-IV TR) criteria.
-
Results
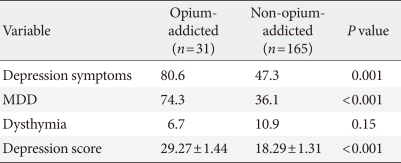
- The mean depression score was higher in the opium abuse group than in the non-abuser group (29.27±1.44 vs. 18.29±1.31, P<0.001). In general, a significant association was found between opium abuse and depression among patients (odds ratio [OR], 4.54; 95% confidence interval [CI], 2.87 to 7.44; P=0.001). No significant relationship was found between dysthymia and opium abuse (OR, 0.68; 95% CI, 0.18 to 1.192; P=0.155), while MDD was significantly higher in the opium abuser group (OR, 7.32; 95% CI, 5.20 to 12.01; P<0.001).
-
Conclusion
- Depression is more frequent in opium-dependent diabetic patients, and its severity is also greater. Given these findings, opium-dependent diabetic patients should be advised about the increased risks of depression and related comorbidities.
- Diabetes mellitus is a chronic disease that can have many complications, such as retinopathy, neuropathy, and nephropathy; it affects patients' quality of life, and it is often associated with depression [1-3]. Dietary restrictions, constant dependence on medicine, short- and long-term side effects, and the cost-burden of the disease may help to explain the high prevalence of depression among these patients [4].
- Opium has traditionally been consumed in Iran as a treatment for pain, diarrhea, insomnia, and premature ejaculation. It is also used for enjoyment and elimination of tension [5,6]. Some Iranian patients consume opium for their diabetes, although the reasons and prevalence are not clear. The pain reduction effects of opium and a lack of adequate knowledge may explain their opium usage to some extent, but research is needed to gain a better understanding. However, it is known that opium abuse among diabetics is associated with an increased risk of severe depression [7]. Overall, about 90% of opioid-dependent patients are recognized to have a psychiatric disorder, which most typically include depression [8], antisocial character, anxiety [9], and memory and psychosomatic disorders [10,11].
- Depression is one of the most common psychiatric disorders [12]. Initial presenting symptoms tend to include an increasingly reduced sense of self-efficacy, reduced self-care behaviors, mood swings, and in some cases increased suicidal ideation [9,13]. These symptoms, although significant in and of themselves, can also exacerbate problems for patients with diabetes [3], such as poor medical adherence, functional impairment, and high health care costs (up to 86% in primary care) [14].
- Although opium abuse is known to have negative effects on diabetic patients [7], few studies have specifically assessed the impact of opium abuse on depression in this population. Therefore, the current study is designed to investigate the association between opium abuse and severity of depression in Iranian diabetic patients.
INTRODUCTION
- In this prospective case-control study, we recruited 642 type 2 diabetic patients presenting at two outpatient clinics of the Akhavan Hospital in Kashan, Iran: 173 with a diagnosis of opium abuse (cases) and 469 without this diagnosis (controls). We obtained clinical and demographic information about all participants through a detailed questionnaire. Opium use status was ascertained by self report; however, because of variation in opium quality and difficulty in uniform measurement across all study patients, the quantity of opium intake was not estimated. However, all opium abusers reported having regularly used opium for at least 12 months prior to admission (as indicated by the question, "Has the client continued to use opium despite recurrent problems aggravated by the substance use within the last 12-month period?") [15]. Data on the frequency and duration of opium intake was missing or incomplete in some cases.
- Depressive symptomalogy and severity were assessed through administration of the Beck Depression Inventory (BDI) [16], and patients scoring over 16 were considered to be depressed. A corresponding diagnosis was made based on the Diagnostic and Statistical Manual of Mental Disorders-IV, Text Revision, 2000 (DSM-IV TR) criteria [17]. To distinguish opium-induced major depressive disorder (MDD) from independent MDD, patients with a history of depression before beginning opium use, such as those who had used antidepressants or been hospitalized due to depression, were excluded. Patients were considered to have hypertension if they were currently using antihypertensive medications or if they had blood pressure readings above 140/90 mm Hg on two different occasions. Hypercholesterolemia was defined as having one or more serum cholesterol level readings above 6.2 mmol/L (240 mg/dL) [18]. Blood glucose control was assessed by measuring HbA1c levels, and values above 7% were considered uncontrolled [19]. The study protocol was approved by the ethics committee of Kashan University of Medical Sciences (Kashan, Iran). Informed consent was obtained from the patients before enrollment.
- Statistical analysis
- In a case-control study, cases and controls ideally match on all characteristics except the variable of interest. In order to consider the confounding effects of unmatched characteristics, we treated them as covariates in a multiple logistic regression analysis. Variables with P values less than 0.2 for differences between the two groups were considered to be covariates: these included age, marital status, literacy, hypertension, and hyperlipidemia. Results were reported as mean±standard deviation for quantitative variables and percentages for categorical variables. The groups were compared using the Student's t-test for continuous variables and the chi-square test (or Fisher's exact test as appropriate) for categorical variables. Odds ratios (OR) and 95% confidence intervals (CI) for OR were calculated. To decrease the effects of probable confounders, adjusted ORs were estimated when appropriate. Results for which P values were 0.05 or less were considered statistically significant. All the statistical analyses were performed using SPSS version 13.0 for Windows (SPSS Inc., Chicago, IL, USA).
METHODS
- Of the 642 Iranian subjects with type 2 diabetes who satisfied the inclusion criteria, 600 (150 opium-abusers and 450 nonopium-abusers) patients were selected for analysis in this study. Their demographic and clinical characteristics are summarized in Table 1.
- Depression and MDD were found to be more prevalent among opium-abusing diabetic patients than in non-abusers, and the mean depression score was higher in the opium abuse group. In contrast, dysthymia was diagnosed in 6.7% of abusers and 10.9% of non-abusers. Table 2 shows the prevalence of the different types of depression detected in the two groups.
- In general, depression was found to be over four times more likely among opium abusers than non-abusers (OR, 4.54; 95% CI, 2.87 to 7.44; P=0.001). No significant relationship was found between dysthymia and opium abuse (OR, 0.68; 95% CI, 0.18 to 1.192; P=0.155). However, MDD was over seven times as likely in the opium abuse group (OR, 7.32; 95% CI, 5.20 to 12.01; P<0.001).
RESULTS
- Depression is frequently comorbid in type 2 diabetic patients, with a prevalence of unipolar depression of about 10.9% and that of significant depressive symptoms of about 32.9% [20]. However, depression is under-recognized, and it is estimated to be untreated in approximately two-thirds of depressed diabetic patients. This is unfortunate, since the course of depression tends to be chronic and severe in patients with diabetes [21].
- The association between diabetes and depression seems to be interactional, with some studies indicating that diabetes increases the incidence of depression [1-3], and others reporting that depressed adults have an increased risk of developing type 2 diabetes [22-24]. The latter might be explained by an increased likelihood for depressed adults to have a sedentary lifestyle, obesity, and smoking [21]. Conversely, Golden et al. [25] described a reduced risk of depression in patients with impaired fasting glucose levels and untreated type 2 diabetes. In addition, depression has been seen as a predictive factor for the severity and number of diabetic complications in some studies [26,27], while others have not found any such associations [28].
- A surprising report states that up to 17.9% of Iranians have used opium at least once, and 8.8% are regular users [29]. Opium contains as much as 12% morphine and is the crudest and least potent form of opiate. Opium abuse has propagated among youth and in various nations for reasons such as ample accessibility and affordability of the drug, unemployment, and mental health problems.
- From a physiopathological point of view, opiates act at the basomedial hypothalamus to inhibit the release of gonadotropin releasing factor and thus decrease the circulating concentrations of luteizing hormone and follicular-stimulating hormone. As a result of these decreased pituitary hormone concentrations, the testosterone plasma level declines [30]. McIntyre et al. [31] found that depressed men had lower mean bioavailable and total testosterone levels; likewise, several lines of evidence suggest that testosterone might be effective for the treatment of depression [32,33].
- In several studies, it was established that opiate addicts were significantly more depressed than normal controls [8] and even slightly more depressed than psychiatric patients [34]. The reason why opium abuse appears to worsen depression severity in diabetic patients has not been clarified. Although opium use was once recommended as a treatment for diabetes mellitus [35], studies have demonstrated that opium addiction adversely affects some serum factors in diabetic patients by increasing serum glucose, decreasing high density lipoprotein cholesterol, and thus adding to metabolic derangements [36]. Further, serum glucose elevation in diabetics appears to be correlated with MDD [3].
- Association between opium and depression or diabetes and depression has been discussed in several studies, and recent studies have found a significant association between opium abuse and depression in diabetic patients [7]. The current study also found this association to be significant, with 74.3% of opium abusing diabetic patients diagnosed with MDD, compared to 36.1% of non-abusing diabetic patients. In contrast, Shiri et al. [7] detected MDD in only 22.8% of diabetic men which was 1.7 times more than non-abuser diabetics. The reason for this considerable difference in results is not clear, but study design differences and confounders are likely contributors. In another study, Khamseh et al. [1] reported a 71.8% prevalence of MDD in all diabetic patients (abuser or non-abuser), just slightly less than the 74.3% in our study's abuser group. Their study included both type 1 and type 2 diabetic patients, while only type 2 diabetics were enrolled in our study. This appears to highlight the direct impact of opium consumption on diabetics' depressive symptoms compared to the effects of diabetes alone.
- There is a fallacy among Iranian people that opium has favorable effects on diabetes mellitus. A previous study that addressed this possibility found that opium temporarily decreased blood glucose, but it had no distinct, long-lasting effects on blood glucose, as determined by HbA1c measurements [37]. Cerebral vascular involvement [38], vascular impairment following retinopathy, multiple hospital admissions, and sexual dysfunction seem to be worsened with comorbid depression among diabetic patients [39]. This may be related to the negative effects of psychiatric disorders on blood glucose control, which implies the importance of treatment for comorbid depression [40].
- In the present study, opium use was ascertained by self report, and the quality of the opium intake was not quantified. It is possible that varying quality and frequency of opium use among abusing patients affected study outcomes. Additionally, while one of the exclusion criteria was a documented history of depression before beginning opium use, it is difficult to distinguish opium-induced major MDD from independent MDD, and there may have been pre-existing MDD that was not diagnosed. Group differences were adjusted for unmatched variables, but there are many possible confounders which were not measured or presented in this study. These include other aspects of physical health, mental health, and social support which might have modified the observed association between opium addiction and depression. Similarly, other possible confounders, such as thyroid function and folate levels, which may impact depression, were not matched between the groups. Findings could also have been enhanced by adding another control group consisting of non-diabetic opium abusers to assess the direct effects of opium abuse on depression.
- We found that depression is both more frequent and more severe in diabetic patients who regularly use opium than in those who do not. Thus, our results suggest that opium-dependent diabetic patients should be advised about the associated increased risks of depression and related comorbidities. Further research is indicated to assess whether consultation about opium abuse in diabetic patients with depression can reduce the severity of depressive symptoms.
DISCUSSION
-
Acknowledgements
- The authors would like to thank Dr. Siamak Mehdizadeh Seraj for language revision of the manuscript.
ACKNOWLEDGMENTS
- 1. Khamseh ME, Baradaran HR, Rajabali H. Depression and diabetes in Iranian patients: a comparative study. Int J Psychiatry Med 2007;37:81-86. ArticlePubMedPDF
- 2. Nau DP, Aikens JE, Pacholski AM. Effects of gender and depression on oral medication adherence in persons with type 2 diabetes mellitus. Gend Med 2007;4:205-213. ArticlePubMed
- 3. Larijani B, Khoram Shahi Baiat M, Khalili Gorgani M, Bandarian F, Akhondzadeh S, Sadjadi SA. Association between depression and diabetes. German J Psychiatry 2004;7:62-65.
- 4. Braunwald E, Fauci AS, Kasper DL, Hauser SL, Longo DL, Jameson JL. Harrison's principal of internal medicine. 2001. 15th ed. New York: McGrow-Hill.
- 5. Ahmadi J, Sharifi M, Mohagheghzadeh S, Dehbozorgi GR, Farrashbandi H, Moosavinasab M, Firoozabadi A, Pridmore S, Evren C, Busch S, Farash S. Pattern of cocaine and heroin abuse in a sample of Iranian population. German J Psychiatry 2005;8:1-4.
- 6. Ahmadi J, Maharlooy N, Alishahi M. Substance abuse: prevalence in a sample of nursing students. J Clin Nurs 2004;13:60-64. ArticlePubMed
- 7. Shiri R, Hassani KF, Ansari M. Association between opium abuse and comorbidity in diabetic men. Am J Addict 2006;15:468-472. ArticlePubMed
- 8. Gharagozlou H, Behin MT. Frequency of psychiatric symptoms among 150 opium addicts in Shiraz, Iran. Int J Addict 1979;14:1145-1149. ArticlePubMed
- 9. Kaplan H, Sadock B. Kaplan and Sadock's synopsis of psychiatry. 2002. 9th ed. Baltimore: Lippincott Williams & Wilkins.
- 10. Ahmadi J, Toobaee S, Kharras M, Radmehr M. Psychiatric disorders in opioid dependants. Int J Soc Psychiatry 2003;49:185-191. ArticlePubMedPDF
- 11. Pavarin RM. Substance use and related problems: a study on the abuse of recreational and not recreational drugs in Northern Italy. Ann Ist Super Sanita 2006;42:477-484. PubMed
- 12. Ciechanowski PS, Katon WJ, Russo JE. Depression and diabetes: impact of depressive symptoms on adherence, function, and costs. Arch Intern Med 2000;160:3278-3285. ArticlePubMed
- 13. Kivela SL, Pahkala K. Clinician-rated symptoms and signs of depression in aged Finns. Int J Soc Psychiatry 1988;34:274-284. ArticlePubMedPDF
- 14. Kawakami N, Takatsuka N, Shimizu H, Ishibashi H. Depressive symptoms and occurrence of type 2 diabetes among Japanese men. Diabetes Care 1999;22:1071-1076. ArticlePubMedPDF
- 15. American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 1994. 4th ed. Washington, DC: American Psychiatric Association.
- 16. Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry 1961;4:561-571. ArticlePubMed
- 17. Othmer E, Othmer SC. The clinical interview using DSM-IV-TR. Fundamentals. 2002. Vol. 1:1st ed. Washington, DC: American Psychiatric Publishing.
- 18. The Expert Panel. Report of the National Cholesterol Education Program Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Arch Intern Med 1988;148:36-69. ArticlePubMed
- 19. American Diabetes Association. Standards of medical care for patients with diabetes mellitus. Diabetes Care 2003;26(Suppl 1):S33-S50. ArticlePubMedPDF
- 20. Ali S, Stone MA, Peters JL, Davies MJ, Khunti K. The prevalence of co-morbid depression in adults with type 2 diabetes: a systematic review and meta-analysis. Diabet Med 2006;23:1165-1173. ArticlePubMed
- 21. Katon WJ. The comorbidity of diabetes mellitus and depression. Am J Med 2008;121(11 Suppl 2):S8-S15. ArticlePMC
- 22. Williams MM, Clouse RE, Lustman PJ. Treating depression to prevent diabetes and its complications: understanding depression as a medical risk factor. Clin Diabetes 2006;24:79-86.ArticlePDF
- 23. Musselman DL, Betan E, Larsen H, Phillips LS. Relationship of depression to diabetes types 1 and 2: epidemiology, biology, and treatment. Biol Psychiatry 2003;54:317-329. ArticlePubMed
- 24. Knol MJ, Twisk JW, Beekman AT, Heine RJ, Snoek FJ, Pouwer F. Depression as a risk factor for the onset of type 2 diabetes mellitus. A meta-analysis. Diabetologia 2006;49:837-845. ArticlePubMedPDF
- 25. Golden SH, Lazo M, Carnethon M, Bertoni AG, Schreiner PJ, Diez Roux AV, Lee HB, Lyketsos C. Examining a bidirectional association between depressive symptoms and diabetes. JAMA 2008;299:2751-2759. ArticlePubMedPMC
- 26. Harris MD. Psychosocial aspects of diabetes with an emphasis on depression. Curr Diab Rep 2003;3:49-55. ArticlePubMedPDF
- 27. Ludman EJ, Katon W, Russo J, Von Korff M, Simon G, Ciechanowski P, Lin E, Bush T, Walker E, Young B. Depression and diabetes symptom burden. Gen Hosp Psychiatry 2004;26:430-436. ArticlePubMed
- 28. Bryan CJ, Songer TJ, Brooks MM, Thase ME, Gaynes BN, Klinkman M, Rush AJ, Trivedi MH, Fava M, Wisniewski SR. A comparison of baseline sociodemographic and clinical characteristics between major depressive disorder patients with and without diabetes: a STAR*D report. J Affect Disord 2008;108:113-120. ArticlePubMed
- 29. Ahmadi J, Pridmore S, Alimi A, Cheraghi A, Arad A, Parsaeyan H, Mohagheghzadeh MS, Kianpour M. Epidemiology of opium use in the general population. Am J Drug Alcohol Abuse 2007;33:483-491. ArticlePubMed
- 30. Goodman LS, Gilman A. The pharmacological basis of therapeuticsv. 1985. 7th ed. New York: McGraw Hill.
- 31. McIntyre RS, Mancini D, Eisfeld BS, Soczynska JK, Grupp L, Konarski JZ, Kennedy SH. Calculated bioavailable testosterone levels and depression in middle-aged men. Psychoneuroendocrinology 2006;31:1029-1035. ArticlePubMed
- 32. Kanayama G, Amiaz R, Seidman S, Pope HG Jr. Testosterone supplementation for depressed men: current research and suggested treatment guidelines. Exp Clin Psychopharmacol 2007;15:529-538. ArticlePubMed
- 33. Kumano H. Hormone replacement up-to-date. The effects of androgen replacement therapy on brain function. Clin Calcium 2007;17:1378-1383. PubMed
- 34. Blatt SJ, Rounsaville B, Eyre SL, Wilber C. The psychodynamics of opiate addiction. J Nerv Ment Dis 1984;172:342-352. ArticlePubMed
- 35. Elson DF, Meredith M. Therapy for type 2 diabetes mellitus. WMJ 1998;97:49-54.
- 36. Karam GA, Reisi M, Kaseb AA, Khaksari M, Mohammadi A, Mahmoodi M. Effects of opium addiction on some serum factors in addicts with non-insulin-dependent diabetes mellitus. Addict Biol 2004;9:53-58. ArticlePubMedPDF
- 37. Azod L, Rashidi M, Afkhami-Ardekani M, Kiani G, Khoshkam F. Effect of opium addiction on diabetes. Am J Drug Alcohol Abuse 2008;34:383-388. ArticlePubMed
- 38. D'Mello DA, Rooker GM. Refractory depression, cardiovascular risk factors, and leukoariosis. J Clin Psychiatry 1997;58:274ArticlePubMed
- 39. Lishman WA. Organic psychiatry: the psychological consequences of cerebral disorder. 1998. 3rd ed. London: Blackwell Science.
- 40. Lustman PJ, Clouse RE, Griffith LS, Carney RM, Freedland KE. Screening for depression in diabetes using the Beck Depression Inventory. Psychosom Med 1997;59:24-31. ArticlePubMed
REFERENCES
Figure & Data
References
Citations
Citations to this article as recorded by 

- Prevalence of and factors associated with depression among hill tribe individuals aged 30 years and over in Thailand
Chalitar Chomchoei, Tawatchai Apidechkul, Vivat Keawdounglek, Chanyanut Wongfu, Siriyaporn Khunthason, Niwed Kullawong, Ratipark Tamornpark, Panupong Upala, Fartima Yeemard
Heliyon.2020; 6(6): e04273. CrossRef - The prevalence of comorbid depression in patients with type 2 diabetes: an updated systematic review and meta-analysis on huge number of observational studies
Mohammad Khaledi, Fahimeh Haghighatdoost, Awat Feizi, Ashraf Aminorroaya
Acta Diabetologica.2019; 56(6): 631. CrossRef - Psychometrics of the PHQ-9 as a measure of depressive symptoms in patients with heart failure
Muna H Hammash, Lynne A Hall, Terry A Lennie, Seongkum Heo, Misook L Chung, Kyoung Suk Lee, Debra K Moser
European Journal of Cardiovascular Nursing.2013; 12(5): 446. CrossRef

 KDA
KDA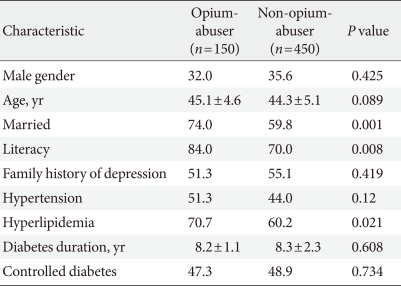
 PubReader
PubReader Cite
Cite



