- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Articles
- Page Path
- HOME > Diabetes Metab J > Volume 45(4); 2021 > Article
-
ReviewGuideline/Fact Sheet 2021 Clinical Practice Guidelines for Diabetes Mellitus in Korea
-
Kyu Yeon Hur1
 , Min Kyong Moon2, Jong Suk Park3, Soo-Kyung Kim4, Seung-Hwan Lee5, Jae-Seung Yun5, Jong Ha Baek6, Junghyun Noh7, Byung-Wan Lee3, Tae Jung Oh8, Suk Chon9, Ye Seul Yang5, Jang Won Son5, Jong Han Choi10, Kee Ho Song10, Nam Hoon Kim11, Sang Yong Kim12, Jin Wha Kim12, Sang Youl Rhee9, You-Bin Lee1, Sang-Man Jin1, Jae Hyeon Kim1, Chong Hwa Kim13, Dae Jung Kim14, SungWan Chun15, Eun-Jung Rhee16, Hyun Min Kim17, Hyun Jung Kim18, Donghyun Jee19, Jae Hyun Kim20, Won Seok Choi21, Eun-Young Lee5, Kun-Ho Yoon5, Seung-Hyun Ko5
, Min Kyong Moon2, Jong Suk Park3, Soo-Kyung Kim4, Seung-Hwan Lee5, Jae-Seung Yun5, Jong Ha Baek6, Junghyun Noh7, Byung-Wan Lee3, Tae Jung Oh8, Suk Chon9, Ye Seul Yang5, Jang Won Son5, Jong Han Choi10, Kee Ho Song10, Nam Hoon Kim11, Sang Yong Kim12, Jin Wha Kim12, Sang Youl Rhee9, You-Bin Lee1, Sang-Man Jin1, Jae Hyeon Kim1, Chong Hwa Kim13, Dae Jung Kim14, SungWan Chun15, Eun-Jung Rhee16, Hyun Min Kim17, Hyun Jung Kim18, Donghyun Jee19, Jae Hyun Kim20, Won Seok Choi21, Eun-Young Lee5, Kun-Ho Yoon5, Seung-Hyun Ko5 , Committee of Clinical Practice Guidelines, Korean Diabetes Association
, Committee of Clinical Practice Guidelines, Korean Diabetes Association -
Diabetes & Metabolism Journal 2021;45(4):461-481.
DOI: https://doi.org/10.4093/dmj.2021.0156
Published online: July 30, 2021
1Division of Endocrinology and Metabolism, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
2Department of Internal Medicine, Seoul Metropolitan Government Seoul National University Boramae Medical Center, Seoul National University College of Medicine, Seoul, Korea
3Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea
4Division of Endocrinology and Metabolism, Department of Internal Medicine, CHA Bundang Medical Center, CHA University, Seongnam, Korea
5Division of Endocrinology and Metabolism, Department of Internal Medicine, College of Medicine, The Catholic University of Korea, Seoul, Korea
6Division of Endocrinology & Metabolism, Department of Medicine, Gyeongsang National University Changwon Hospital, Gyeongsang National University College of Medicine, Changwon, Korea
7Division of Endocrinology and Metabolism, Department of Internal Medicine, Inje University Ilsan Paik Hospital, Inje University College of Medicine, Goyang, Korea
8Department of Internal Medicine, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea
9Department of Endocrinology and Metabolism, Kyung Hee University College of Medicine, Kyung Hee University Hospital, Seoul, Korea
10Division of Endocrinology and Metabolism, Konkuk University Medical Center, Konkuk University School of Medicine, Seoul, Korea
11Department of Internal Medicine, Korea University College of Medicine, Seoul, Korea
12Division of Endocrinology and Metabolism, Department of Internal Medicine, Chosun University College of Medicine, Gwangju, Korea
13Division of Endocrinology & Metabolism, Department of Internal Medicine, Sejong General Hospital, Bucheon, Korea
14Department of Endocrinology and Metabolism, Ajou University School of Medicine, Suwon, Korea
15Department of Internal Medicine, Soonchunhyang University Cheonan Hospital, Soonchunhyang University College of Medicine, Cheonan, Korea
16Division of Endocrinology and Metabolism, Department of Internal Medicine, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea
17Division of Endocrinology and Metabolism, Department of Internal Medicine, Chung-Ang University College of Medicine, Seoul, Korea
18Institute for Evidence-based Medicine, Cochrane Korea, Department of Preventive Medicine, Korea University College of Medicine, Seoul, Korea
19Division of Vitreous and Retina, Department of Ophthalmology, St. Vincent’s Hospital, College of Medicine, The Catholic University of Korea, Suwon, Korea
20Department of Pediatrics, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea
21Division of Infectious Diseases, Department of Internal Medicine, Korea University College of Medicine, Korea University Ansan Hospital, Ansan, Korea
-
Corresponding author: Seung-Hyun Ko
 Division of Endocrinology & Metabolism, Department of Internal Medicine, St. Vincent’s Hospital, College of Medicine, The Catholic University of Korea, 93 Jungbu-daero, Paldal-gu, Suwon 16247, Korea E-mail: kosh@catholic.ac.kr
Division of Endocrinology & Metabolism, Department of Internal Medicine, St. Vincent’s Hospital, College of Medicine, The Catholic University of Korea, 93 Jungbu-daero, Paldal-gu, Suwon 16247, Korea E-mail: kosh@catholic.ac.kr
Copyright © 2021 Korean Diabetes Association
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- ABSTRACT
- INTRODUCTION
- DIAGNOSIS OF DIABETES MELLITUS
- SCREENING AND PREVENTION OF DIABETES
- GLYCEMIC, BLOOD PRESSURE, AND LIPID CONTROL TARGETS IN T2DM
- OBESITY MANAGEMENT IN T2DM
- ANTIHYPERGLYCEMIC THERAPY FOR ADULT PATIENTS WITH TYPE 1 DIABETES MELLITUS
- ANTIHYPERGLYCEMIC THERAPY FOR ADULT PATIENTS WITH T2DM
- DIABETIC KIDNEY DISEASE (DIABETIC NEPHROPATHY)
- DIABETIC NEUROPATHY AND FOOT CARE
- DIABETIC RETINOPATHY
- NAFLD IN T2DM
- CGM SYSTEM AND INSULIN PUMP USE
- VACCINATION FOR PATIENTS WITH T2DM
- CONCLUSIONS
- NOTES
- REFERENCES
ABSTRACT
- The Committee of Clinical Practice Guidelines of the Korean Diabetes Association (KDA) updated the previous clinical practice guidelines for Korean adults with diabetes and prediabetes and published the seventh edition in May 2021. We performed a comprehensive systematic review of recent clinical trials and evidence that could be applicable in real-world practice and suitable for the Korean population. The guideline is provided for all healthcare providers including physicians, diabetes experts, and certified diabetes educators across the country who manage patients with diabetes or the individuals at the risk of developing diabetes mellitus. The recommendations for screening diabetes and glucose-lowering agents have been revised and updated. New sections for continuous glucose monitoring, insulin pump use, and non-alcoholic fatty liver disease in patients with diabetes mellitus have been added. The KDA recommends active vaccination for coronavirus disease 2019 in patients with diabetes during the pandemic. An abridgement that contains practical information for patient education and systematic management in the clinic was published separately.
- According to data from the Korea National Health and Nutrition Examination Survey, 13.8% of Korean adults aged ≥30 years had diabetes in 2018 [1]. The prevalence of impaired fasting glucose (IFG) was 26.9%. From 2016 to 2018, 35% of patients with diabetes were not diagnosed with diabetes. Moreover, among Koreans with diabetes, 53.2%, 61.3%, and 72% were obese, hypertensive, and had hypercholesterolemia, respectively [1]. Therefore, early detection, prevention, and proper management are important issues for diabetes. As an expert group, the Korean Diabetes Association (KDA) has tried to provide appropriate guidelines for these purposes and has cooperated with the government, stakeholders, and related organizations. As a part of this policy, the Committee of Clinical Practice Guidelines of KDA has regularly updated its practice guidelines [2] and recently published the 7th edition.
- In this revised edition, the evidence level was classified into four categories: randomized controlled trials (RCTs), including systematic reviews, meta-analyses, and RCTs; non-randomized controlled studies (NRS); others; and expert opinions. According to the target population, each recommendation was classified as a “general recommendation” or a “limited recommendation” if it can be recommended for most subjects or specific subjects, respectively (Table 1).
- The section on “Antihyperglycemic therapy for adult patients with diabetes” describes the general principles of treatment, including both oral and injectable medications, have been updated. Furthermore, new sections on the “Diagnosis, evaluation, and treatment of non-alcoholic fatty liver disease (NAFLD)” and “Continuous glucose monitoring (CGM) and insulin pump use in subjects with diabetes” have been added. Additionally, visual leaflets for practical information, video materials and web-based clinical decision supporting systems were developed at the same time to facilitate the implementation and widespread use of these guidelines [3]. A checklist for the comprehensive management of patients with diabetes is provided in the appendix.
INTRODUCTION
- The diagnostic criteria for diabetes are based on fasting plasma glucose (FPG), 2-hour plasma glucose (2h-PG) during a 75 g oral glucose tolerance test (OGTT), or glycosylated hemoglobin (A1C) value (Table 2) [4]. The A1C test should be performed using a method that is certified by the National Glycohemoglobin Standardization Program and should be standardized or traceable to the Diabetes Control and Complications Trial reference assay [4]. Since 2011, A1C has been included as a diagnostic criterion in the KDA clinical practice guidelines [5].
- Prediabetes is defined as an FPG value of 100 to 125 mg/dL (IFG), a 2h-PG value of 140 to 199 mg/dL after a 75 g oral glucose load (impaired glucose tolerance [IGT]), or A1C levels of 5.7% to 6.4% [2,4]. Specifically, the KDA has classified people with IFG into two stages according to their FPG levels: stage 1 IFG (FPG 100 to 109 mg/dL or A1C 5.7% to 6.0%) and stage 2 IFG (FPG 110 to 125 mg/dL or A1C 6.1% to 6.4%) [6].
DIAGNOSIS OF DIABETES MELLITUS
- A screening test for diabetes mellitus should be conducted annually for adults over 40 years of age and adults over 30 years of age with risk factors by using the FPG, A1C, and 2h-PG levels during 75 g OGTT. For subjects with stage 1 IFG, the KDA had previously recommended annual FPG and A1C monitoring to screen for undiagnosed diabetes in the general population. The Korean Diabetes Prevention Study (KDPS), which is the first randomized controlled clinical trial on the prevention of diabetes in high-risk individuals by using intensive lifestyle modifications, is currently ongoing in Korea [7,8]. In the KDPS, 75 g OGTT results were obtained from 1,637 overweight or obese Korean adult participants. Among them, 27.2% had type 2 diabetes mellitus (T2DM), and 59.3% had IFG and/or IGT. On the basis of the FPG criteria, 31.4% and 21.5% of subjects were classified as having stage 1 IFG and stage 2 IFG, respectively. More importantly, 19.0% of stage 1 and 43.5% of stage 2 IFG subjects showed 2h-PG levels in the diabetic range [8]. Therefore, the 75 g OGTT should be actively considered for Korean adults who are overweight or obese (body mass index [BMI] ≥23.0 kg/m2) with stage 1 IFG (FPG 100 to 109 mg/dL) and for all adults with stage 2 IFG independent of BMI for early detection and prompt intervention (Expert opinion, General).
- To prevent diabetes in subjects with prediabetes, prompt individualized lifestyle modification and education should be introduced according to their BMI. Pre-diabetic adults with a BMI <23 kg/m2 should modify their lifestyle by using medical nutrition and exercise therapies (medium or high-level physical activity for at least 150 minutes per week) (Expert opinion, General). If adults have prediabetes and a BMI ≥23 kg/m2, they should be asked to lose 5% to 10% of their body weight and maintain the reduced weight by lifestyle modification with medical nutrition and exercise therapies (RCT, General) [9-12]. The use of metformin may be considered to prevent the development of T2DM among adults with a BMI ≥23 kg/m2 and aged 30 to 70 years (RCT, Limited) [13]. To maintain lifestyle modification, the patient should be continuously motivated and monitored in various ways, including individualized diabetes education and use of aids based on information and communications technology (Expert opinion, General) [12].
SCREENING AND PREVENTION OF DIABETES
- Intensive and strict glycemic control with lifestyle modifications has been emphasized to prevent microvascular and macrovascular diabetic complications in T2DM (RCT, General) [14-16]. Therefore, the long-term maintenance of glycemic control status within the near-normal range is very important for patients with diabetes [17]. The optimal A1C target for patients with T2DM is <6.5% via lifestyle modification and glucose-lowering agents (RCT, General) (Table 3), particularly in recently diagnosed, young patients with T2DM without severe complications or hypoglycemia (Expert opinion, General). However, the glycemic target should be individualized on the basis of the patients’ clinical characteristics. In patients with a history of severe hypoglycemia, advanced diabetic complications, short life expectancy, or advanced age, the glycemic target must be set by considering the risks of complications, such as hypoglycemia (NRS, General) [18-20].
- Blood pressure (BP) should be measured at every clinic visit and at home (NRS, General). For patients with diabetes and BP >120/80 mm Hg, lifestyle modification should be initiated (RCT, General). The target BP level for patients with diabetes is <140 mm Hg systolic BP and 85 mm Hg diastolic BP (RCT, General) [21-25]. The target BP for patients with diabetes and cardiovascular disease (CVD) should be <130/80 mm Hg (Expert opinion, Limited) [26]. Patients with diabetes and high BP could be prescribed any class of antihypertensive medication as the primary medication for BP control to achieve the target range (RCT, General). There was no difference on the effect of preventing CVD between angiotensin-converting enzyme (ACE) inhibitors, angiotensin II receptor blockers (ARBs), calcium channel blockers, and beta-blockers, which can all be used as first-line antihypertensive agents in patients with T2DM [27,28]. If BP is not controlled by the initial antihypertensive medication, combination therapy using drugs with different mechanisms is recommended. However, the combination of ACE inhibitors and ARBs is not recommended (RCT, General). Patients with coronary artery disease and diabetes are advised to use ACE inhibitors or ARBs as a first-line therapy (RCT, General) [29]. ACE inhibitors or ARBs are also recommended when high BP is accompanied by albuminuria (NRS, General) [30-32].
- To assess the risk of CVD, a serum lipid test, which measures total cholesterol, high-density lipoprotein cholesterol, triglycerides, and low-density lipoprotein cholesterol (LDL-C), should be performed at least once a year when diagnosing diabetes (Expert opinion, General). LDL-C concentration was graded according to risk level. Patients with diabetes and CVD are classified as very high risk, and LDL-C targets should be adjusted to <70 mg/dL (RCT, General) [33,34]. In individuals with diabetes who also have target organ damage (albuminuria or estimated glomerular filtration rate [eGFR] <60 mL/min/1.73 m2) or risk factors for CVD (hypertension, smoking, or family history of premature atherosclerotic CVD), an LDL-C target of <70 mg/dL should be considered (RCT, General) [35]. In patients with diabetes but without CVD, the recommended target for LDL-C is <100 mg/dL (RCT, General).
- The primary drug of choice for the treatment of dyslipidemia is statins; however, if target lipid levels are not reached even with the maximum tolerated dose of statins, the addition of ezetimibe could be considered (RCT, General) [36-39]. For patients with CVD, a combination of statins and proprotein convertase subtilisin/kexin type 9 inhibitors is recommended if the LDL-C target has not been reached even after adding ezetimibe to the maximally tolerated doses of statins (RCT, Limited) [40,41]. For severe hypertriglyceridemia (>500 mg/dL), treatment with fenofibrate or omega-3 could be considered in the evaluation of secondary causes (NRS, General) [42]. Serum lipid tests need to be performed 4 to 12 weeks after the initiation of lipid-lowering therapy to evaluate the drug response and patient compliance (Expert opinion, General).
GLYCEMIC, BLOOD PRESSURE, AND LIPID CONTROL TARGETS IN T2DM
- According to the 2020 Korean Society for the Study of Obesity Guidelines, the classification of obesity into classes I, II, and III relies on adult BMI and the World Health Organization guidelines for the Asia-Pacific region. Classes I and II obesity are defined as BMIs of 25.0 to 29.9 kg/m2 and 30.0 to 34.9 kg/m2, respectively; class III obesity was newly defined in 2018 as a BMI ≥35 kg/m2 [43].
- Obese adults with T2DM should lose at least 5% of their body weight via lifestyle modifications and maintain their reduced weight (RCT, General) [44,45]. If patients with T2DM and BMI ≥25 kg/m2 (class I) fail to lose weight with diet, physical activity, and behavior counseling, anti-obesity medications can be considered (Others, Limited) [46-48]. If the use of antiobesity drugs does not result in a loss of more than 5% of body weight within 3 months, it is judged that the effect of the drug is not significant, and changing or discontinuing the drug should be considered (RCT, General). Bariatric surgery can be considered in patients with T2DM with a BMI ≥35 kg/m2 (class III obesity). Bariatric surgery may also be considered in patients with T2DM with BMI ≥30 kg/m2 (class II obesity) if nonsurgical treatment fails to lose weight or control glycemia (Table 4) [49,50]. Multidisciplinary care is needed before and after surgery to enhance the effectiveness and safety of bariatric surgery (Expert opinion, General).
OBESITY MANAGEMENT IN T2DM
- The recommended glycemic target for patients with type 1 diabetes mellitus (T1DM) is an A1C <7.0% (RCT, General). All patients with T1DM should be systematically trained to adjust their insulin dose so that they can have flexible meals (RCT, General) [51-54]. The level of understanding following education and performance among patients with T1DM should be evaluated, and patients should receive regular feedback following a diagnosis of diabetes (Expert opinion, General). In addition, individualized self-management education should be provided upon diagnosis not only to patients with T1DM but also to parents/caregivers, and regular reassessment should be performed according to the patient’s growth and independent development of self-management (Expert opinion, Limited). In particular, patients with T1DM who develop hypoglycemia unawareness or severe hypoglycemia should receive specialized and professional education to prevent hypoglycemia and restore cognitive abilities in case of hypoglycemia (RCT, Limited) [55].
- Individuals with T1DM should be treated with intensive insulin replacement using multiple daily injections (MDIs) of prandial and basal insulin or insulin pumps (RCT, General). Rapid-acting insulin analogs (lispro, glulisine, or aspart) and long-acting insulin analogs (glargine, detemir, or degludec) should be used first in patients with T1DM when treated with MDIs (RCT, General) [14,56-61].
ANTIHYPERGLYCEMIC THERAPY FOR ADULT PATIENTS WITH TYPE 1 DIABETES MELLITUS
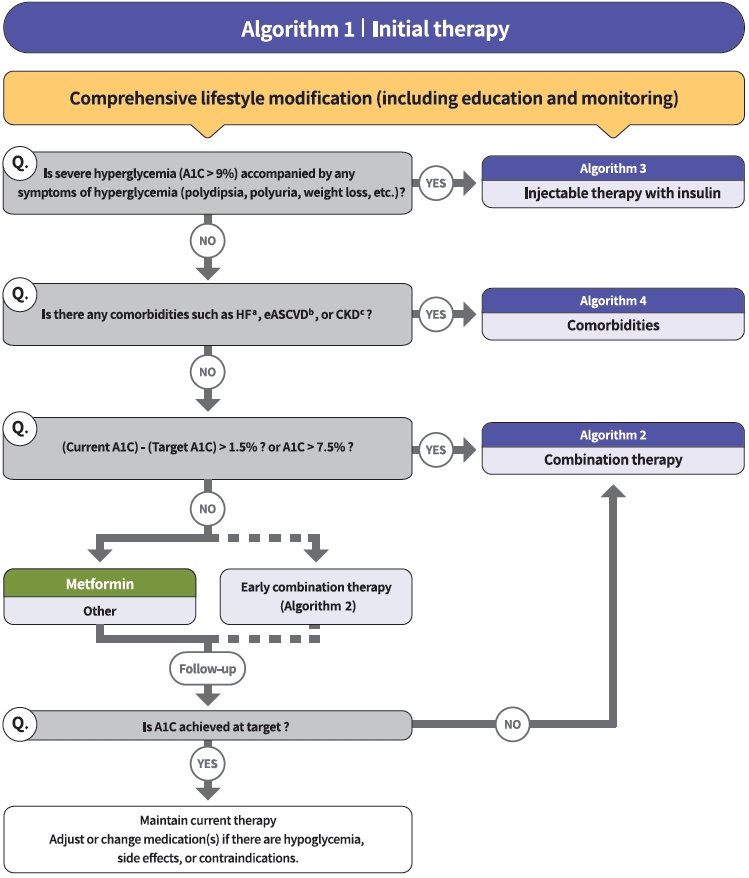
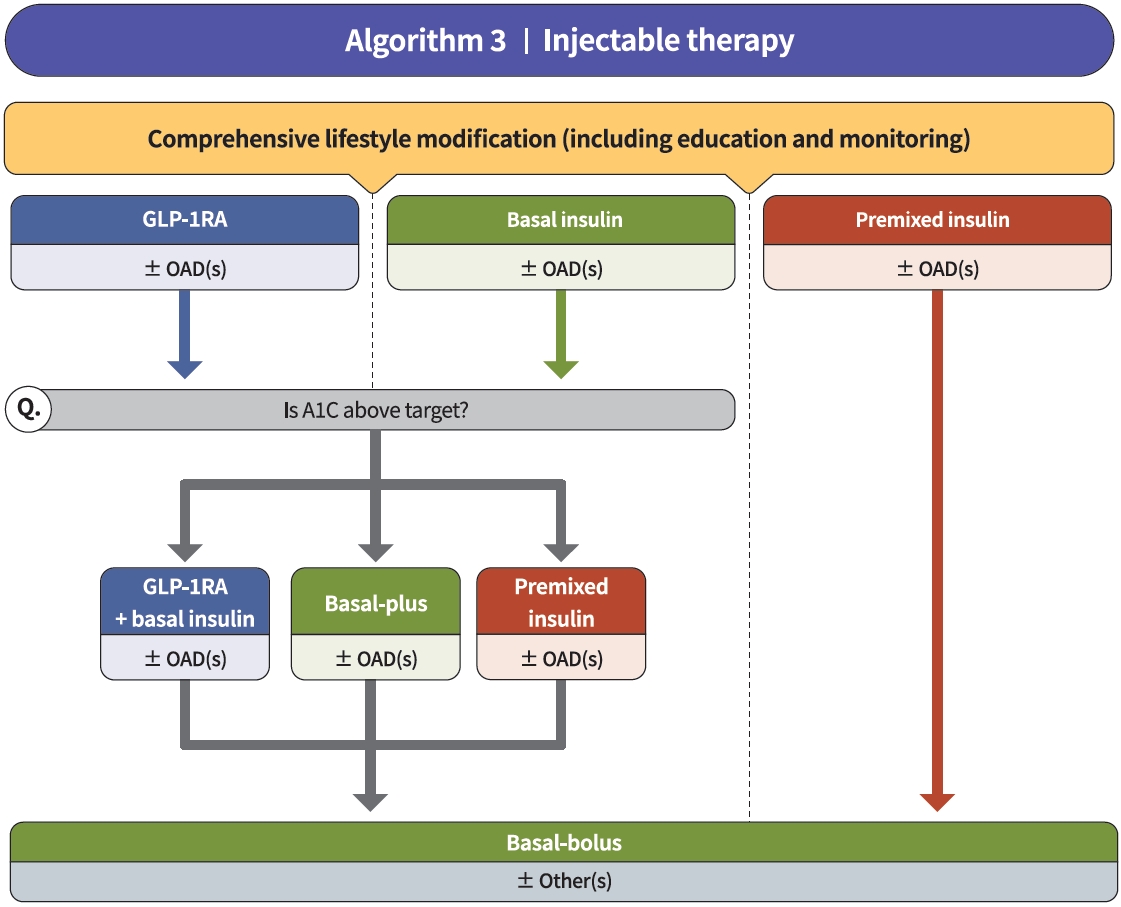
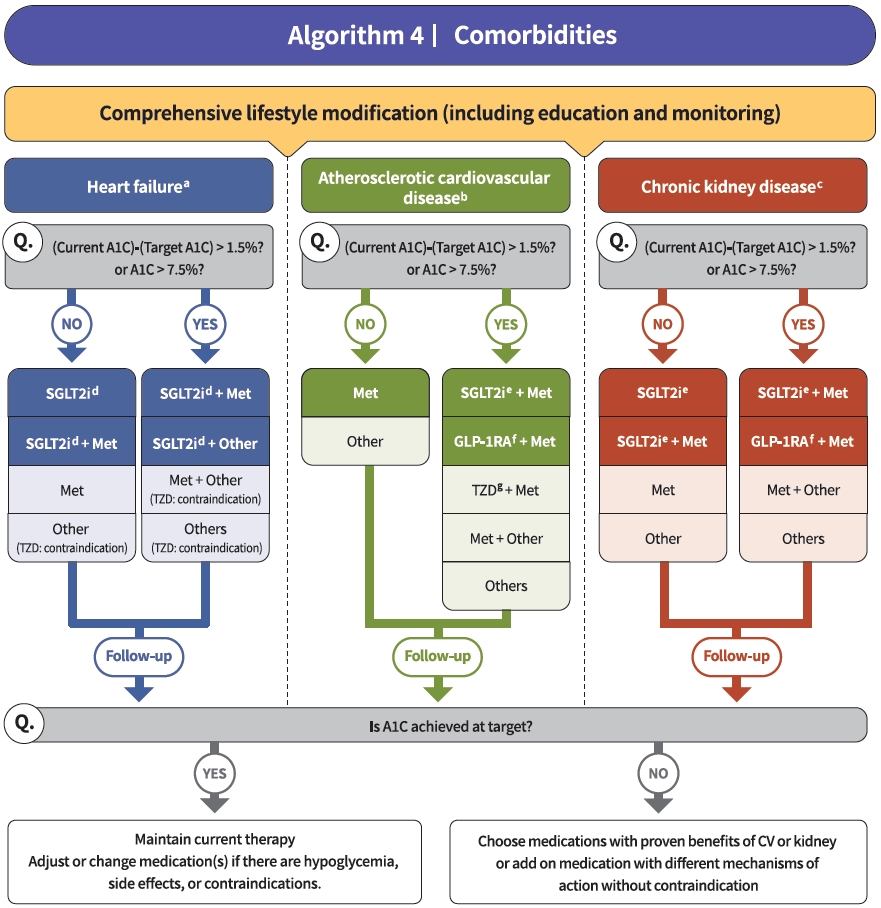
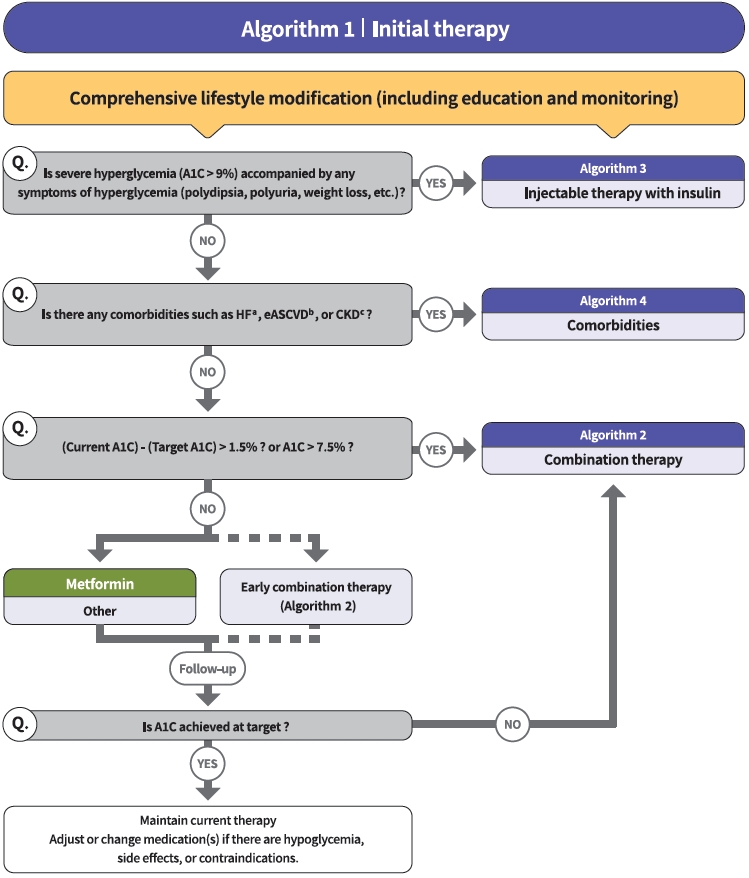
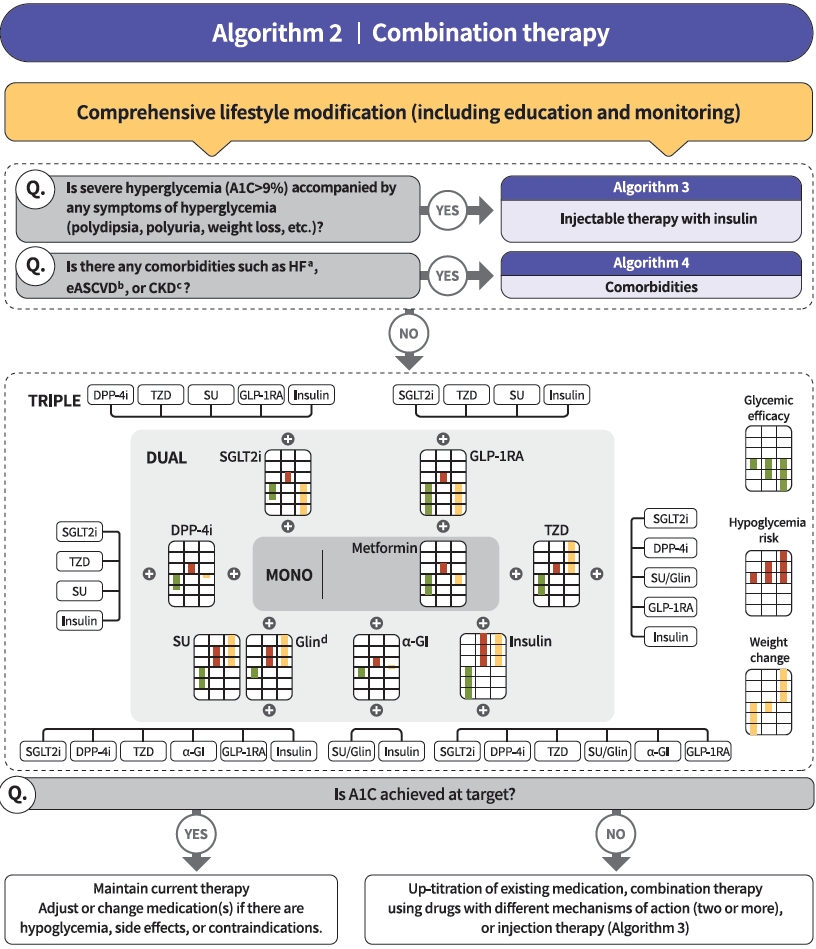
- Diabetes education for patients with T2DM is an essential and fundamental part of diabetes management. Certified healthcare professionals should actively educate the patient on lifestyle modification immediately upon diagnosis, and this should be continuously monitored (RCT, General). Following glucose-lowering agent initiation, monotherapy or combination therapy should be provided on the basis of the target and current A1C levels (RCT, General). In general, the glucoselowering efficacy (reduction of A1C) of monotherapy with an oral anti-diabetic drug is estimated to be 1.0%; therefore, if the current A1C is 1.5% to 2.0% higher than that of the target A1C, initial combination therapy is recommended (Figs. 1 and 2) [15,62]. However, insulin treatment should be prioritized if severe hyperglycemia (A1C >9.0%) is accompanied by symptoms of hyperglycemia (polydipsia, polyuria, weight loss, etc.) (Expert opinion, General) (Fig. 3). When choosing a glucoselowering agent, consider whether there are any associated comorbidities (heart failure [HF], established atherosclerotic cardiovascular disease [eASCVD], or chronic kidney disease [CKD]) (Fig. 4) as well as the glucose-lowering efficacy, hypoglycemia risk or weight change, side effects, treatment acceptability, age, personal value of life, and cost (Expert opinion, General) (Figs. 1 to 3).
- Metformin is recommended as a first-line glucose-lowering agent in patients with T2DM and is maintained as long as there are no contraindications or intolerable side effects (RCT, General) (Fig. 1). In the Practical Evidence of Antidiabetic Monotherapy study, the glucose-lowering efficacy of sulfonylureas, metformin, and thiazolidinediones as a monotherapy administered for 48 weeks were similar in drug-naïve Korean patients with T2DM [63]. Metformin has adequate efficacy, low hypoglycemia risk, weight neutrality, and cost-effectiveness. However, if there are contraindications or intolerable side effects related to metformin use, a different class of medications can be considered (Expert opinion, General). If the target A1C level has not been achieved, the up-titration of existing medication, combination therapy using medications with different mechanisms of action, or use of injectable medication should actively be considered as soon as possible; however, dipeptidyl peptidase-4 (DPP-4) inhibitor and glucagon-like peptide 1 receptor agonist (GLP-1RA) should not be combined at the same time (RCT, General) (Fig. 2).
- The recent Vildagliptin Efficacy in combination with metfoRmIn for earlY treatment of type 2 diabetes (VERIFY) trial demonstrated that an early intervention strategy with a combination therapy of vildagliptin plus metformin in treatment-naïve patients with T2DM provides more durable long-term clinical benefits than metformin monotherapy with a traditional stepwise increase in dosage [64]. According to a subgroup analysis of Korean patients with newly diagnosed T2DM among VERIFY trial participants, early combination treatment significantly and consistently improved long-term glycemic durability compared with monotherapy with metformin [65]. Therefore, to reduce the risk of failure of glycemic control, early initial combination therapy is recommended following the diagnosis of T2DM (RCT, Limited) (Fig. 1).
- There is a strong correlation between adherence to glucose-lowering agents and metabolic control in T2DM. For each 10% increment in drug adherence, the A1C level decreases by 0.16% [66]. Therefore, adherence to medication should be checked regularly, and medication adjustment should not be delayed if necessary (Expert opinion, General).
- Injectable therapy (GLP-1RA or insulin) is recommended when potent glucose-lowering efficacy is required (RCT, General) (Fig. 3) [67]. The addition of GLP-1RA, basal insulin, or premixed insulin is recommended equally [68-77]. If A1C target is not achieved with GLP-1RA or basal insulin-based therapy, free or fixed-ratio combination therapy of GLP-1RA and basal insulin could be considered (RCT, Limited) [72,78-94]. Intensification of insulin therapy with premixed insulin twice daily, basal-plus, or basal-bolus is also recommended to enhance blood glucose control (RCT, Limited) [95-99].
- Regimens that include sodium-glucose cotransporter 2 (SGLT2) inhibitors or GLP-1RAs with proven cardiovascular (CV) benefits should be prioritized for combination therapy in patients with eASCVD (RCT, Limited) (Fig. 4). T2DM patients with CV risk factors who received empagliflozin treatment had a remarkably lower three-point major adverse cardiovascular event (MACE), including CV death, nonfatal myocardial infarction, and nonfatal stroke, compared with the placebo treatment group (hazard ratio, 0.86; 95% confidence interval, 0.74 to 0.99; EMPA-REG OUTCOME trial) (Table 5) [100]. The effects of empagliflozin on the components of MACE, all-cause mortality, and HF outcomes in Asian patients were also consistent with the overall population of the EMPA-REG study [101]. Dulaglutide [102], liraglutide [103], and semaglutide [104] significantly led to a lower composite three-point MACE outcome compared with the placebo (Table 6).
- In patients with T2DM who had or were at risk for atherosclerotic CVD, SGLT2 inhibitor treatment with empagliflozin (EMPEROR-Reduced study), dapagliflozin (DECLARE-TIMI 58 study, DAPA-HF study), and ertugliflozin (VERTIS-CV study) showed lower rates of CV death and hospitalization for HF than placebo treatment regardless of the presence of diabetes [105-107]. For patients with HF, glucose-lowering agents, including SGLT2 inhibitors with proven CV benefits, should be prioritized (RCT, Limited). The use of SGLT2 inhibitors could be beneficial in patients with T2DM suffering from HF symptoms and reduced ejection fraction to reduce the aggravation of HF and death from CV causes (Table 5).
- For patients with albuminuria or reduced eGFR, glucose-lowering agents, including SGLT2 inhibitors with proven renal and CV benefits, should be prioritized (RCT, Limited). According to the EMPA-REG OUTCOME trial, empagliflozin treatment led to a significantly lower risk of composite renal outcome (persistent doubling of serum creatinine level, end-stage kidney disease [i.e., the need for long-term dialysis or renal transplantation], or death from renal causes) compared with placebo [108]. The DECLARE-TIMI 58 study confirmed that dapagliflozin also led to a lower renal end-point compared with placebo treatment [105]. A recently published DAPACKD study showed that regardless of the presence or absence of diabetes, the risk of renal composite outcomes was significantly lower with dapagliflozin treatment than with placebo in patients with pre-existing CKD (eGFR, 25 to 75 mL/min/1.73 m2; urine albumin-to-creatinine ratio [UACR], 200 to 5,000) [109]. According to recent evidence from large-scale RCTs and meta-analyses, SGLT2 inhibitor treatment in patients with T2DM has proven beneficial effects on renal outcomes, such as progression of albuminuria, aggravation of eGFR, or initiation of renal replacement therapy. Most clinical trials were performed in patients with eASCVD, high risk of CVD, or CKD; therefore, we recommend SGLT2 inhibitors preferentially in these populations.
ANTIHYPERGLYCEMIC THERAPY FOR ADULT PATIENTS WITH T2DM
- UACR and eGFR should be evaluated in people with T2DM at the time of diagnosis and at least once a year (NRS, General) [110]. Blood glucose and BP should be controlled optimally to suppress the development and progression of diabetic kidney disease (RCT, General) [24]. Patients with diabetic kidney disease should avoid excessively high or low (≤0.8 g/kg/day) consumption of protein (RCT, General) [111,112]. In patients with diabetes, high BP, and albuminuria, ACE inhibitors or ARBs should be prescribed (RCT, General) [113-115]. The use of ACE inhibitors or ARBs to prevent the progression of diabetic kidney disease is not recommended in patients with normal BP (RCT, General) [116]. Treatment with SGLT2 inhibitors, which have proven CV and renal benefits, should be prioritized in cases of albuminuria or reduced eGFR (RCT, General) [105,109,117]. If the cause of kidney disease is unclear or if renal dysfunction has progressed (GFR <30 mL/min/1.73 m2), a nephrologist should be consulted (NRS, General).
DIABETIC KIDNEY DISEASE (DIABETIC NEPHROPATHY)
- Patients with T1DM should be screened 5 years after diagnosis; patients with T2DM should be screened for diabetes-related peripheral and autonomic neuropathy at the time of diagnosis of diabetes and then repeatedly tested every year (RCT, General). Screening tests for diabetic peripheral neuropathy include the Michigan Neuropathy Screening Instrument Questionnaire and a neurological physical examination (Michigan Neuropathy Screening Instrument Examination, a pinching test, and a temperature perception test) (RCT, General) [118,119]. In patients with symptoms or signs such as orthostatic hypotension and stable tachycardia, the patient should be tested for CV autonomic neuropathy (RCT, Limited). Strict glycemic control is recommended to prevent or delay the development of peripheral neuropathy and CV autonomic neuropathy in both T1DM and T2DM (RCT, General) [120]. In patients with neuropathic pain, pharmacologic treatment should be used to control the pain and improve the patient’s quality of life (RCT, General) [121,122]. Annual comprehensive evaluation to check for risk factors of foot ulcer and amputation and education for foot management should be conducted in adult patients with diabetes (RCT, General) [123]. Peripheral angiography examination should be performed in patients with severe claudication, weak pulse of the dorsalis pedis artery, or ankle brachial index ≤0.9 (Expert opinion, Limited) [124].
DIABETIC NEUROPATHY AND FOOT CARE
- The optimization of blood glucose, BP, and lipid control is recommended to reduce the risk of developing diabetic retinopathy or to prevent its progression (RCT, General) [15,125]. Patients with T1DM should undergo ophthalmic and comprehensive eye examinations, including the periphery of the retina, within 5 years of diagnosis. Patients with T2DM should undergo ophthalmic and comprehensive eye examinations, including the periphery of the retina, at the time of diagnosis and then every year (Expert opinion, General). If there are no signs of retinopathy and glycemia is well controlled, the patient can be examined at an interval of 1 to 2 years. If a woman with diabetes plans to become pregnant, she must undergo an eye examination in advance; if she becomes pregnant, she should undergo an eye examination within the first 3 months of pregnancy and receive counseling on the development and risk of diabetic retinopathy (RCT, General). The patient should be followed up every 3 months during pregnancy and up to 1 year postpartum [126]. The use of aspirin to prevent CVD does not increase the risk of retinal bleeding (RCT, General) [127]. When the illness progresses to proliferative diabetic retinopathy, panretinal photocoagulation should be performed by a specialist (Expert opinion, General) [128].
DIABETIC RETINOPATHY
- All adults with T2DM should undergo NAFLD evaluation by using alanine aminotransferase or abdominal ultrasonography (Expert opinion, General) [129]. For adults with T2DM and NAFLD, lifestyle modification is required to treat CV risk factors and fatty liver diseases (RCT, General). Adults with BMI ≥23 kg/m2, NAFLD, and T2DM should lose at least 7% of their body weight to reduce liver inflammation (RCT, General) [130]. Thiazolidinedione can be used as the initial medication for NAFLD in adults with T2DM (RCT, Limited) [131]. GLP-1RAs can be used for the treatment of NAFLD in adults with T2DM (RCT, Limited) [132]. Metformin, DPP-4 inhibitors, vitamin E, statins, ursodeoxycholic acid, and pentoxifyline should not be used for the treatment of NAFLD (RCT, General) [133].
NAFLD IN T2DM
- CGM results and an ambulatory glucose profile should be analyzed using internationally standardized core metrics (NRS, General) [134]. The clinical benefits of CGM and insulin pump use can only be expected if the patients are trained to use these devices properly and if the information obtained for blood glucose control are properly applied (NRS, General). For adults who treated with MDIs of prandial and basal insulin or insulin pumps, training should be provided professionally and systematically (NRS, General). All adult T1DM patients should be encouraged to use real-time CGM devices to control blood glucose and lower the risk of hypoglycemia (RCT, General) [135-137]. Adults with T2DM who require MDIs may use realtime CGM devices for glycemic control (RCT, Limited) [138]. Adults with T2DM who receive treatment other than MDI can periodically perform real-time CGM to control blood glucose (RCT, Limited) [139]. The daily use of real-time CGM devices is recommended for pregnant women with T1DM to control blood glucose, lower the risk of hypoglycemia, and improve perinatal performance (RCT, General) [140].
- To reduce the risk of severe hypoglycemia for adult patients with T1DM who experience severe hypoglycemia or hypoglycemia unawareness at least twice a year, insulin pumps rather than MDIs are also recommended even in the absence of CGM (RCT, Limited) [141]. Sensor-enhanced insulin pumps with built-in basal insulin injection interruption algorithms are recommended for adults with T1DM with a high risk of hypoglycemia despite the everyday use of CGM devices (RCT, Limited) [142]. Insulin pumps should only be considered if intensive education is preceded by a professional education system for adults with T2DM whose blood glucose is not controlled by MDIs (RCT, Limited) [143].
CGM SYSTEM AND INSULIN PUMP USE
- An annual flu vaccine is recommended for patients with diabetes. Streptococcus pneumoniae vaccines should also be considered. Coronavirus disease 2019 (COVID-19) vaccination is recommended for patients with diabetes (Expert opinion, General) [144].
VACCINATION FOR PATIENTS WITH T2DM
- Despite the constant efforts of the KDA, healthcare professionals, and the Korean government, the achievement rates for the comprehensive management of diabetes via ABC clinical targets (A, A1C <6.5%; B, BP <140/85 mm Hg; C, LDL-C <100 mg/dL) to prevent and delay vascular complications are still low in Korean adult patients with T2DM. Only 11.5% of subjects achieved all three ABC targets from 2016 to 2018, although there was an increasing trend (9.4% in 2013–2014, 8.4% in 2013–2016, and 11.5% in 2016–2018) [145]. Patients with diabetes should receive active education from multidisciplinary certified and professional education teams and constant monitoring for adherence to self-management, nutrition, and exercise. To encourage and maintain a properly modified lifestyle, technology-based behavior therapy could be useful. Individualized glycemic target, early and intensive glucose control, clinical strategies to improve adherence to glucoselowering agents and to overcome clinical inertia are necessary to optimize glycemic control in patients with diabetes. In addition, regular check-up and early detection of acute or chronic vascular complications as well as treatment strategies with CV benefit-proven medications should be considered to reduce comorbidities or mortality in patients with diabetes. The timely updated Clinical Practice Guidelines of KDA will provide evidence-based recommendations to healthcare professionals and contribute the improvement of diabetes care in Korea.
CONCLUSIONS
-
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
-
FUNDING
This work was supported by the Korean Diabetes Association.
NOTES
-
Acknowledgements
- We would like to thank the following for their thoughtful peer review and endorsement of the guidelines: the Study group of Neuropathy, Nephropathy, Gestational Diabetes, Beta-cell and islet transplantation, Geriatrics of KDA; Committee of Education, Committee of Food and Nutrition, and Committee of Medical Practitioners of KDA; Korean Association of Internal Medicine; Korean Society of Nephrology; Korean Association of Obesity; Korean Society of Hypertension; Korean Association of Infection; Korean Society of Pediatric Endocrinology; Korean Association of Ophthalmology; Korean Association of Diabetes Dietetic Educators; Korean Association of Diabetes Nurse Educators; and Korean Society of Social Workers for Diabetes Education. We would also like to thank Esther Kim for her contribution to the systematic literature search.


| 1. A1C level ≥6.5%a or |
| 2. Fasting plasma glucose of ≥126 mg/dL for >8 hoursa or |
| 3. 2 hours plasma glucose of ≥200 mg/dL during 75 g oral glucose tolerance testa or |
| 4. Classic symptoms of hyperglycemia (polyuria, polydipsia, unexplained weight loss) with a random plasma glucose of ≥200 mg/dL |
| Cardiovascular disease | Present | Absent |
|---|---|---|
| A1C, % | <6.5a | <6.5a |
| Blood pressure, mm Hg | <130/80 | <140/85 |
| LDL-C, mg/dL | <70 | <100b |
| Triglycerides, mg/dL | <150 | <150 |
| HDL-C, mg/dL | >40 (men), >50 (women) | |
A1C, glycosylated hemoglobin; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol.
a A1C target should be individualized according to the patient’s clinical condition,
b Target LDL-C is also <70 mg/dL in the presence of target organ damage or cardiovascular risk factors.
| Classification | BMI, kg/m2 | Lifestyle therapy | Medical therapya | Bariatric surgeryb |
|---|---|---|---|---|
| Normal | 18.5–22.9 | - | - | - |
| Overweight | 23.0–24.9 | ⊙ | - | - |
| Class I obesity | 25.0–29.9 | ⊙ | ◎ | - |
| Class II obesity | 30.0–34.9 | ⊙ | ◎ | ◎ |
| Class III obesity | ≥35.0 | ⊙ | ◎ | ◎ |
| Variable | EMPA-REG OUTCOME | DECLARE-TIMI 58 | DAPA-HFa | VERTIS-CV | DAPA-CKD | EMPEROR-R |
|---|---|---|---|---|---|---|
| No. of patients enrolled | 7,020 | 17,160 | 4,744 | 8,246 | 4,304 | 3,730 |
| Drug | Empagliflozin | Dapagliflozin | Dapagliflozin | Ertugliflozin | Dapagliflozin | Empagliflozin |
| Median follow-up, yr | 3.1 | 4.2 | 1.5 | 3 | 2.4 | 1.3 |
| Mean baseline A1C, % | 8.1 | 8.3 | NA | 8.2 | NA | NA |
| Mean duration of diabetes, yr | NA | 11 | NA | 13 | NA | NA |
| Baseline statin use, % | 77 | 75 | NA | 82.3 | 65 | NA |
| Baseline CVD/HF, % | 99 | 41 | Not reported | 100 | 37 | Not reported |
| Baseline HF, % | 10 | 10 | 100 | 23.7 | 10.8 | 100 |
| MACE outcomeb | 0.86 (0.74–0.99) | 0.93 (0.84–1.03) | Not reported | 0.97 (0.85–1.11) | Not reported | Not reported |
| Hospitalization for HF or CV death | 0.66 (0.55–0.79) | 0.83 (0.73–0.95) | 0.75 (0.65– 0.85) | 0.88 (0.75–1.03) | 0.71 (0.55–0.92) | 0.75 (0.65–0.86) |
| CV death | 0.62 (0.49–0.77) | 0.98 (0.82–1.17) | 0.82 (0.69– 0.98) | 0.92 (0.77–1.11) | 0.81 (0.58–1.12) | 0.92 (0.75–1.12) |
| Fatal or nonfatal MI | 0.87 (0.70–1.09) | 0.89 (0.77–1.01) | Not reported | 1.04 (0.86–1.26) | Not reported | Not reported |
| Fatal or nonfatal stroke | 1.18 (0.89–1.56) | 1.01 (0.84–1.21) | Not reported | 1.06 (0.82–1.37) | Not reported | Not reported |
| All-cause mortality | 0.68 (0.57–0.82) | 0.93 (0.82–1.04) | 0.83 (0.71–0.97) | 0.93 (0.80–1.08) | 0.69 (0.53–0.88) | 0.92 (0.77–1.10) |
| HF hospitalization | 0.65 (0.50–0.85) | 0.73 (0.61–0.88) | 0.70 (0.59– 0.83) | 0.70 (0.54–0.90) | Not reported | 0.69 (0.59–0.81) |
| Renal composite endpoint | 0.54 (0.40–0.75) | 0.53 (0.43–0.66) | 0.71 (0.44–1.16) | 0.81 (0.63–1.04) | 0.61 (0.51–0.72) | 0.50 (0.32–0.77) |
| ESKD | 0.45 (0.21–0.97) | 0.31 (0.13–0.79) | 1.00 (0.50–1.99) | 0.81 (0.63–1.04) | 0.64 (0.50–0.82) | Not reported |
| Renal death | Not reported | 0.60 (0.22–1.65) | NA | NA | NA | Not reported |
Values are presented as hazard ratio (95% confidence interval).
SGLT2, sodium-glucose cotransporter 2; A1C, glycosylated hemoglobin; NA, not available; CVD, cardiovascular disease; HF, heart failure; MACE, major adverse cardiovascular events; CV, cardiovascular; MI, myocardial infarction; ESKD, end-stage kidney disease.
a 58.2% of patients enrolled in DAPA-HF did not have diabetes mellitus. All patients enrolled in DAPA-HF had HF with reduced ejection fraction,
b Mean duration of diabetes was not provided for EMPA-REG OUTCOME, but 57% of patients enrolled had diabetes for more than 10 years.
| REWIND | LEADER | SUSTAIN-6 | |
|---|---|---|---|
| Patients enrolled | 9,901 | 9,340 | 3,297 |
| Drug | Dulaglutide | Liraglutide | Semaglutide SC |
| Dose | 1.5 mg per week | 1.8 mg or max tolerated dose per day | 0.5 mg or 1 mg per week |
| Median duration of follow-up, yr | 5.4 | 3.8 | 2.1 |
| Mean baseline A1C, % | 7.2 | 8.7 | 8.7 |
| Mean duration of diabetes, yr | 9.5 | 12.8 | 13.9 |
| Baseline statin use, % | 66 | 72 | 73 |
| Baseline prevalence of ASCVD/HF, % | 31 | 81 | 72 |
| Baseline prevalence of HF, % | 9 | 18 | 24 |
| Primary outcome, 3-MACEa | 0.88 (0.79–0.99) | 0.87 (0.78–0.97) | 0.74 (0.58–0.95) |
| CV death | 0.91 (0.78–1.06) | 0.78 (0.66–0.93) | 0.98 (0.65–1.48) |
| Fatal or nonfatal MIb | 0.96 (0.79–1.15) | 0.86 (0.73–1.00) | 0.74 (0.51–1.08) |
| Fatal or nonfatal strokeb | 0.76 (0.62–0.94) | 0.86 (0.71–1.06) | 0.61 (0.38–0.99) |
| All-cause mortality | 0.90 (0.80–1.01) | 0.85 (0.74–0.97) | 1.05 (0.74–1.50) |
| HF hospitalizationc | 0.93 (0.77–1.12)c | 0.87 (0.73–1.05) | 0.86 (0.48–1.55) |
| Renal composite outcomed | 0.85 (0.77–0.93) | 0.78 (0.67–0.92) | 0.64 (0.46–0.88) |
Values are presented as hazard ratio (95% confidence interval).
GLP-1, glucagon-like peptide-1; SC, subcutaneous; A1C, glycosylated hemoglobin; ASCVD, atherosclerotic cardiovascular disease; HF, heart failure; 3-MACE, 3-point major adverse cardiovascular event; CV, cardiovascular; MI, myocardial infarction.
a Three-point MACE is a composite of CV death, MI, or stroke,
b The risk estimates and 95% CIs for SUSTAIN-6 is for nonfatal MI (excluding fatal MI) or nonfatal stroke (excluding fatal stroke). The effect estimates for the composite endpoints of fatal or nonfatal MI and fatal or nonfatal stroke were not available in the primary manuscripts,
c Urgent HF visit or hospitalization for HF,
d The renal composite outcome reported in a recent meta-analysis was a composite of the development of macroalbuminuria, doubling of serum creatinine, a ≥40% decline in estimated glomerular filtration rate (eGFR), development of end-stage kidney disease, or death due to renal causes. For SUSTAIN-6, the renal composite was persistent macroalbuminuria, persistent doubling of serum creatinine with an eGFR <45 mL/min/1.73 m2 or need for continuous renal replacement therapy.
- 1. Jung CH, Son JW, Kang S, Kim WJ, Kim HS, Kim HS, et al. Diabetes fact sheets in Korea, 2020: an appraisal of current status. Diabetes Metab J 2021;45:1-10.ArticlePubMedPMCPDF
- 2. Kim MK, Ko SH, Kim BY, Kang ES, Noh J, Kim SK, et al. 2019 Clinical practice guidelines for type 2 diabetes mellitus in Korea. Diabetes Metab J 2019;43:398-406.ArticlePubMedPMCPDF
- 3. Korean Diabetes Association. Treatment guideline for diabetes 6th ed Available from: kdaguideline.com (cited 2021 Jul 16).
- 4. American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2018. Diabetes Care 2018;41(Suppl 1):S13-27.ArticlePubMedPDF
- 5. Ko SH, Kim SR, Kim DJ, Oh SJ, Lee HJ, Shim KH, et al. 2011 Clinical practice guidelines for type 2 diabetes in Korea. Diabetes Metab J 2011;35:431-6.ArticlePubMedPMC
- 6. Oh JY, Lim S, Kim DJ, Kim NH, Kim DJ, Moon SD, et al. A report on the diagnosis of intermediate hyperglycemia in Korea: a pooled analysis of four community-based cohort studies. Diabetes Res Clin Pract 2008;80:463-8.ArticlePubMed
- 7. Rhee SY, Chon S, Ahn KJ, Woo JT; Korean Diabetes Prevention Study Investigators. Hospital-based Korean Diabetes Prevention Study: a prospective, multi-center, randomized, openlabel controlled study. Diabetes Metab J 2019;43:49-58.ArticlePubMedPDF
- 8. Lee JH, Chon S, Cha SA, Lim SY, Kim KR, Yun JS, et al. Impaired fasting glucose levels in overweight or obese subjects for screening of type 2 diabetes in Korea. Korean J Intern Med 2021;36:382-91.ArticlePubMedPDF
- 9. Pan XR, Li GW, Hu YH, Wang JX, Yang WY, An ZX, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance: the Da Qing IGT and Diabetes Study. Diabetes Care 1997;20:537-44.ArticlePubMedPDF
- 10. Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, Ilanne-Parikka P, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 2001;344:1343-50.ArticlePubMed
- 11. Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393-403.ArticlePubMedPMC
- 12. Lee JH, Lim SY, Cha SA, Han CJ, Jung AR, Kim KR, et al. Short-term effects of the Internet-based Korea Diabetes Prevention Study: 6-month results of a community-based randomized controlled trial. Diabetes Metab J 2021 Mar 17 [Epub]. https://doi.org/10.4093/dmj.2020.0225.Article
- 13. Ramachandran A, Snehalatha C, Mary S, Mukesh B, Bhaskar AD, Vijay V, et al. The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1). Diabetologia 2006;49:289-97.ArticlePubMedPDF
- 14. Diabetes Control and Complications Trial Research Group, Nathan DM, Genuth S, Lachin J, Cleary P, Crofford O, et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulindependent diabetes mellitus. N Engl J Med 1993;329:977-86.ArticlePubMed
- 15. UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352:837-53.ArticlePubMed
- 16. Ohkubo Y, Kishikawa H, Araki E, Miyata T, Isami S, Motoyoshi S, et al. Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: a randomized prospective 6-year study. Diabetes Res Clin Pract 1995;28:103-17.ArticlePubMed
- 17. Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-Year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008;359:1577-89.ArticlePubMed
- 18. Hayward RA, Reaven PD, Wiitala WL, Bahn GD, Reda DJ, Ge L, et al. Follow-up of glycemic control and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2015;372:2197-206.ArticlePubMed
- 19. Lee AK, Warren B, Lee CJ, McEvoy JW, Matsushita K, Huang ES, et al. The association of severe hypoglycemia with incident cardiovascular events and mortality in adults with type 2 diabetes. Diabetes Care 2018;41:104-11.ArticlePubMedPDF
- 20. Writing Group for the DCCT/EDIC Research Group, Orchard TJ, Nathan DM, Zinman B, Cleary P, Brillon D, et al. Association between 7 years of intensive treatment of type 1 diabetes and long-term mortality. JAMA 2015;313:45-53.ArticlePubMedPMC
- 21. ACCORD Study Group, Cushman WC, Evans GW, Byington RP, Goff DC Jr, Grimm RH Jr, et al. Effects of intensive bloodpressure control in type 2 diabetes mellitus. N Engl J Med 2010;362:1575-85.ArticlePubMedPMC
- 22. SPRINT Research Group, Wright JT Jr, Williamson JD, Whelton PK, Snyder JK, Sink KM, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015;373:2103-16.ArticlePubMedPMC
- 23. Buckley LF, Dixon DL, Wohlford GF 4th, Wijesinghe DS, Baker WL, Van Tassell BW. Intensive versus standard blood pressure control in SPRINT-eligible participants of AC-CORD-BP. Diabetes Care 2017;40:1733-8.ArticlePubMedPDF
- 24. UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. BMJ 1998;317:703-13.ArticlePubMedPMC
- 25. Hansson L, Zanchetti A, Carruthers SG, Dahlof B, Elmfeldt D, Julius S, et al. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. HOT Study Group. Lancet 1998;351:1755-62.PubMed
- 26. Bakris GL, Gaxiola E, Messerli FH, Mancia G, Erdine S, Cooper-DeHoff R, et al. Clinical outcomes in the diabetes cohort of the INternational VErapamil SR-Trandolapril study. Hypertension 2004;44:637-42.ArticlePubMed
- 27. Turnbull F, Neal B, Algert C, Chalmers J, Chapman N, Cutler J, et al. Effects of different blood pressure-lowering regimens on major cardiovascular events in individuals with and without diabetes mellitus: results of prospectively designed overviews of randomized trials. Arch Intern Med 2005;165:1410-9.ArticlePubMed
- 28. Bangalore S, Fakheri R, Toklu B, Messerli FH. Diabetes mellitus as a compelling indication for use of renin angiotensin system blockers: systematic review and meta-analysis of randomized trials. BMJ 2016;352:i438.ArticlePubMedPMC
- 29. Arnold SV, Bhatt DL, Barsness GW, Beatty AL, Deedwania PC, Inzucchi SE, et al. Clinical management of stable coronary artery disease in patients with type 2 diabetes mellitus: a scientific statement from the American Heart Association. Circulation 2020;141:e779-806.ArticlePubMed
- 30. Lindholm LH, Ibsen H, Dahlof B, Devereux RB, Beevers G, de Faire U, et al. Cardiovascular morbidity and mortality in patients with diabetes in the Losartan Intervention for Endpoint Reduction in Hypertension Study (LIFE): a randomised trial against atenolol. Lancet 2002;359:1004-10.ArticlePubMed
- 31. Berl T, Hunsicker LG, Lewis JB, Pfeffer MA, Porush JG, Rouleau JL, et al. Cardiovascular outcomes in the Irbesartan Diabetic Nephropathy Trial of patients with type 2 diabetes and overt nephropathy. Ann Intern Med 2003;138:542-9.ArticlePubMed
- 32. Palmer SC, Mavridis D, Navarese E, Craig JC, Tonelli M, Salanti G, et al. Comparative efficacy and safety of blood pressure-lowering agents in adults with diabetes and kidney disease: a network meta-analysis. Lancet 2015;385:2047-56.ArticlePubMed
- 33. Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebocontrolled trial. Lancet 2002;360:7-22.ArticlePubMed
- 34. Cholesterol Treatment Trialists’ (CTT) Collaboration, Fulcher J, O’Connell R, Voysey M, Emberson J, Blackwell L, et al. Efficacy and safety of LDL-lowering therapy among men and women: meta-analysis of individual data from 174,000 participants in 27 randomised trials. Lancet 2015;385:1397-405.ArticlePubMed
- 35. Brugts JJ, Yetgin T, Hoeks SE, Gotto AM, Shepherd J, Westendorp RG, et al. The benefits of statins in people without established cardiovascular disease but with cardiovascular risk factors: meta-analysis of randomised controlled trials. BMJ 2009;338:b2376.ArticlePubMedPMC
- 36. Colhoun HM, Betteridge DJ, Durrington PN, Hitman GA, Neil HA, Livingstone SJ, et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet 2004;364:685-96.ArticlePubMed
- 37. Cholesterol Treatment Trialists’ (CTT) Collaborators, Kearney PM, Blackwell L, Collins R, Keech A, Simes J, et al. Efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta-analysis. Lancet 2008;371:117-25.ArticlePubMed
- 38. Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med 2015;372:2387-97.ArticlePubMed
- 39. Hong N, Lee YH, Tsujita K, Gonzalez JA, Kramer CM, Kovarnik T, et al. Comparison of the effects of ezetimibe-statin combination therapy on major adverse cardiovascular events in patients with and without diabetes: a meta-analysis. Endocrinol Metab (Seoul) 2018;33:219-27.ArticlePubMedPMCPDF
- 40. Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med 2017;376:1713-22.ArticlePubMed
- 41. Schwartz GG, Steg PG, Szarek M, Bhatt DL, Bittner VA, Diaz R, et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med 2018;379:2097-107.ArticlePubMed
- 42. American Diabetes Association. 10. Cardiovascular disease and risk management: standards of medical care in diabetes-2020. Diabetes Care 2020;43(Suppl 1):S111-34.ArticlePubMedPDF
- 43. Kim BY, Kang SM, Kang JH, Kang SY, Kim KK, Kim KB, et al. 2020 Korean Society for the Study of Obesity guidelines for the management of obesity in Korea. J Obes Metab Syndr 2021;30:81-92.ArticlePubMedPMC
- 44. Look AHEAD Research Group, Wing RR, Bolin P, Brancati FL, Bray GA, Clark JM, et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med 2013;369:145-54.ArticlePubMedPMC
- 45. Lean ME, Leslie WS, Barnes AC, Brosnahan N, Thom G, McCombie L, et al. Primary care-led weight management for remission of type 2 diabetes (DiRECT): an open-label, clusterrandomised trial. Lancet 2018;391:541-51.ArticlePubMed
- 46. Gadde KM, Allison DB, Ryan DH, Peterson CA, Troupin B, Schwiers ML, et al. Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): a randomised, placebo-controlled, phase 3 trial. Lancet 2011;377:1341-52.ArticlePubMed
- 47. Davies MJ, Bergenstal R, Bode B, Kushner RF, Lewin A, Skjoth TV, et al. Efficacy of liraglutide for weight loss among patients with type 2 diabetes: the SCALE diabetes randomized clinical trial. JAMA 2015;314:687-99.ArticlePubMed
- 48. Hollander P, Gupta AK, Plodkowski R, Greenway F, Bays H, Burns C, et al. Effects of naltrexone sustained-release/bupropion sustained-release combination therapy on body weight and glycemic parameters in overweight and obese patients with type 2 diabetes. Diabetes Care 2013;36:4022-9.ArticlePubMedPMCPDF
- 49. Cohen R, Le Roux CW, Junqueira S, Ribeiro RA, Luque A. Roux-en-Y gastric bypass in type 2 diabetes patients with mild obesity: a systematic review and meta-analysis. Obes Surg 2017;27:2733-9.ArticlePubMedPDF
- 50. Yeo D, Yeo C, Low TY, Ahmed S, Phua S, Oo AM, et al. Outcomes after metabolic surgery in asians-a meta-analysis. Obes Surg 2019;29:114-26.ArticlePubMedPDF
- 51. Bell KJ, Barclay AW, Petocz P, Colagiuri S, Brand-Miller JC. Efficacy of carbohydrate counting in type 1 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol 2014;2:133-40.ArticlePubMed
- 52. Rytter K, Schmidt S, Rasmussen LN, Pedersen-Bjergaard U, Norgaard K. Education programmes for persons with type 1 diabetes using an insulin pump: a systematic review. Diabetes Metab Res Rev 2021;37:e3412.ArticlePubMedPDF
- 53. DAFNE Study Group. Training in flexible, intensive insulin management to enable dietary freedom in people with type 1 diabetes: dose adjustment for normal eating (DAFNE) randomised controlled trial. BMJ 2002;325:746.ArticlePubMedPMC
- 54. Sanchez-Hernandez RM, Alvarado-Martel D, Lopez-Plasencia Y, Carrillo-Dominguez A, Jimenez-Rodriguez A, Rodriguez-Cordero J, et al. Assessment of Alimentacion Normal con Ajuste de Insulina (ANAIS), a Spanish version of the DAFNE programme, in people with type 1 diabetes: a randomized controlled parallel trial. Diabet Med 2019;36:1037-45.ArticlePubMedPDF
- 55. Little SA, Speight J, Leelarathna L, Walkinshaw E, Tan HK, Bowes A, et al. Sustained reduction in severe hypoglycemia in adults with type 1 diabetes complicated by impaired awareness of hypoglycemia: two-year follow-up in the HypoCOMPaSS randomized clinical trial. Diabetes Care 2018;41:1600-7.ArticlePubMedPDF
- 56. Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005;353:2643-53.ArticlePubMedPMC
- 57. Writing Team for the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. Effect of intensive therapy on the microvascular complications of type 1 diabetes mellitus. JAMA 2002;287:2563-9.ArticlePubMedPMC
- 58. Chico A, Corcoy R. Intensive insulin therapy (≃ Basal-Bolus). Am J Ther 2020 Oct 6 [Epub]. https://doi.org/10.1097/MJT.0000000000001152.Article
- 59. Bergenstal RM, Tamborlane WV, Ahmann A, Buse JB, Dailey G, Davis SN, et al. Effectiveness of sensor-augmented insulinpump therapy in type 1 diabetes. N Engl J Med 2010;363:311-20.ArticlePubMed
- 60. Laranjeira FO, de Andrade KR, Figueiredo AC, Silva EN, Pereira MG. Long-acting insulin analogues for type 1 diabetes: an overview of systematic reviews and meta-analysis of randomized controlled trials. PLoS One 2018;13:e0194801.ArticlePubMedPMC
- 61. Melo KF, Bahia LR, Pasinato B, Porfirio GJ, Martimbianco AL, Riera R, et al. Short-acting insulin analogues versus regular human insulin on postprandial glucose and hypoglycemia in type 1 diabetes mellitus: a systematic review and meta-analysis. Diabetol Metab Syndr 2019;11:2.ArticlePubMedPMCPDF
- 62. Holman RR, Thorne KI, Farmer AJ, Davies MJ, Keenan JF, Paul S, et al. Addition of biphasic, prandial, or basal insulin to oral therapy in type 2 diabetes. N Engl J Med 2007;357:1716-30.ArticlePubMed
- 63. Yoon KH, Shin JA, Kwon HS, Lee SH, Min KW, Ahn YB, et al. Comparison of the efficacy of glimepiride, metformin, and rosiglitazone monotherapy in Korean drug-naive type 2 diabetic patients: the practical evidence of antidiabetic monotherapy study. Diabetes Metab J 2011;35:26-33.ArticlePubMedPMC
- 64. Matthews DR, Paldanius PM, Proot P, Chiang Y, Stumvoll M, Del Prato S, et al. Glycaemic durability of an early combination therapy with vildagliptin and metformin versus sequential metformin monotherapy in newly diagnosed type 2 diabetes (VERIFY): a 5-year, multicentre, randomised, doubleblind trial. Lancet 2019;394:1519-29.ArticlePubMed
- 65. Yoo SJ, Chang SA, Sohn TS, Kwon HS, Lee JM, Moon S, et al. Long-term glycaemic durability of early combination therapy strategy versus metformin monotherapy in Korean patients with newly diagnosed type 2 diabetes mellitus. Diabetes Metab J 2020 Nov 12 [Epub]. https://doi.org/10.4093/dmj.2020.0173.Article
- 66. Schectman JM, Nadkarni MM, Voss JD. The association between diabetes metabolic control and drug adherence in an indigent population. Diabetes Care 2002;25:1015-21.ArticlePubMedPDF
- 67. Tsapas A, Avgerinos I, Karagiannis T, Malandris K, Manolopoulos A, Andreadis P, et al. Comparative effectiveness of glucose-lowering drugs for type 2 diabetes: a systematic review and network meta-analysis. Ann Intern Med 2020;173:278-86.PubMed
- 68. Davies MJ, Donnelly R, Barnett AH, Jones S, Nicolay C, Kilcoyne A. Exenatide compared with long-acting insulin to achieve glycaemic control with minimal weight gain in patients with type 2 diabetes: results of the Helping Evaluate Exenatide in patients with diabetes compared with Long-Acting insulin (HEELA) study. Diabetes Obes Metab 2009;11:1153-62.ArticlePubMedPMC
- 69. Heine RJ, Van Gaal LF, Johns D, Mihm MJ, Widel MH, Brodows RG, et al. Exenatide versus insulin glargine in patients with suboptimally controlled type 2 diabetes: a randomized trial. Ann Intern Med 2005;143:559-69.ArticlePubMed
- 70. Russell-Jones D, Vaag A, Schmitz O, Sethi BK, Lalic N, Antic S, et al. Liraglutide vs insulin glargine and placebo in combination with metformin and sulfonylurea therapy in type 2 diabetes mellitus (LEAD-5 met+SU): a randomised controlled trial. Diabetologia 2009;52:2046-55.ArticlePubMedPMC
- 71. D’Alessio D, Haring HU, Charbonnel B, de Pablos-Velasco P, Candelas C, Dain MP, et al. Comparison of insulin glargine and liraglutide added to oral agents in patients with poorly controlled type 2 diabetes. Diabetes Obes Metab 2015;17:170-8.ArticlePubMedPDF
- 72. Gough SC, Bode B, Woo V, Rodbard HW, Linjawi S, Poulsen P, et al. Efficacy and safety of a fixed-ratio combination of insulin degludec and liraglutide (IDegLira) compared with its components given alone: results of a phase 3, open-label, randomised, 26-week, treat-to-target trial in insulin-naive patients with type 2 diabetes. Lancet Diabetes Endocrinol 2014;2:885-93.ArticlePubMed
- 73. Gough SC, Bode BW, Woo VC, Rodbard HW, Linjawi S, Zacho M, et al. One-year efficacy and safety of a fixed combination of insulin degludec and liraglutide in patients with type 2 diabetes: results of a 26-week extension to a 26-week main trial. Diabetes Obes Metab 2015;17:965-73.ArticlePubMedPMCPDF
- 74. Araki E, Inagaki N, Tanizawa Y, Oura T, Takeuchi M, Imaoka T. Efficacy and safety of once-weekly dulaglutide in combination with sulphonylurea and/or biguanide compared with once-daily insulin glargine in Japanese patients with type 2 diabetes: a randomized, open-label, phase III, non-inferiority study. Diabetes Obes Metab 2015;17:994-1002.ArticlePubMedPMCPDF
- 75. Blonde L, Jendle J, Gross J, Woo V, Jiang H, Fahrbach JL, et al. Once-weekly dulaglutide versus bedtime insulin glargine, both in combination with prandial insulin lispro, in patients with type 2 diabetes (AWARD-4): a randomised, open-label, phase 3, non-inferiority study. Lancet 2015;385:2057-66.ArticlePubMed
- 76. Giorgino F, Benroubi M, Sun JH, Zimmermann AG, Pechtner V. Efficacy and safety of once-weekly dulaglutide versus insulin glargine in patients with type 2 diabetes on metformin and glimepiride (AWARD-2). Diabetes Care 2015;38:2241-9.ArticlePubMedPDF
- 77. Aroda VR, Bain SC, Cariou B, Piletic M, Rose L, Axelsen M, et al. Efficacy and safety of once-weekly semaglutide versus once-daily insulin glargine as add-on to metformin (with or without sulfonylureas) in insulin-naive patients with type 2 diabetes (SUSTAIN 4): a randomised, open-label, parallelgroup, multicentre, multinational, phase 3a trial. Lancet Diabetes Endocrinol 2017;5:355-66.ArticlePubMed
- 78. DeVries JH, Bain SC, Rodbard HW, Seufert J, D’Alessio D, Thomsen AB, et al. Sequential intensification of metformin treatment in type 2 diabetes with liraglutide followed by randomized addition of basal insulin prompted by A1C targets. Diabetes Care 2012;35:1446-54.ArticlePubMedPMCPDF
- 79. Aroda VR, Bailey TS, Cariou B, Kumar S, Leiter LA, Raskin P, et al. Effect of adding insulin degludec to treatment in patients with type 2 diabetes inadequately controlled with metformin and liraglutide: a double-blind randomized controlled trial (BEGIN: ADD TO GLP-1 Study). Diabetes Obes Metab 2016;18:663-70.ArticlePubMedPMCPDF
- 80. Rosenstock J, Aronson R, Grunberger G, Hanefeld M, Piatti P, Serusclat P, et al. Benefits of lixilan, a titratable fixed-ratio combination of insulin glargine plus lixisenatide, versus insulin glargine and lixisenatide monocomponents in type 2 diabetes inadequately controlled on oral agents: the LixiLan-O Randomized Trial. Diabetes Care 2016;39:2026-35.ArticlePubMedPDF
- 81. Blonde L, Rosenstock J, Del Prato S, Henry R, Shehadeh N, Frias J, et al. Switching to iGlarLixi versus continuing daily or weekly GLP-1 RA in type 2 diabetes inadequately controlled by GLP-1 RA and oral antihyperglycemic therapy: the LixiLan-G Randomized Clinical Trial. Diabetes Care 2019;42:2108-16.ArticlePubMedPDF
- 82. Linjawi S, Bode BW, Chaykin LB, Courreges JP, Handelsman Y, Lehmann LM, et al. The efficacy of IDegLira (insulin degludec/liraglutide combination) in adults with type 2 diabetes inadequately controlled with a GLP-1 receptor agonist and oral therapy: DUAL III Randomized Clinical Trial. Diabetes Ther 2017;8:101-14.ArticlePubMedPDF
- 83. Buse JB, Bergenstal RM, Glass LC, Heilmann CR, Lewis MS, Kwan AY, et al. Use of twice-daily exenatide in basal insulintreated patients with type 2 diabetes: a randomized, controlled trial. Ann Intern Med 2011;154:103-12.ArticlePubMed
- 84. Seino Y, Min KW, Niemoeller E, Takami A A; EFC10887 GET-GOAL-L Asia Study Investigators. Randomized, double-blind, placebo-controlled trial of the once-daily GLP-1 receptor agonist lixisenatide in Asian patients with type 2 diabetes insufficiently controlled on basal insulin with or without a sulfonylurea (GetGoal-L-Asia). Diabetes Obes Metab 2012;14:910-7.PubMedPMC
- 85. Riddle MC, Aronson R, Home P, Marre M, Niemoeller E, Miossec P, et al. Adding once-daily lixisenatide for type 2 diabetes inadequately controlled by established basal insulin: a 24-week, randomized, placebo-controlled comparison (GetGoal-L). Diabetes Care 2013;36:2489-96.PubMedPMC
- 86. Riddle MC, Forst T, Aronson R, Sauque-Reyna L, Souhami E, Silvestre L, et al. Adding once-daily lixisenatide for type 2 diabetes inadequately controlled with newly initiated and continuously titrated basal insulin glargine: a 24-week, randomized, placebo-controlled study (GetGoal-Duo 1). Diabetes Care 2013;36:2497-503.PubMedPMC
- 87. Yang W, Min K, Zhou Z, Li L, Xu X, Zhu D, et al. Efficacy and safety of lixisenatide in a predominantly Asian population with type 2 diabetes insufficiently controlled with basal insulin: the GetGoal-L-C randomized trial. Diabetes Obes Metab 2018;20:335-43.PubMed
- 88. Ahmann A, Rodbard HW, Rosenstock J, Lahtela JT, de Loredo L, Tornoe K, et al. Efficacy and safety of liraglutide versus placebo added to basal insulin analogues (with or without metformin) in patients with type 2 diabetes: a randomized, placebo-controlled trial. Diabetes Obes Metab 2015;17:1056-64.ArticlePubMedPMCPDF
- 89. Pozzilli P, Norwood P, Jodar E, Davies MJ, Ivanyi T, Jiang H, et al. Placebo-controlled, randomized trial of the addition of once-weekly glucagon-like peptide-1 receptor agonist dulaglutide to titrated daily insulin glargine in patients with type 2 diabetes (AWARD-9). Diabetes Obes Metab 2017;19:1024-31.ArticlePubMedPDF
- 90. Rodbard HW, Lingvay I, Reed J, de la Rosa R, Rose L, Sugimoto D, et al. Semaglutide added to basal insulin in type 2 diabetes (SUSTAIN 5): a randomized, controlled trial. J Clin Endocrinol Metab 2018;103:2291-301.ArticlePubMedPMC
- 91. Aroda VR, Rosenstock J, Wysham C, Unger J, Bellido D, Gonzalez-Galvez G, et al. Efficacy and safety of lixilan, a titratable fixed-ratio combination of insulin glargine plus lixisenatide in type 2 diabetes inadequately controlled on basal insulin and metformin: the LixiLan-L randomized trial. Diabetes Care 2016;39:1972-80.ArticlePubMedPDF
- 92. Rosenstock J, Diamant M, Aroda VR, Silvestre L, Souhami E, Zhou T, et al. Efficacy and safety of lixilan, a titratable fixedratio combination of lixisenatide and insulin glargine, versus insulin glargine in type 2 diabetes inadequately controlled on metformin monotherapy: the lixilan proof-of-concept randomized trial. Diabetes Care 2016;39:1579-86.ArticlePubMedPMCPDF
- 93. Buse JB, Vilsboll T, Thurman J, Blevins TC, Langbakke IH, Bottcher SG, et al. Contribution of liraglutide in the fixed-ratio combination of insulin degludec and liraglutide (IDegLira). Diabetes Care 2014;37:2926-33.ArticlePubMedPDF
- 94. Lingvay I, Perez Manghi F, Garcia-Hernandez P, Norwood P, Lehmann L, Tarp-Johansen MJ, et al. Effect of insulin glargine up-titration vs insulin degludec/liraglutide on glycated hemoglobin levels in patients with uncontrolled type 2 diabetes: the DUAL V Randomized Clinical Trial. JAMA 2016;315:898-907.ArticlePubMed
- 95. Lankisch MR, Ferlinz KC, Leahy JL, Scherbaum WA; Orals Plus Apidra and LANTUS (OPAL) study group. Introducing a simplified approach to insulin therapy in type 2 diabetes: a comparison of two single-dose regimens of insulin glulisine plus insulin glargine and oral antidiabetic drugs. Diabetes Obes Metab 2008;10:1178-85.ArticlePubMed
- 96. Leahy JL. Insulin therapy in type 2 diabetes mellitus. Endocrinol Metab Clin North Am 2012;41:119-44.ArticlePubMed
- 97. Rodbard HW, Visco VE, Andersen H, Hiort LC, Shu DH. Treatment intensification with stepwise addition of prandial insulin aspart boluses compared with full basal-bolus therapy (FullSTEP Study): a randomised, treat-to-target clinical trial. Lancet Diabetes Endocrinol 2014;2:30-7.ArticlePubMed
- 98. Aschner P, Sethi B, Gomez-Peralta F, Landgraf W, Loizeau V, Dain MP, et al. Insulin glargine compared with premixed insulin for management of insulin-naïve type 2 diabetes patients uncontrolled on oral antidiabetic drugs: the open-label, randomized GALAPAGOS study. J Diabetes Complications 2015;29:838-45.ArticlePubMed
- 99. Owens DR, Luzio SD, Sert-Langeron C, Riddle MC. Effects of initiation and titration of a single pre-prandial dose of insulin glulisine while continuing titrated insulin glargine in type 2 diabetes: a 6-month ‘proof-of-concept’ study. Diabetes Obes Metab 2011;13:1020-7.ArticlePubMedPMC
- 100. Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015;373:2117-28.ArticlePubMed
- 101. Kaku K, Lee J, Mattheus M, Kaspers S, George J, Woerle HJ, et al. Empagliflozin and cardiovascular outcomes in asian patients with type 2 diabetes and established cardiovascular disease: results from EMPA-REG OUTCOME®. Circ J 2017;81:227-34.ArticlePubMed
- 102. Gerstein HC, Colhoun HM, Dagenais GR, Diaz R, Lakshmanan M, Pais P, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet 2019;394:121-30.PubMed
- 103. Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016;375:311-22.ArticlePubMedPMC
- 104. Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jodar E, Leiter LA, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2016;375:1834-44.ArticlePubMed
- 105. Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2019;380:347-57.ArticlePubMed
- 106. Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med 2020;383:1413-24.PubMed
- 107. Cannon CP, Pratley R, Dagogo-Jack S, Mancuso J, Huyck S, Masiukiewicz U, et al. Cardiovascular outcomes with ertugliflozin in type 2 diabetes. N Engl J Med 2020;383:1425-35.ArticlePubMed
- 108. Wanner Ch, Inzucchi SE, Zinman B. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med 2016;375:1801-2.ArticlePubMed
- 109. Heerspink HJ, Stefansson BV, Correa-Rotter R, Chertow GM, Greene T, Hou FF, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med 2020;383:1436-46.ArticlePubMed
- 110. Levin A, Stevens PE, Bilous RW, Coresh J, De Francisco AL, De Jong PE, et al. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2013;3:1-150.
- 111. Kasiske BL, Lakatua JD, Ma JZ, Louis TA. A meta-analysis of the effects of dietary protein restriction on the rate of decline in renal function. Am J Kidney Dis 1998;31:954-61.ArticlePubMed
- 112. Wheeler ML, Dunbar SA, Jaacks LM, Karmally W, Mayer-Davis EJ, Wylie-Rosett J, et al. Macronutrients, food groups, and eating patterns in the management of diabetes: a systematic review of the literature, 2010. Diabetes Care 2012;35:434-45.PubMedPMC
- 113. Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med 1993;329:1456-62.ArticlePubMed
- 114. Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001;345:861-9.ArticlePubMed
- 115. Heart Outcomes Prevention Evaluation Study Investigators. Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Lancet 2000;355:253-9.ArticlePubMed
- 116. Mauer M, Zinman B, Gardiner R, Suissa S, Sinaiko A, Strand T, et al. Renal and retinal effects of enalapril and losartan in type 1 diabetes. N Engl J Med 2009;361:40-51.ArticlePubMedPMC
- 117. Wanner C, Inzucchi SE, Lachin JM, Fitchett D, von Eynatten M, Mattheus M, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med 2016;375:323-34.ArticlePubMed
- 118. Diabetes Canada Clinical Practice Guidelines Expert Committee, Punthakee Z, Goldenberg R, Katz P. Definition, classification and diagnosis of diabetes, prediabetes and metabolic syndrome. Can J Diabetes 2018;42 Suppl 1:S10-5.ArticlePubMed
- 119. Saudek CD, Herman WH, Sacks DB, Bergenstal RM, Edelman D, Davidson MB. A new look at screening and diagnosing diabetes mellitus. J Clin Endocrinol Metab 2008;93:2447-53.ArticlePubMedPDF
- 120. Pop-Busui R, Low PA, Waberski BH, Martin CL, Albers JW, Feldman EL, et al. Effects of prior intensive insulin therapy on cardiac autonomic nervous system function in type 1 diabetes mellitus: the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study (DCCT/EDIC). Circulation 2009;119:2886-93.ArticlePubMedPMC
- 121. Waldfogel JM, Nesbit SA, Dy SM, Sharma R, Zhang A, Wilson LM, et al. Pharmacotherapy for diabetic peripheral neuropathy pain and quality of life: a systematic review. Neurology 2017;88:1958-67.ArticlePubMed
- 122. Finnerup NB, Attal N, Haroutounian S, McNicol E, Baron R, Dworkin RH, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol 2015;14:162-73.ArticlePubMedPMC
- 123. Boulton AJ, Armstrong DG, Albert SF, Frykberg RG, Hellman R, Kirkman MS, et al. Comprehensive foot examination and risk assessment: a report of the task force of the foot care interest group of the American Diabetes Association, with endorsement by the American Association of Clinical Endocrinologists. Diabetes Care 2008;31:1679-85.PubMedPMC
- 124. Hingorani A, LaMuraglia GM, Henke P, Meissner MH, Loretz L, Zinszer KM, et al. The management of diabetic foot: a clinical practice guideline by the Society for Vascular Surgery in collaboration with the American Podiatric Medical Association and the Society for Vascular Medicine. J Vasc Surg 2016;63(2 Suppl):3S-21S.ArticlePubMed
- 125. The effect of intensive diabetes treatment on the progression of diabetic retinopathy in insulin-dependent diabetes mellitus: the diabetes control and complications trial. Arch Ophthalmol 1995;113:36-51.ArticlePubMed
- 126. Diabetes Control and Complications Trial Research Group. Effect of pregnancy on microvascular complications in the diabetes control and complications trial. The Diabetes Control and Complications Trial Research Group. Diabetes Care 2000;23:1084-91.ArticlePubMedPDF
- 127. Chew EY, Klein ML, Murphy RP, Remaley NA, Ferris FL 3rd. Effects of aspirin on vitreous/preretinal hemorrhage in patients with diabetes mellitus: Early Treatment Diabetic Retinopathy Study report no. 20. Arch Ophthalmol 1995;113:52-5.ArticlePubMed
- 128. The Diabetic Retinopathy Study Research Group. Preliminary report on effects of photocoagulation therapy. Am J Ophthalmol 1976;81:383-96.ArticlePubMed
- 129. Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018;67:328-57.ArticlePubMedPDF
- 130. Koutoukidis DA, Astbury NM, Tudor KE, Morris E, Henry JA, Noreik M, et al. Association of weight loss interventions with changes in biomarkers of nonalcoholic fatty liver disease: a systematic review and meta-analysis. JAMA Intern Med 2019;179:1262-71.ArticlePubMedPMC
- 131. Musso G, Cassader M, Paschetta E, Gambino R. Thiazolidinediones and advanced liver fibrosis in nonalcoholic steatohepatitis: a meta-analysis. JAMA Intern Med 2017;177:633-40.ArticlePubMedPMC
- 132. Armstrong MJ, Gaunt P, Aithal GP, Barton D, Hull D, Parker R, et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet 2016;387:679-90.ArticlePubMed
- 133. Tang W, Xu Q, Hong T, Tong G, Feng W, Shen S, et al. Comparative efficacy of anti-diabetic agents on nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus: a systematic review and meta-analysis of randomized and nonrandomized studies. Diabetes Metab Res Rev 2016;32:200-16.ArticlePubMed
- 134. Battelino T, Danne T, Bergenstal RM, Amiel SA, Beck R, Biester T, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the International Consensus on Time in Range. Diabetes Care 2019;42:1593-603.PubMedPMC
- 135. Heinemann L, Freckmann G, Ehrmann D, Faber-Heinemann G, Guerra S, Waldenmaier D, et al. Real-time continuous glucose monitoring in adults with type 1 diabetes and impaired hypoglycaemia awareness or severe hypoglycaemia treated with multiple daily insulin injections (HypoDE): a multicentre, randomised controlled trial. Lancet 2018;391:1367-77.ArticlePubMed
- 136. Battelino T, Phillip M, Bratina N, Nimri R, Oskarsson P, Bolinder J. Effect of continuous glucose monitoring on hypoglycemia in type 1 diabetes. Diabetes Care 2011;34:795-800.ArticlePubMedPMCPDF
- 137. Seyed Ahmadi S, Westman K, Pivodic A, Olafsdottir AF, Dahlqvist S, Hirsch IB, et al. The association between HbA1c and time in hypoglycemia during CGM and self-monitoring of blood glucose in people with type 1 diabetes and multiple daily insulin injections: a randomized clinical trial (GOLD-4). Diabetes Care 2020;43:2017-24.ArticlePubMedPMCPDF
- 138. Beck RW, Riddlesworth TD, Ruedy K, Ahmann A, Haller S, Kruger D, et al. Continuous glucose monitoring versus usual care in patients with type 2 diabetes receiving multiple daily insulin injections: a randomized trial. Ann Intern Med 2017;167:365-74.ArticlePubMed
- 139. Vigersky RA, Fonda SJ, Chellappa M, Walker MS, Ehrhardt NM. Short- and long-term effects of real-time continuous glucose monitoring in patients with type 2 diabetes. Diabetes Care 2012;35:32-8.ArticlePubMedPDF
- 140. Feig DS, Donovan LE, Corcoy R, Murphy KE, Amiel SA, Hunt KF, et al. Continuous glucose monitoring in pregnant women with type 1 diabetes (CONCEPTT): a multicentre international randomised controlled trial. Lancet 2017;390:2347-59.PubMedPMC
- 141. Yeh HC, Brown TT, Maruthur N, Ranasinghe P, Berger Z, Suh YD, et al. Comparative effectiveness and safety of methods of insulin delivery and glucose monitoring for diabetes mellitus: a systematic review and meta-analysis. Ann Intern Med 2012;157:336-47.ArticlePubMed
- 142. Forlenza GP, Li Z, Buckingham BA, Pinsker JE, Cengiz E, Wadwa RP, et al. Predictive low-glucose suspend reduces hypoglycemia in adults, adolescents, and children with type 1 diabetes in an at-home randomized crossover study: results of the PROLOG trial. Diabetes Care 2018;41:2155-61.ArticlePubMedPDF
- 143. Reznik Y, Cohen O, Aronson R, Conget I, Runzis S, Castaneda J, et al. Insulin pump treatment compared with multiple daily injections for treatment of type 2 diabetes (OpT2mise): a randomised open-label controlled trial. Lancet 2014;384:1265-72.ArticlePubMed
- 144. Powers AC, Aronoff DM, Eckel RH. COVID-19 vaccine prioritisation for type 1 and type 2 diabetes. Lancet Diabetes Endocrinol 2021;9:140-1.ArticlePubMedPMC
- 145. Korean Diabetes Association. Diabetes fact sheet in Korea Available from: www.diabetes.or.kr (cited 2021 Jul 16).
REFERENCES
Figure & Data
References
Citations

- Impact of Subclinical Atrial Function on the Prognosis of Patients With Atrial Fibrillation and Metabolic Syndrome
Hyun-Jin Kim
CardioMetabolic Syndrome Journal.2024; 4(1): 36. CrossRef - Associations of omega-3 fatty acids vs. fenofibrate with adverse cardiovascular outcomes in people with metabolic syndrome: propensity matched cohort study
Nam Hoon Kim, Ji Yoon Kim, Jimi Choi, Sin Gon Kim
European Heart Journal - Cardiovascular Pharmacotherapy.2024; 10(2): 118. CrossRef - A Multicenter, Randomized, Open-Label Study to Compare the Effects of Gemigliptin Add-on or Escalation of Metformin Dose on Glycemic Control and Safety in Patients with Inadequately Controlled Type 2 Diabetes Mellitus Treated with Metformin and SGLT-2 Inh
Hae Jin Kim, Jung Hyun Noh, Min Kyong Moon, Sung Hee Choi, Seung-Hyun Ko, Eun-Jung Rhee, Kyu Yeon Hur, In-Kyung Jeong, Mark Yorek
Journal of Diabetes Research.2024; 2024: 1. CrossRef - Efficacy and Safety of Once-Weekly Semaglutide Versus Once-Daily Sitagliptin as Metformin Add-on in a Korean Population with Type 2 Diabetes
Byung-Wan Lee, Young Min Cho, Sin Gon Kim, Seung-Hyun Ko, Soo Lim, Amine Dahaoui, Jin Sook Jeong, Hyo Jin Lim, Jae Myung Yu
Diabetes Therapy.2024; 15(2): 547. CrossRef - Real-World Continuous Glucose Monitoring Data from a Population with Type 1 Diabetes in South Korea: Nationwide Single-System Analysis
Ji Yoon Kim, Sang-Man Jin, Sarah Andrade, Boyang Chen, Jae Hyeon Kim
Diabetes Technology & Therapeutics.2024;[Epub] CrossRef - Association between Dyslipidemia and Glycated Hemoglobin in a Population-Based Study
Purum Kang, Ka Young Kim, Hye Young Shin
Metabolites.2024; 14(2): 92. CrossRef - Outcomes of Various Classes of Oral Antidiabetic Drugs on Nonalcoholic Fatty Liver Disease
Heejoon Jang, Yeonjin Kim, Dong Hyeon Lee, Sae Kyung Joo, Bo Kyung Koo, Soo Lim, Woojoo Lee, Won Kim
JAMA Internal Medicine.2024; 184(4): 375. CrossRef - View on Metformin: Antidiabetic and Pleiotropic Effects, Pharmacokinetics, Side Effects, and Sex-Related Differences
Guglielmina Froldi
Pharmaceuticals.2024; 17(4): 478. CrossRef - Efficacy of intermittent short‐term use of a real‐time continuous glucose monitoring system in non‐insulin–treated patients with type 2 diabetes: A randomized controlled trial
Sun Joon Moon, Kyung‐Soo Kim, Woo Je Lee, Mi Yeon Lee, Robert Vigersky, Cheol‐Young Park
Diabetes, Obesity and Metabolism.2023; 25(1): 110. CrossRef - Therapeutic Effects of Switching to Anagliptin from Other DPP-4 Inhibitors in T2DM Patients with Inadequate Glycemic Control: A Non-interventional, Single-Arm, Open-Label, Multicenter Observational Study
Sang-Yong Kim, Sungrae Kim
Diabetes Therapy.2023; 14(1): 109. CrossRef - Low Skeletal Muscle Mass Accompanied by Abdominal Obesity Additively Increases the Risk of Incident Type 2 Diabetes
Ji Eun Jun, Seung-Eun Lee, You-Bin Lee, Gyuri Kim, Sang-Man Jin, Jae Hwan Jee, Jae Hyeon Kim
The Journal of Clinical Endocrinology & Metabolism.2023; 108(5): 1173. CrossRef - Diabetes screening in South Korea: a new estimate of the number needed to screen to detect diabetes
Kyoung Hwa Ha, Kyung Ae Lee, Kyung-Do Han, Min Kyong Moon, Dae Jung Kim
The Korean Journal of Internal Medicine.2023; 38(1): 93. CrossRef - Justicia carnea extracts ameliorated hepatocellular damage in streptozotocin-induced type 1 diabetic male rats via decrease in oxidative stress, inflammation and increasing other risk markers
John Adeolu Falode, Oluwaseun Igbekele Ajayi, Tolulope Victoria Isinkaye, Akinwunmi Oluwaseun Adeoye, Basiru Olaitan Ajiboye, Bartholomew I. C. Brai
Biomarkers.2023; 28(2): 177. CrossRef - Sex differences in the impact of diabetes mellitus on tuberculosis recurrence: a retrospective national cohort study
Dararat Eksombatchai, Dawoon Jeong, Jeongha Mok, Doosoo Jeon, Hee-Yeon Kang, Hee Jin Kim, Hee-Sun Kim, Hongjo Choi, Young Ae Kang
International Journal of Infectious Diseases.2023; 127: 1. CrossRef - The Predictive Ability of C-Peptide in Distinguishing Type 1 Diabetes From Type 2 Diabetes: A Systematic Review and Meta-Analysis
Sajid Iqbal, Abdulrahim Abu Jayyab, Ayah Mohammad Alrashdi, Silvia Reverté-Villarroya
Endocrine Practice.2023; 29(5): 379. CrossRef - Anagliptin twice‐daily regimen improves glycaemic variability in subjects with type 2 diabetes: A double‐blind, randomized controlled trial
Yong‐ho Lee, Doo‐Man Kim, Jae Myung Yu, Kyung Mook Choi, Sin Gon Kim, Kang Seo Park, Hyun‐Shik Son, Choon Hee Chung, Kyu Jeung Ahn, Soon Hee Lee, Ki‐Ho Song, Su Kyoung Kwon, Hyeong Kyu Park, Kyu Chang Won, Hak Chul Jang
Diabetes, Obesity and Metabolism.2023; 25(5): 1174. CrossRef - Implementation of five machine learning methods to predict the 52-week blood glucose level in patients with type 2 diabetes
Xiaomin Fu, Yuhan Wang, Ryan S. Cates, Nan Li, Jing Liu, Dianshan Ke, Jinghua Liu, Hongzhou Liu, Shuangtong Yan
Frontiers in Endocrinology.2023;[Epub] CrossRef - Lipid Management in Korean People With Type 2 Diabetes Mellitus: Korean Diabetes Association and Korean Society of Lipid and Atherosclerosis Consensus Statement
Ye Seul Yang, Hack-Lyoung Kim, Sang-Hyun Kim, Min Kyong Moon
Journal of Lipid and Atherosclerosis.2023; 12(1): 12. CrossRef - The Efficacy of Treatment Intensification by Quadruple Oral Therapy Compared to GLP-1RA Therapy in Poorly Controlled Type 2 Diabetes Mellitus Patients: A Real-world Data Study
Minyoung Kim, Hosu Kim, Kyong Young Kim, Soo Kyoung Kim, Junghwa Jung, Jong Ryeal Hahm, Jaehoon Jung, Jong Ha Baek
Diabetes & Metabolism Journal.2023; 47(1): 135. CrossRef - Safety and Effectiveness of Empagliflozin in Korean Patients with Type 2 Diabetes Mellitus: Results from a Nationwide Post-Marketing Surveillance
Jun Sung Moon, Nam Hoon Kim, Jin Oh Na, Jae Hyoung Cho, In-Kyung Jeong, Soon Hee Lee, Ji-Oh Mok, Nan Hee Kim, Dong Jin Chung, Jinhong Cho, Dong Woo Lee, Sun Woo Lee, Kyu Chang Won
Diabetes & Metabolism Journal.2023; 47(1): 82. CrossRef - Evaluation and Management of Patients With Diabetes and Heart Failure: A Korean Diabetes Association and Korean Society of Heart Failure Consensus Statement
Kyu-Sun Lee, Junghyun Noh, Seong-Mi Park, Kyung Mook Choi, Seok-Min Kang, Kyu-Chang Won, Hyun-Jai Cho, Min Kyong Moon
International Journal of Heart Failure.2023; 5(1): 1. CrossRef - Influenza vaccination trend and related factors among patients with diabetes in Korea: Analysis using a nationwide database
Dong-Hwa Lee, Bumhee Yang, Seonhye Gu, Eung-Gook Kim, Youlim Kim, Hyung Koo Kang, Yeong Hun Choe, Hyun Jeong Jeon, Seungyong Park, Hyun Lee
Frontiers in Endocrinology.2023;[Epub] CrossRef - Optimal Low-Density Lipoprotein Cholesterol Level for Primary Prevention in Koreans with Type 2 Diabetes Mellitus
Ji Yoon Kim, Nam Hoon Kim
Diabetes & Metabolism Journal.2023; 47(1): 42. CrossRef - Evaluation and Management of Patients with Diabetes and Heart Failure: A Korean Diabetes Association and Korean Society of Heart Failure Consensus Statement
Kyu-Sun Lee, Junghyun Noh, Seong-Mi Park, Kyung Mook Choi, Seok-Min Kang, Kyu-Chang Won, Hyun-Jai Cho, Min Kyong Moon
Diabetes & Metabolism Journal.2023; 47(1): 10. CrossRef - Lipid Management in Korean People with Type 2 Diabetes Mellitus: Korean Diabetes Association and Korean Society of Lipid and Atherosclerosis Consensus Statement
Ye Seul Yang, Hack-Lyoung Kim, Sang-Hyun Kim, Min Kyong Moon
Diabetes & Metabolism Journal.2023; 47(1): 1. CrossRef - Association between Low-Density Lipoprotein Cholesterol Level and Cardiovascular Outcomes in Korean Adults: A Nationwide Cohort Study
Junghyun Noh, Min Kyong Moon, Eun-Jung Rhee, Sang Hyun Park, Hyeon Chang Kim, Byung Jin Kim, Hae Jin Kim, Seonghoon Choi, Jin Oh Na, Young Youl Hyun, Bum Joon Kim, Kyung-Do Han, In-Kyung Jeong
Diabetes & Metabolism Journal.2023; 47(1): 59. CrossRef - Analysis of the Incidence of Type 2 Diabetes, Requirement of Insulin Treatment, and Diabetes-Related Complications among Patients with Cancer
Su Jung Lee, Chulho Kim, Hyunjae Yu, Dong-Kyu Kim
Cancers.2023; 15(4): 1094. CrossRef - The 2022 focused update of the 2018 Korean Hypertension Society Guidelines for the management of hypertension
Hack-Lyoung Kim, Eun Mi Lee, Shin Young Ahn, Kwang-il Kim, Hyeon Chang Kim, Ju Han Kim, Hae-Young Lee, Jang Hoon Lee, Jong-Moo Park, Eun Joo Cho, Sungha Park, Jinho Shin, Young-Kwon Kim
Clinical Hypertension.2023;[Epub] CrossRef - Consistency of 1-day and 3-day average dietary intake and the relationship of dietary intake with blood glucose, hbA1c, BMI, and lipids in patients with type 2 diabetes
DaeEun Lee, Haejung Lee, Sangeun Lee, MinJin Lee, Ah Reum Khang
Journal of Korean Biological Nursing Science.2023; 25(1): 20. CrossRef - Efficacy and safety of enavogliflozin versus dapagliflozin added to metformin plus gemigliptin treatment in patients with type 2 diabetes: A double-blind, randomized, comparator-active study: ENHANCE-D study
Kyung-Soo Kim, Kyung Ah Han, Tae Nyun Kim, Cheol-Young Park, Jung Hwan Park, Sang Yong Kim, Yong Hyun Kim, Kee Ho Song, Eun Seok Kang, Chul Sik Kim, Gwanpyo Koh, Jun Goo Kang, Mi Kyung Kim, Ji Min Han, Nan Hee Kim, Ji Oh Mok, Jae Hyuk Lee, Soo Lim, Sang S
Diabetes & Metabolism.2023; 49(4): 101440. CrossRef - Effect of olmesartan and amlodipine on serum angiotensin-(1–7) levels and kidney and vascular function in patients with type 2 diabetes and hypertension
Kyuho Kim, Ji Hye Moon, Chang Ho Ahn, Soo Lim
Diabetology & Metabolic Syndrome.2023;[Epub] CrossRef - Menopausal hormone therapy and the risk of type 2 diabetes mellitus: Health Insurance Database in South Korea–based retrospective cohort study
Jin-Sung Yuk, Jung Min Kim
Menopause.2023; 30(5): 497. CrossRef - Intensified Multifactorial Intervention in Patients with Type 2 Diabetes Mellitus
Takayoshi Sasako, Toshimasa Yamauchi, Kohjiro Ueki
Diabetes & Metabolism Journal.2023; 47(2): 185. CrossRef - Association between antidiabetic drugs and the incidence of atrial fibrillation in patients with type 2 diabetes: A nationwide cohort study in South Korea
Sunyoung Kim, So Young Park, Bongseong Kim, Chanyang Min, Wonyoung Cho, Dong Keon Yon, Joo Young Kim, Kyung-Do Han, Eun-Jung Rhee, Won-Young Lee, Sang Youl Rhee
Diabetes Research and Clinical Practice.2023; 198: 110626. CrossRef - Totally robotic Roux-en-Y gastric bypass in a morbidly obese patient in Korea: a case report
Ji Won Seo, Kyong-Hwa Jun
Journal of Minimally Invasive Surgery.2023; 26(1): 40. CrossRef - Effect of diabetes-specific oral nutritional supplements with allulose on weight and glycemic profiles in overweight or obese type 2 diabetic patients
Jihye Tak, Minkyung Bok, Hyunkyung Rho, Ju Hyun Park, Yunsook Lim, Suk Chon, Hyunjung Lim
Nutrition Research and Practice.2023; 17(2): 241. CrossRef - Associations Between Modifiable Risk Factors and Changes in Glycemic Status Among Individuals With Prediabetes
Salma Nabila, Ji-Eun Kim, Jaesung Choi, JooYong Park, Aesun Shin, Sang-Ah Lee, Jong-koo Lee, Daehee Kang, Ji-Yeob Choi
Diabetes Care.2023; 46(3): 535. CrossRef - Efficacy and safety of enavogliflozin, a novel SGLT2 inhibitor, in Korean people with type 2 diabetes: A 24‐week, multicentre, randomized, double‐blind, placebo‐controlled, phase III trial
Soo Heon Kwak, Kyung Ah Han, Kyung‐Soo Kim, Jae Myung Yu, EunSook Kim, Jong Chul Won, Jun Goo Kang, Choon Hee Chung, Seungjoon Oh, Sung Hee Choi, Kyu Chang Won, Sin Gon Kim, Seung Ah Cho, Bo Young Cho, Kyong Soo Park
Diabetes, Obesity and Metabolism.2023; 25(7): 1865. CrossRef - Adjusting the Use of Glucose-Lowering Agents in the Real-World Clinical Management of People with Type 2 Diabetes: A Narrative Review
Siew Pheng Chan, Lee-Ling Lim, Juliana C. N. Chan, David R. Matthews
Diabetes Therapy.2023; 14(5): 823. CrossRef - The association of perfluoroalkyl substances (PFAS) exposure and kidney function in Korean adolescents using data from Korean National Environmental Health Survey (KoNEHS) cycle 4 (2018–2020): a cross-sectional study
Jisuk Yun, Eun-Chul Jang, Soon-Chan Kwon, Young-Sun Min, Yong-Jin Lee
Annals of Occupational and Environmental Medicine.2023;[Epub] CrossRef - A Comparison of the Pharmacokinetics and Safety of Dapagliflozin Formate, an Ester Prodrug of Dapagliflozin, to Dapagliflozin Propanediol Monohydrate in Healthy Subjects
Hyun Chul Kim, Sangmi Lee, Siyoung Sung, Eunjin Kim, In-Jin Jang, Jae-Yong Chung
Drug Design, Development and Therapy.2023; Volume 17: 1203. CrossRef - Efficacy and safety of monotherapy with enavogliflozin in Korean patients with type 2 diabetes mellitus: Results of a 12‐week, multicentre, randomized, double‐blind, placebo‐controlled, phase 2 trial
Ye Seul Yang, Kyung Wan Min, Seok‐O Park, Kyung‐Soo Kim, Jae Myung Yu, Eun‐Gyoung Hong, Sung Rae Cho, Kyu Chang Won, Yong Hyun Kim, Seungjoon Oh, Sung Hee Choi, Gwanpyo Koh, Wan Huh, Su Young Kim, Kyong Soo Park
Diabetes, Obesity and Metabolism.2023; 25(8): 2096. CrossRef - An Integrated Digital Health Care Platform for Diabetes Management With AI-Based Dietary Management: 48-Week Results From a Randomized Controlled Trial
You-Bin Lee, Gyuri Kim, Ji Eun Jun, Hyunjin Park, Woo Je Lee, You-Cheol Hwang, Jae Hyeon Kim
Diabetes Care.2023; 46(5): 959. CrossRef - Performance of Simple Fibrosis Score in Non-Alcoholic Fatty Liver Disease with and without Type 2 Diabetes
Seung Min Chung, Min Kyu Kang, Jun Sung Moon, Jung Gil Park
Endocrinology and Metabolism.2023; 38(2): 277. CrossRef - Correlation analysis of cancer incidence after pravastatin treatment
Jin Yu, Raeun Kim, Jiwon Shinn, Man Young Park, Hun-Sung Kim
Cardiovascular Prevention and Pharmacotherapy.2023; 5(2): 61. CrossRef - Comparison of the effects of gemigliptin versus glimepiride on cardiac function in patients with type 2 diabetes uncontrolled with metformin: The gemi‐heart study
Seung Min Chung, Jun Sung Moon, Jun Hwa Hong, In‐Chang Hwang, Soo Lim
Diabetes, Obesity and Metabolism.2023; 25(8): 2181. CrossRef - The era of continuous glucose monitoring and its expanded role in type 2 diabetes
Jin Yu, Jae‐Hyoung Cho, Seung‐Hwan Lee
Journal of Diabetes Investigation.2023; 14(7): 841. CrossRef - Impact of continuous glucose monitoring on glycemic control and its derived metrics in type 1 diabetes: a longitudinal study
So Hyun Cho, Seohyun Kim, You-Bin Lee, Sang-Man Jin, Kyu Yeon Hur, Gyuri Kim, Jae Hyeon Kim
Frontiers in Endocrinology.2023;[Epub] CrossRef - Impact of mental disorders on the risk of heart failure among Korean patients with diabetes: a cohort study
Tae Kyung Yoo, Kyung-Do Han, Eun-Jung Rhee, Won-Young Lee
Cardiovascular Diabetology.2023;[Epub] CrossRef - Asia-Pacific consensus recommendations for application of continuous glucose monitoring in diabetes management
Alice P.S. Kong, Soo Lim, Seung-Hyun Yoo, Linong Ji, Liming Chen, Yuqian Bao, Ester Yeoh, Siew-Pheng Chan, Chih-Yuan Wang, Viswanathan Mohan, Neale Cohen, Margaret J. McGill, Stephen M. Twigg
Diabetes Research and Clinical Practice.2023; 201: 110718. CrossRef - Chronic disease management program applied to type 2 diabetes patients and prevention of diabetic complications: a retrospective cohort study using nationwide data
Min Kyung Hyun, Jang Won Lee, Seung-Hyun Ko
BMC Public Health.2023;[Epub] CrossRef - Fatty Liver & Diabetes Statistics in Korea: Nationwide Data 2009 to 2017
Eugene Han, Kyung-Do Han, Yong-ho Lee, Kyung-Soo Kim, Sangmo Hong, Jung Hwan Park, Cheol-Young Park
Diabetes & Metabolism Journal.2023; 47(3): 347. CrossRef - Opening the Precision Diabetes Care through Digital Healthcare
Joonyub Lee, Jin Yu, Kun-Ho Yoon
Diabetes & Metabolism Journal.2023; 47(3): 307. CrossRef - Glycemia according to the Use of Continuous Glucose Monitoring among Adults with Type 1 Diabetes Mellitus in Korea: A Real-World Study
You-Bin Lee, Minjee Kim, Jae Hyeon Kim
Diabetes & Metabolism Journal.2023; 47(3): 405. CrossRef - Navigating the Seas of Glycemic Control: The Role of Continuous Glucose Monitoring in Type 1 Diabetes Mellitus
Jun Sung Moon
Diabetes & Metabolism Journal.2023; 47(3): 345. CrossRef - Lost in translation: assessing the nomenclature change for diabetic kidney disease in Japan
Tetsuya Babazono, Tatsumi Moriya
Diabetology International.2023; 14(4): 319. CrossRef - Effects of dapagliflozin compared with glimepiride on body composition in Asian patients with type 2 diabetes inadequately controlled with metformin: The BEYOND study
Hyeong Kyu Park, Kyoung‐Ah Kim, Kyung‐Wan Min, Tae‐Seo Sohn, In Kyung Jeong, Chul Woo Ahn, Nan‐Hee Kim, Ie Byung Park, Ho Chan Cho, Choon Hee Chung, Sung Hee Choi, Kang Seo Park, Seoung‐Oh Yang, Kwan Woo Lee
Diabetes, Obesity and Metabolism.2023; 25(9): 2743. CrossRef - Topic Modeling Analysis of Diabetes-Related Health Information during the Coronavirus Disease Pandemic
Soyoon Min, Jeongwon Han
Healthcare.2023; 11(13): 1871. CrossRef - Screening Test for Evaluation of Cardiovascular Disease in Patients with Diabetes
Ji-Oh Mok, Chan-Hee Jung
The Journal of Korean Diabetes.2023; 24(2): 76. CrossRef - Paradigm Shift in Management of Hyperglycemia in Patients with Type 2 Diabetes: Glucocentric versus Organ Protection
Jong Chul Won
The Journal of Korean Diabetes.2023; 24(2): 59. CrossRef - Association between type 2 diabetes mellitus and depression among Korean midlife women: a cross-sectional analysis study
You Lee Yang, Eun-Ok Im, Yunmi Kim
BMC Nursing.2023;[Epub] CrossRef - Fibrotic Burden in the Liver Differs Across Metabolic Dysfunction-Associated Fatty Liver Disease Subtypes
Tae Seop Lim, Ho Soo Chun, Soon Sun Kim, Ja Kyung Kim, Minjong Lee, Hyo Jung Cho, Seung Up Kim, Jae Youn Cheong
Gut and Liver.2023; 17(4): 610. CrossRef - Association between the number of pregnancies and cardiac target organ damages: a cross-sectional analysis of data from the Korean women’s chest pain registry (KoROSE)
Hack-Lyoung Kim, Hyun-Jin Kim, Mina Kim, Sang Min Park, Hyun Ju Yoon, Young Sup Byun, Seong-Mi Park, Mi-Seung Shin, Kyung-Soon Hong, Myung-A Kim
BMC Women's Health.2023;[Epub] CrossRef - Exercise therapy for diabetes mellitus
Chaiho Jeong, Tae-Seo Sohn
Journal of the Korean Medical Association.2023; 66(7): 427. CrossRef - Medical nutrition therapy for diabetes mellitus
Suk Chon
Journal of the Korean Medical Association.2023; 66(7): 421. CrossRef - Identification of individuals at risk of hepatocellular carcinoma: screening for clinically significant liver fibrosis in patients with T2DM
Tina Reinson, Ryan M Buchanan, Christopher D Byrne
Expert Review of Endocrinology & Metabolism.2023; 18(5): 355. CrossRef - Additive impact of diabetes and sarcopenia on all-cause and cardiovascular mortality: A longitudinal nationwide population-based study
Eyun Song, Soon Young Hwang, Min Jeong Park, Ahreum Jang, Kyeong Jin Kim, Ji Hee Yu, Nam Hoon Kim, Hye Jin Yoo, Ji A. Seo, Sin Gon Kim, Nan Hee Kim, Sei Hyun Baik, Kyung Mook Choi
Metabolism.2023; 148: 155678. CrossRef - Exposure to perfluoroalkyl and polyfluoroalkyl substances and risk of stroke in adults: a meta-analysis
Min Cheol Chang, Seung Min Chung, Sang Gyu Kwak
Reviews on Environmental Health.2023;[Epub] CrossRef - Risk of Pancreatic Cancer and Use of Dipeptidyl Peptidase 4 Inhibitors in Patients with Type 2 Diabetes: A Propensity Score-Matching Analysis
Mee Kyoung Kim, Kyungdo Han, Hyuk-Sang Kwon, Soon Jib Yoo
Endocrinology and Metabolism.2023; 38(4): 426. CrossRef - Incident infection risks depending on oral antidiabetic exposure in insulin-treated type 2 diabetes patients
Sanghwa Park, Jiseon Jeong, Yunna Woo, Yeo Jin Choi, Sooyoung Shin
Scientific Reports.2023;[Epub] CrossRef - Dyslipidemia Fact Sheet in South Korea, 2022
Eun-Sun Jin, Jee-Seon Shim, Sung Eun Kim, Jae Hyun Bae, Shinae Kang, Jong Chul Won, Min-Jeong Shin, Heung Yong Jin, Jenny Moon, Hokyou Lee, Hyeon Chang Kim, In-Kyung Jeong
Journal of Lipid and Atherosclerosis.2023; 12(3): 237. CrossRef - Diabetes Mellitus in the Elderly Adults in Korea: Based on Data from the Korea National Health and Nutrition Examination Survey 2019 to 2020
Seung-Hyun Ko, Kyung Do Han, Yong-Moon Park, Jae-Seung Yun, Kyuho Kim, Jae-Hyun Bae, Hyuk-Sang Kwon, Nan-Hee Kim
Diabetes & Metabolism Journal.2023; 47(5): 643. CrossRef - Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD): A State-of-the-Art Review
Wah-Kheong Chan, Kee-Huat Chuah, Ruveena Bhavani Rajaram, Lee-Ling Lim, Jeyakantha Ratnasingam, Shireene Ratna Vethakkan
Journal of Obesity & Metabolic Syndrome.2023; 32(3): 197. CrossRef - Management of Dyslipidemia in Patients with Diabetes Mellitus
Kyung Ae Lee
The Journal of Korean Diabetes.2023; 24(3): 111. CrossRef - 2023 Clinical Practice Guidelines for Diabetes: Management of Cardiovascular Risk Factors
Ye Seul Yang
The Journal of Korean Diabetes.2023; 24(3): 135. CrossRef - Dyslipidemia Fact Sheet in South Korea, 2022
Eun-Sun Jin, Jee-Seon Shim, Sung Eun Kim, Jae Hyun Bae, Shinae Kang, Jong Chul Won, Min-Jeong Shin, Heung Yong Jin, Jenny Moon, Hokyou Lee, Hyeon Chang Kim, In-Kyung Jeong
Diabetes & Metabolism Journal.2023; 47(5): 632. CrossRef - 2023 Clinical Practice Guidelines for Diabetes Mellitus of the Korean Diabetes Association
Jong Han Choi, Kyung Ae Lee, Joon Ho Moon, Suk Chon, Dae Jung Kim, Hyun Jin Kim, Nan Hee Kim, Ji A Seo, Mee Kyoung Kim, Jeong Hyun Lim, YoonJu Song, Ye Seul Yang, Jae Hyeon Kim, You-Bin Lee, Junghyun Noh, Kyu Yeon Hur, Jong Suk Park, Sang Youl Rhee, Hae J
Diabetes & Metabolism Journal.2023; 47(5): 575. CrossRef - Riesgo residual. Conclusiones
Ángel Cequier, José Luis Zamorano
Revista Española de Cardiología Suplementos.2023; 23: 25. CrossRef - Intake of Fruit and Glycemic Control in Korean Patients with Diabetes Mellitus Using the Korea National Health and Nutrition Examination Survey
Eunju Yoon, Ji Cheol Bae, Sunghwan Suh
Endocrinology and Metabolism.2023; 38(5): 538. CrossRef - 2023 Clinical Practice Guidelines for Diabetes
Min Kyong Moon
The Journal of Korean Diabetes.2023; 24(3): 120. CrossRef - Cumulative effect of impaired fasting glucose on the risk of dementia in middle-aged and elderly people: a nationwide cohort study
Jin Yu, Kyu-Na Lee, Hun-Sung Kim, Kyungdo Han, Seung-Hwan Lee
Scientific Reports.2023;[Epub] CrossRef - Coleus forskohlii Root Extract (ForcslimTM) as a Prospective Antidiabetic Agent: In vitro Glucose Uptake Stimulation and α-Amylase Inhibitory Effects
Firoz Hirehal Hussain Mi, Channangihalli Thimmegowda Sadashiva, Neethumol Benny, Sreedrisya Ayippakkari Kuttiattu, Ravi Subban
International Journal of Pharmacology.2023; 19(5): 730. CrossRef - Comparison of on-Statin Lipid and Lipoprotein Levels for the Prediction of First Cardiovascular Event in Type 2 Diabetes Mellitus
Ji Yoon Kim, Jimi Choi, Sin Gon Kim, Nam Hoon Kim
Diabetes & Metabolism Journal.2023; 47(6): 837. CrossRef - Differential Impact of Obesity on the Risk of Diabetes Development in Two Age Groups: Analysis from the National Health Screening Program
Tae Kyung Yoo, Kyung-Do Han, Yang-Hyun Kim, Ga Eun Nam, Sang Hyun Park, Eun-Jung Rhee, Won-Young Lee
Diabetes & Metabolism Journal.2023; 47(6): 846. CrossRef - Strategies to Maintain the Remission of Diabetes Following Metabolic Surgery
Mi Kyung Kim, Hye Soon Kim
Journal of Metabolic and Bariatric Surgery.2023; 12(2): 26. CrossRef - East Asian perspectives in metabolic and bariatric surgery
Tae Jung Oh, Hyuk‐Joon Lee, Young Min Cho
Journal of Diabetes Investigation.2022; 13(5): 756. CrossRef - Recent Updates to Clinical Practice Guidelines for Diabetes Mellitus
Jin Yu, Seung-Hwan Lee, Mee Kyoung Kim
Endocrinology and Metabolism.2022; 37(1): 26. CrossRef - Association between Physical Exercise and Glycated Hemoglobin Levels in Korean Patients Diagnosed with Diabetes
Il Yun, Hye Jin Joo, Yu Shin Park, Eun-Cheol Park
International Journal of Environmental Research and Public Health.2022; 19(6): 3280. CrossRef - Effectiveness and safety of teneligliptin added to patients with type 2 diabetes inadequately controlled by oral triple combination therapy: A multicentre, randomized, double‐blind, and placebo‐controlled study
Minyoung Lee, Woo‐je Lee, Jae Hyeon Kim, Byung‐Wan Lee
Diabetes, Obesity and Metabolism.2022; 24(6): 1105. CrossRef - Trends of severe hypoglycemia in patients with type 2 diabetes in Korea: A longitudinal nationwide cohort study
Jae‐Seung Yun, Kyungdo Han, Seung‐Hyun Ko
Journal of Diabetes Investigation.2022; 13(8): 1438. CrossRef - GLP-1 receptor agonists in diabetic kidney disease: current evidence and future directions
Ji Hee Yu, So Young Park, Da Young Lee, Nan Hee Kim, Ji A Seo
Kidney Research and Clinical Practice.2022; 41(2): 136. CrossRef - Cardiorenal Risk Profiles Among Data-Driven Type 2 Diabetes Sub-Phenotypes: A Post-Hoc Analysis of the China Health and Nutrition Survey
Hui Gao, Kan Wang, Wensui Zhao, Jianlin Zhuang, Yu Jiang, Lei Zhang, Qingping Liu, Fariba Ahmadizar
Frontiers in Endocrinology.2022;[Epub] CrossRef - Individualized Medical Nutrition Therapy for Diabetic Patients according to Diabetes Medication
Juyeon Park
The Journal of Korean Diabetes.2022; 23(1): 50. CrossRef - Critical shear stress of red blood cells as a novel integrated biomarker for screening chronic kidney diseases in cases of type 2 diabetes
Il Rae Park, Jimi Choi, Eun Young Ha, Seung Min Chung, Jun Sung Moon, Sehyun Shin, Sin Gon Kim, Kyu Chang Won
Clinical Hemorheology and Microcirculation.2022; 81(4): 293. CrossRef - Effects of exercise on reducing diabetes risk in Korean women according to menopausal status
Jung-Hwan Cho, Hye-Mi Kwon, Se-Eun Park, Ju-Hwan Yoo, Kyung-Do Han, Eun-Jung Rhee, Won-Young Lee
Cardiovascular Prevention and Pharmacotherapy.2022; 4(2): 75. CrossRef - Novel Glycemic Index Based on Continuous Glucose Monitoring to Predict Poor Clinical Outcomes in Critically Ill Patients: A Pilot Study
Eun Yeong Ha, Seung Min Chung, Il Rae Park, Yin Young Lee, Eun Young Choi, Jun Sung Moon
Frontiers in Endocrinology.2022;[Epub] CrossRef - Free Versus Fixed-Ratio Combination of Basal Insulin and GLP-1 Receptor Agonists in Type 2 Diabetes Uncontrolled With GLP-1 Receptor Agonists: A Systematic Review and Indirect Treatment Comparison
Han Na Jung, Yun Kyung Cho, Se Hee Min, Hwi Seung Kim, Ye-Jee Kim, Joong-Yeol Park, Woo Je Lee, Chang Hee Jung
Frontiers in Endocrinology.2022;[Epub] CrossRef - Obesity, hypertension, diabetes mellitus, and hypercholesterolemia in Korean adults before and during the COVID-19 pandemic: a special report of the 2020 Korea National Health and Nutrition Examination Survey
Ga Bin Lee, Yoonjung Kim, Suyeon Park, Hyeon Chang Kim, Kyungwon Oh
Epidemiology and Health.2022; 44: e2022041. CrossRef - Adherence to healthy lifestyle behaviors as a preventable risk factor for severe hypoglycemia in people with type 2 diabetes: A longitudinal nationwide cohort study
Jae‐Seung Yun, Kyungdo Han, Yong‐Moon Park, Eugene Han, Yong‐ho Lee, Seung‐Hyun Ko
Journal of Diabetes Investigation.2022; 13(9): 1533. CrossRef - Diabetes Fact Sheet in Korea 2021
Jae Hyun Bae, Kyung-Do Han, Seung-Hyun Ko, Ye Seul Yang, Jong Han Choi, Kyung Mook Choi, Hyuk-Sang Kwon, Kyu Chang Won
Diabetes & Metabolism Journal.2022; 46(3): 417. CrossRef - Comprehensive Understanding for Application in Korean Patients with Type 2 Diabetes Mellitus of the Consensus Statement on Carbohydrate-Restricted Diets by Korean Diabetes Association, Korean Society for the Study of Obesity, and Korean Society of Hyperte
Jong Han Choi, Jee-Hyun Kang, Suk Chon
Diabetes & Metabolism Journal.2022; 46(3): 377. CrossRef - Effect of Carbohydrate-Restricted Diets and Intermittent Fasting on Obesity, Type 2 Diabetes Mellitus, and Hypertension Management: Consensus Statement of the Korean Society for the Study of Obesity, Korean Diabetes Association, and Korean Society of Hype
Jong Han Choi, Yoon Jeong Cho, Hyun-Jin Kim, Seung-Hyun Ko, Suk Chon, Jee-Hyun Kang, Kyoung-Kon Kim, Eun Mi Kim, Hyun Jung Kim, Kee-Ho Song, Ga Eun Nam, Kwang Il Kim
Diabetes & Metabolism Journal.2022; 46(3): 355. CrossRef - Effect of carbohydrate-restricted diets and intermittent fasting on obesity, type 2 diabetes mellitus, and hypertension management: consensus statement of the Korean Society for the Study of obesity, Korean Diabetes Association, and Korean Society of Hype
Jong Han Choi, Yoon Jeong Cho, Hyun-Jin Kim, Seung-Hyun Ko, Suk Chon, Jee-Hyun Kang, Kyoung-Kon Kim, Eun Mi Kim, Hyun Jung Kim, Kee-Ho Song, Ga Eun Nam, Kwang Il Kim
Clinical Hypertension.2022;[Epub] CrossRef - Efficacy of Personalized Diabetes Self-care Using an Electronic Medical Record–Integrated Mobile App in Patients With Type 2 Diabetes: 6-Month Randomized Controlled Trial
Eun Young Lee, Seon-Ah Cha, Jae-Seung Yun, Sun-Young Lim, Jin-Hee Lee, Yu-Bae Ahn, Kun-Ho Yoon, Min Kyung Hyun, Seung-Hyun Ko
Journal of Medical Internet Research.2022; 24(7): e37430. CrossRef - A double‐blind, Randomized controlled trial on glucose‐lowering EFfects and safety of adding 0.25 or 0.5 mg lobeglitazone in type 2 diabetes patients with INadequate control on metformin and dipeptidyl peptidase‐4 inhibitor therapy: REFIND study
Soree Ryang, Sang Soo Kim, Ji Cheol Bae, Ji Min Han, Su Kyoung Kwon, Young Il Kim, Il Seong Nam‐Goong, Eun Sook Kim, Mi‐kyung Kim, Chang Won Lee, Soyeon Yoo, Gwanpyo Koh, Min Jeong Kwon, Jeong Hyun Park, In Joo Kim
Diabetes, Obesity and Metabolism.2022; 24(9): 1800. CrossRef - Hypoglycemic agents and glycemic variability in individuals with type 2 diabetes: A systematic review and network meta-analysis
SuA Oh, Sujata Purja, Hocheol Shin, Minji Kim, Eunyoung Kim
Diabetes and Vascular Disease Research.2022; 19(3): 147916412211068. CrossRef - Tolerability and Effectiveness of Switching to Dulaglutide in Patients With Type 2 Diabetes Inadequately Controlled With Insulin Therapy
Youngsook Kim, Ji Hye Huh, Minyoung Lee, Eun Seok Kang, Bong-Soo Cha, Byung-Wan Lee
Frontiers in Endocrinology.2022;[Epub] CrossRef - Factors Influencing the Utilization of Diabetes Complication Tests Under the COVID-19 Pandemic: Machine Learning Approach
Haewon Byeon
Frontiers in Endocrinology.2022;[Epub] CrossRef - Association of prediabetes with death and diabetic complications in older adults: the pros and cons of active screening for prediabetes
Giwoong Choi, Hojun Yoon, Hyun Ho Choi, Kyoung Hwa Ha, Dae Jung Kim
Age and Ageing.2022;[Epub] CrossRef - Recent information on test utilization and intraindividual change in anti-glutamic acid decarboxylase antibody in Korea: a retrospective study
Rihwa Choi, Wonseo Park, Gayoung Chun, Jiwon Lee, Sang Gon Lee, Eun Hee Lee
BMJ Open Diabetes Research & Care.2022; 10(3): e002739. CrossRef - Extra-Glycemic Effects of Anti-Diabetic Medications: Two Birds with One Stone?
Eun-Jung Rhee
Endocrinology and Metabolism.2022; 37(3): 415. CrossRef - Pharmacological Treatment of Nonalcoholic Fatty Liver Disease: Antidiabetic Agents
Kyung-Soo Kim
The Journal of Korean Diabetes.2022; 23(2): 83. CrossRef - Maintaining Physical Activity Is Associated with Reduced Major Adverse Cardiovascular Events in People Newly Diagnosed with Diabetes
Duhoe Kim, Jaehun Seo, Kyoung Hwa Ha, Dae Jung Kim
Journal of Obesity & Metabolic Syndrome.2022; 31(2): 187. CrossRef - Effect of Carbohydrate-Restricted Diets and Intermittent Fasting on Obesity, Type 2 Diabetes Mellitus, and Hypertension Management: Consensus Statement of the Korean Society for the Study of Obesity, Korean Diabetes Association, and Korean Society of Hype
Jong Han Choi, Yoon Jeong Cho, Hyun-Jin Kim, Seung-Hyun Ko, Suk Chon, Jee-Hyun Kang, Kyoung-Kon Kim, Eun Mi Kim, Hyun Jung Kim, Kee-Ho Song, Ga Eun Nam, Kwang Il Kim
Journal of Obesity & Metabolic Syndrome.2022; 31(2): 100. CrossRef - Advanced Glycation End Products and Their Effect on Vascular Complications in Type 2 Diabetes Mellitus
Jeongmin Lee, Jae-Seung Yun, Seung-Hyun Ko
Nutrients.2022; 14(15): 3086. CrossRef - Severe hypoglycemia as a risk factor for cardiovascular outcomes in patients with type 2 diabetes: is it preventable?
Seung-Hyun Ko
Cardiovascular Prevention and Pharmacotherapy.2022; 4(3): 106. CrossRef - New, Novel Lipid-Lowering Agents for Reducing Cardiovascular Risk: Beyond Statins
Kyuho Kim, Henry N. Ginsberg, Sung Hee Choi
Diabetes & Metabolism Journal.2022; 46(4): 517. CrossRef - Current status of obesity treatment in Korea: based on the 2020 Korean Society for the Study of Obesity guidelines for obesity management
Eun-Jung Rhee
Journal of the Korean Medical Association.2022; 65(7): 388. CrossRef - Experiences of Using Wearable Continuous Glucose Monitors in Adults With Diabetes: A Qualitative Descriptive Study
Hee Sun Kang, Hyang Rang Park, Chun-Ja Kim, Savitri Singh-Carlson
The Science of Diabetes Self-Management and Care.2022; 48(5): 362. CrossRef - 젊은 2형 당뇨병 환자의 관리
재현 배
Public Health Weekly Report.2022; 15(35): 2474. CrossRef - Real-World Prescription Patterns and Barriers Related to the Use of Sodium-Glucose Cotransporter 2 Inhibitors among Korean Patients with Type 2 Diabetes Mellitus and Cardiovascular Disease
Jong Ha Baek, Ye Seul Yang, Seung-Hyun Ko, Kyung Do Han, Jae Hyeon Kim, Min Kyong Moon, Jong Suk Park, Byung-Wan Lee, Tae Jung Oh, Suk Chon, Jong Han Choi, Kyu Yeon Hur
Diabetes & Metabolism Journal.2022; 46(5): 701. CrossRef - Low-Density Lipoprotein Cholesterol Level, Statin Use and Myocardial Infarction Risk in Young Adults
Heekyoung Jeong, Kyungdo Han, Soon Jib Yoo, Mee Kyoung Kim
Journal of Lipid and Atherosclerosis.2022; 11(3): 288. CrossRef - Blood Pressure Target in Type 2 Diabetes Mellitus
Hyun-Jin Kim, Kwang-il Kim
Diabetes & Metabolism Journal.2022; 46(5): 667. CrossRef - Association of underweight status with the risk of tuberculosis: a nationwide population-based cohort study
Su Hwan Cho, Hyun Lee, Hyuktae Kwon, Dong Wook Shin, Hee-Kyung Joh, Kyungdo Han, Jin Ho Park, Belong Cho
Scientific Reports.2022;[Epub] CrossRef - Exploring the risk factors of impaired fasting glucose in middle-aged population living in South Korean communities by using categorical boosting machine
Haewon Byeon
Frontiers in Endocrinology.2022;[Epub] CrossRef - External validation and clinical application of the predictive model for severe hypoglycemia
Jae-Seung Yun, Kyungdo Han, Soo-Yeon Choi, Seon-Ah Cha, Yu-Bae Ahn, Seung-Hyun Ko
Frontiers in Endocrinology.2022;[Epub] CrossRef - Effect of Euonymus alatus Extracts on Diabetes Related Markers in Pancreatic β-Cells and C57BL/Ksj-db/db Mice
Ye Rin Kim, Eun-young Kim, Seong Uk Lee, Young Wan Kim, Yoon Hee Kim
Journal of the Korean Society of Food Science and Nutrition.2022; 51(9): 894. CrossRef - Muscle fat contents rather than muscle mass determines nonalcoholic steatohepatitis and liver fibrosis in patients with severe obesity
Eugene Han, Mi Kyung Kim, Hye Won Lee, Seungwan Ryu, Hye Soon Kim, Byoung Kuk Jang, Youngsung Suh
Obesity.2022; 30(12): 2440. CrossRef - Correlation between shift work and non-alcoholic fatty liver disease among male workers in the steel manufacturing company of Korea: a cross-sectional study
Kiseok Kim, Yong-Jin Lee, Soon-Chan Kwon, Young-Sun Min, Hyun Kyo Lee, Gwangin Baek, Sang Hyeon Kim, Eun-Chul Jang
Annals of Occupational and Environmental Medicine.2022;[Epub] CrossRef - FGM-based remote intervention for adults with type 1 diabetes: The FRIEND randomized clinical trial
Jinju Lee, Myeong Hoon Lee, Jiyun Park, Kyung-Soo Kim, Soo-Kyung Kim, Yong-Wook Cho, Hyun Wook Han, Young Shin Song
Frontiers in Endocrinology.2022;[Epub] CrossRef - Screening for Prediabetes and Diabetes in Korean Nonpregnant Adults: A Position Statement of the Korean Diabetes Association, 2022
Kyung Ae Lee, Dae Jung Kim, Kyungdo Han, Suk Chon, Min Kyong Moon
Diabetes & Metabolism Journal.2022; 46(6): 819. CrossRef - Blood Pressure Control in Patients with Diabetic Kidney Disease
Yaeni Kim, Won Kim, Jwa-Kyung Kim, Ju Young Moon, Samel Park, Cheol Whee Park, Hoon Suk Park, Sang Heon Song, Tae-Hyun Yoo, So-Young Lee, Eun Young Lee, Jeonghwan Lee, Kyubok Jin, Dae Ryong Cha, Jin Joo Cha, Sang Youb Han
Electrolytes & Blood Pressure.2022; 20(2): 39. CrossRef - The Gangwon Obesity and Metabolic Syndrome Study: Methods and Initial Baseline Data
Yoon Jeong Cho, Sohyun Park, Sung Soo Kim, Hyo Jin Park, Jang Won Son, Tae Kyung Lee, Sangmo Hong, Jee-Hyun Kang, Seon Mee Kim, Yang-Hyun Kim, Won Jun Kim, Young Eun Seo, Yoosuk An, Sang Youl Rhee, Suk Chon, Sookyoung Jeon, Kyungho Park, Bong-Soo Kim, Cha
Journal of Obesity & Metabolic Syndrome.2022; 31(4): 303. CrossRef - Oral Semaglutide, the First Ingestible Glucagon-Like Peptide-1 Receptor Agonist: Could It Be a Magic Bullet for Type 2 Diabetes?
Hwi Seung Kim, Chang Hee Jung
International Journal of Molecular Sciences.2021; 22(18): 9936. CrossRef - Long-term effectiveness and safety of quadruple combination therapy with empagliflozin versus dapagliflozin in patients with type 2 diabetes: 3-year prospective observational study
Eu Jeong Ku, Dong-Hwa Lee, Hyun Jeong Jeon, Tae Keun Oh
Diabetes Research and Clinical Practice.2021; 182: 109123. CrossRef - Albuminuria Is Associated with Steatosis Burden in Patients with Type 2 Diabetes Mellitus and Nonalcoholic Fatty Liver Disease (Diabetes Metab J 2021;45:698-707)
Mi-kyung Kim
Diabetes & Metabolism Journal.2021; 45(6): 968. CrossRef - Incidence and Risk Factors for Progression to Diabetes Mellitus: A Retrospective Cohort Study
Min Kyung Hyun, Jong Heon Park, Kyoung Hoon Kim, Soon-Ki Ahn, Seon Mi Ji
International Journal of Environmental Research and Public Health.2021; 19(1): 123. CrossRef - 2021 Clinical Practice Guidelines for Diabetes: Pharmacotherapy and the Korean Diabetes Association Support System
Kyu Yeon Hur
The Journal of Korean Diabetes.2021; 22(4): 250. CrossRef - 2021 Clinical Practice Guidelines for Diabetes: Management of Cardiovascular Risk
Min Kyong Moon
The Journal of Korean Diabetes.2021; 22(4): 259. CrossRef

 KDA
KDA PubReader
PubReader ePub Link
ePub Link Cite
Cite